Abstract
Objectives:
Small dense low-density lipoprotein (sdLDL) which has a small LDL particle size with greater susceptibility to oxidation is regarded as a risk marker for cardiovascular disease. The diacron reactive oxygen metabolites (d-ROMs) test has recently been introduced as an oxidative stress-related marker in the clinic. The aim of the present study was to investigate the correlation between the mean LDL particle size and the oxidative stress status as evaluated by the d-ROMs in dyslipidemic patients.
Methods:
The study included 278 dyslipidemic patients (121 male and 157 female, mean age, 60 years). Clinical data including the conventional atherosclerotic risk factors in addition to the mean LDL particle size measured with the gel electrophoresis and the d-ROMs were collected.
Results:
Male patients had a significantly smaller mean LDL particle size than females (262.2 ± 7.5 [SD] vs. 264.3 ± 6.7 Å, P<0.05), while female patients had a significantly higher d-ROMs level than males (318 ± 68 vs. 350 ± 72 U. Carr., P<0.01). A multiple regression analysis revealed that there was an independent, significant, and inverse correlation between the mean LDL particle size and the d-ROMs (β=−0.19, P<0.05).
Conclusions:
These findings of the co-existence of both markers suggest that sdLDL and oxidative stress can be cooperative in atherogenesis, possibly leading to the incidence of CVD, in dyslipidemic patients.
Keywords: d-ROMs test, hyperlipidemia, mean LDL particle size, oxidative stress, small dense LDL
INTRODUCTION
Cardiovascular disease (CVD) occurs frequently and remains the most common cause of death in the world; therefore, a deeper understanding of the pathophysiology of CVD, more feasible estimation of CVD risks in the clinical settings, and better development of preventive strategies are necessary in order to control the incidence of CVD.[1] Dyslipidemia is an endocrine and metabolic disorder and has been recognized to identify a target population that is at increased risk of developing CVD.[2,3] In addition to the quantitative levels of low-density lipoprotein (LDL) cholesterol (LDL-C), much attention has been drawn to the qualitative features of LDL particles as a risk factor for CVD.[4,5] The existence of small dense LDL (sdLDL) characterized as a smaller size of LDL particle is regarded as a qualitative feature of LDL in relation to an increased risk of CVD.[4,5] SdLDL is more susceptible to oxidation; thus, the biological modification of sdLDL particles is related to its atherogenic properties.[5,6]
However, the independent role of sdLDL on the development of CVD is still being debated because multiple interdependencies exist between atherogenic and oxidative stress-related pathophysiologies.[5] More data are therefore required to explore the oxidation concept of sdLDL, but there have been few clinical studies showing the association between sdLDL and oxidative stress-related markers.[7,8] In fact, only a few studies have reported a significant and independent correlation between sdLDL and oxidized LDL as an oxidative stress-related marker in diabetic patients[7] or between sdLDL and malondialdehyde or between sdLDL and superoxide anion in healthy middle-aged subjects.[8] This is partially due to the limited indices available to easily analyze the oxidative stress status of patients in daily clinical practice.[9,10] The recently introduced diacron reactive oxygen metabolites (d-ROMs) test (Diacron, Grosseto, Italy) can quantify the oxidative stress status by measuring primarily the levels of hydroperoxides of global organic compounds (lipids, proteins, nucleic acids, etc.) and has been used as a simple clinical marker of oxidative stress.[11–14] The difference in the markers used to measure the oxidative stress status may be associated with different clinical implications, so the relationship between sdLDL and the d-ROMs can be observed in a restricted population of dyslipidemic patients. The aim of the present study was to investigate the correlation between the mean LDL particle size and the oxidative stress status as evaluated by the d-ROMs, in dyslipidemic patients.
METHODS
The study population included 278 dyslipidemic Japanese patients (121 male and 157 female, mean age, 60 years). Dyslipidemia was diagnosed according to the guidelines of the Japan Atherosclerosis Society (circulating concentrations of LDL-C≥3.64 mmol/L, triglycerides [TG]≥1.69 mmol/L, high-density lipoprotein cholesterol [HDL-C]<1.04 mmol/L).[15] The inclusion criteria were patients not taking lipid-lowering medications, (if with diabetes mellitus) patients with a well-controlled glycemic conditions under dietary treatment and/or treatment with oral antihyperglycemic drugs such as biguanides, alpha-glucosidase inhibitors and sulfonylureas, and (if with hypertension) patients taking oral antihypertensive drugs such as beta blockers, calcium channel blockers, angiotensin-converting enzyme inhibitors, and angiotensin-receptor blockers. The exclusion criteria were individuals who were pregnant, had acute infections such as the common cold, were alcohol abusers, or had a history of cardio/cerebrovascular, thyroid, collagen, severe kidney or liver diseases, as well as those treated with insulin injections, oral contraceptives and antioxidant agents. The study was approved by the Institutional Ethics Committee and all subjects gave their informed consent.
The patients’ current smoking habits were based on self-reports. The systolic blood pressure (SBP) and diastolic blood pressure (DBP) levels were measured in the right arm with a mercury sphygmomanometer with the patient in the seated position. Hypertension was defined by SBP≥140 mmHg and/or DBP≥90 mmHg or the use of oral antihypertensive drugs. The fasting serum LDL-C, TG, HDL-C, and fasting plasma glucose (FPG) levels were enzymatically measured (Kyowa Medics Co. Ltd., Tokyo, Japan). Diabetes mellitus was defined by FPG≥7.0 mmol/L or the use of oral antihyperglycemic drugs. The body mass index (BMI) was calculated as the weight in kilograms divided by the square of the height in meters. Obesity was defined as a BMI≥25 kg/m2 for Japanese people.[16]
The mean LDL particle size was measured with a high-resolution, non-gradient polyacrylamide gel electrophoresis system (the Lipoprint system; Quantimetrix, Redondo Beach, CA, USA), which has been validated by using the gold standard method of nuclear magnetic resonance spectroscopy. Briefly, after serum samples (25 μL) were photopolymerized, the samples and loading gels were applied to gel tubes and then electrophoresed. The scanning system calculated the mean LDL particle size based on the fractionalized lipoproteins.[17] On the other hand, the d-ROMs values were obtained using a kinetic spectrophotometric assay (the F.R.E.E system; Diacron, Italy) with intra-and inter-assay coefficients of variation of 2.1% and 3.1%, respectively.[11,12] Briefly, serum samples (25 μL) were mixed with a buffered solution and a chromogenic substrate was added to the mixture. The mixture was centrifuged and then incubated in the thermostatic block of the system. The absorbance was recorded at 505 nm. The measurements are expressed in U. Carr., where 1 U. Carr. corresponds to 0.08 mg/dL H2O2.
The data are expressed as the means±standard deviation (SD) or the medians plus the interquartile range. The data between the groups were compared using unpaired t-tests, Chi-square tests or (if the d-ROMs levels were compared among three groups according to the tertiles of mean LDL particle size) one-way ANOVA with multiple comparison tests. A simple correlation test (Pearson's test) and a multiple linear regression analysis were utilized to observe the correlation between the mean LDL particle size and d-ROMs. All of the atherosclerotic risk factors (age, gender, smoking, obesity, hypertension, hyper-LDL-cholesterolemia, hypertriglyceridemia, hypo-high-density lipoprotein cholesterolemia), or the above-mentioned risk factors plus the use of antihypertensive drugs and antihyperglycemic drugs were entered into the multiple linear regression analysis model as confounding variables. These statistical analyses were performed with the software package SPSS 16.0 (SPSS Inc., Chicago, IL, USA). Statistical significance was defined as a P value <0.05.
RESULTS
The clinical characteristics of the patients are shown in Table 1. Male patients were significantly older and had significantly higher TG levels as well as a higher prevalence of smoking and hypertriglyceridemia than female patients. Males had a significantly smaller mean LDL particle size than females. Female patients had significantly higher levels of LDL-C, HDL-C, and d-ROMs than male patients. In addition, the d-ROMs levels exhibited a significant decrease (P=0.036) from the lowest tertile group with a mean LDL particle size of <261.8 Å (n=90, 350 ± 79 U. Carr.), to the middle tertile group (261.8 to <266.9 Å [n=94], 335 ± 66 U. Carr.) to the highest tertile group (≥266.9 Å [n=94], 323 ± 70 U. Carr.). In particular, a significant difference in the d-ROMs levels was observed between the lowest tertile group and the highest tertile group with regard to the mean LDL particle size (P=0.030).
Table 1.
The clinical characteristics of the dyslipidemic patients
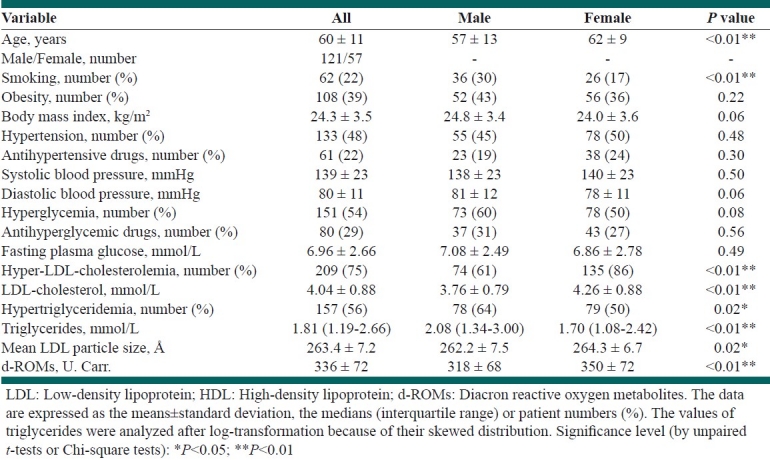
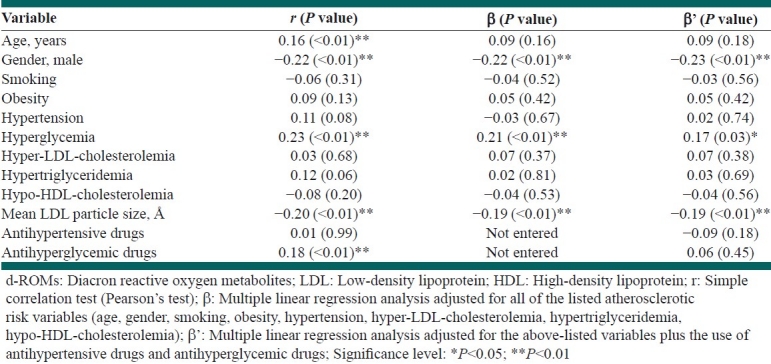
Table 2 shows the correlations of the d-ROMs with other variables including the mean LDL particle size in all patients. A simple correlation analysis showed that age, female gender, hyperglycemia, and the use of antihyperglycemic drugs were significantly and positively correlated with the d-ROMs levels, while the mean LDL particle size was significantly and inversely correlated with the d-ROMs. A subsequent multiple regression analysis, adjusted for all of the listed atherosclerotic risk factors, showed that female gender and hyperglycemia remained independently, significantly, and positively correlated with the d-ROM levels, while the mean LDL particle size also remained independently, significantly, and inversely correlated with the d-ROMs. The same results were observed in a further multiple regression analysis adjusted for the above-mentioned risk factors plus the use of antihypertensive drugs and antihyperglycemic drugs.
Table 2.
The correlation of each variable with the d-ROMs in all patients
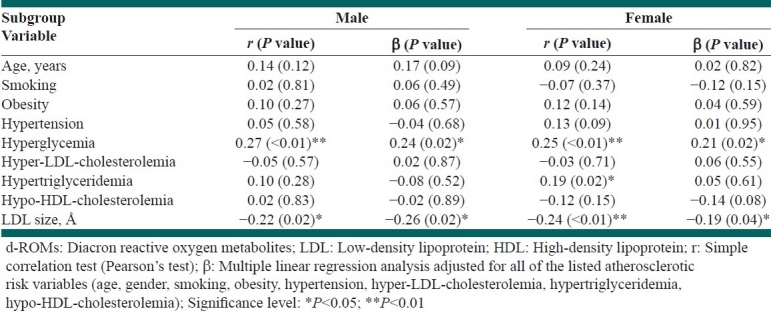
In addition, Table 3 shows the gender-based correlations of the d-ROMs with other variables, including the mean LDL particle size, in the subanalyses. A simple correlation analysis showed that hyperglycemia (in both the genders) and hypertriglyceridemia (in females) were significantly and positively correlated with the d-ROMs levels, while the mean LDL particle size was significantly and inversely correlated with the d-ROMs in both the genders. A subsequent multiple regression analysis, adjusted for all of the listed atherosclerotic risk factors, showed that hyperglycemia remained independently, significantly, and positively correlated with the d-ROM levels, while the mean LDL particle size also remained independently, significantly, and inversely correlated with the d-ROMs in both the genders. The correlation was somewhat greater in males than in females. When the further multiple regression analysis, adjusted for the above-mentioned risk factors plus the use of antihypertensive drugs and antihyperglycemic drugs, was conducted (all data not shown), the correlation between hyperglycemia and the d-ROMs levels was decreased in both the genders (β=0.12, P=0.30 in males, β=0.22, P=0.049 in females). However, there remained an independent, significant, and inverse correlation between the mean LDL particle size and d-ROMs in both the genders (β=-0.27, P=0.02 in males, β=–0.20, P=0.045 in females).
Table 3.
The correlation of each variable with the d-ROMs by gender
DISCUSSION
The present study showed an independent, significant, and inverse correlation between the mean LDL particle size and the oxidative stress status, as evaluated by the d-ROMs test in dyslipidemic patients. The correlation was significant, but relatively weak, so the clinical relevance must be confirmed in the future studies. The present results seem to be consistent with the prior studies on the association between sdLDL and oxidative stress-related markers which were different from the markers used in our present study.[7,8] Of importance, this study on dyslipidemic patients adds clinical information on the correlation between the sdLDL level and the oxidative stress status by using an easy and recent marker under the current situation where few data on the association between sdLDL and oxidative stress-related markers were available in the clinical setting[7,8] and the debate continues about the independency of their associations with the other conventional atherosclerotic risk factors (i.e., hypertriglyceridemia).[5] Moreover, it is important to note that the present findings support the idea that sdLDL and oxidative stress can be cooperative factors in atherogenesis.[4,5]
The association between sdLDL and oxidative stress may be partially explained by the following biological mechanism. SdLDL can be formed in the in vivo environment where there is an increased oxidative stress status, for instance, in the presence of insulin resistance and when patients have a sedentary lifestyle.[5,18] In addition, sdLDL can induce oxidative stress.[5,6,19] Because of their low affinity for the LDL receptor, their prolonged half-life in the circulation and their low resistance to oxidative stress, sdLDL particles are taken up easily in the arterial walls and have an increased oxidative susceptibility with their retention in the walls leading to uptake by macrophages, and thereafter, foam cell formation.[5,6,19] The vascular atherosclerotic process produces oxidative stress.[20]
The gender-based subanalyses showed a somewhat greater correlation between the mean LDL particle size and d-ROMs in males than in females. The reason for this result was unclear. These results may be partially affected by the larger mean LDL particle size and higher d-ROMs levels in females than in males [Table 1], while there have been prior studies reporting that females could have a larger LDL particle size[21] and have a tendency to have high d-ROMs level.[14] Further research is therefore needed to confirm whether there are any gender differences in the relationship between the mean LDL particle size and d-ROMs and whether any such difference may contribute to the gender differences in the incidence of CVD in relation to lipoprotein metabolism.[20,22]
Some limitations of this study merit consideration. The cross-sectional study design did not determine the cause-and-result relationship. The data regarding the CVD-related outcomes were not available in this study. In addition, we did not obtain any data on control populations, such as healthy, non-dyslipidemic or child subjects. The existence of sdLDL is reported to be affected by environmental and genetic factors, so the correlation between sdLDL and oxidative stress-related markers may differ between the studied populations (i.e., adults and children).[23] Therefore, future studies with a prospective and interventional design, the consideration of CVD-related outcomes and various populations will be necessary to confirm the present study findings.
In summary, the present study showed that there was an independent, significant, and inverse correlation between the mean LDL particle size and the oxidative stress status as evaluated by the d-ROMs test in dyslipidemic patients. These findings of the co-existence of both markers suggest that sdLDL and oxidative stress can be cooperative factors in atherogenesis, possibly leading to the incidence of CVD in these patients. Further studies are required to establish the observed relationship.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Franco M, Cooper RS, Bilal U, Fuster V. Challenges and opportunities for cardiovascular disease prevention. Am J Med. 2011;124:95–102. doi: 10.1016/j.amjmed.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 2.LaRosa JC, Gotto AM., Jr Past, present, and future standards for management of dyslipidemia. Am J Med. 2004;116(Suppl 6A):S3–8. doi: 10.1016/j.amjmed.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Ferguson EE., Jr Preventing, stopping, or reversing coronary artery disease - triglyceride-rich lipoproteins and associated lipoprotein and metabolic abnormalities: The need for recognition and treatment. Dis Mon. 2000;46:421–503. doi: 10.1016/s0011-5029(00)90011-7. [DOI] [PubMed] [Google Scholar]
- 4.Packard CJ. Small dense low-density lipoprotein and its role as an independent predictor of cardiovascular disease. Curr Opin Lipidol. 2006;17:412–7. doi: 10.1097/01.mol.0000236367.42755.c1. [DOI] [PubMed] [Google Scholar]
- 5.Rizzo M, Berneis K. Low-density lipoprotein size and cardiovascular risk assessment. QJM. 2006;99:1–14. doi: 10.1093/qjmed/hci154. [DOI] [PubMed] [Google Scholar]
- 6.Chapman MJ, Guérin M, Bruckert E. Atherogenic, dense low-density lipoproteins.Pathophysiology and new therapeutic approaches. Eur Heart J. 1998;19(Suppl A):A24–30. [PubMed] [Google Scholar]
- 7.Lee W, Min WK, Chun S, Jang S, Kim JQ, Lee DH, et al. Low-density lipoprotein subclass and its correlating factors in diabetics. Clin Biochem. 2003;36:657–61. doi: 10.1016/s0009-9120(03)00109-7. [DOI] [PubMed] [Google Scholar]
- 8.Vekic J, Kotur-Stevuljevic J, Jelic-Ivanovic Z, Spasic S, Spasojevic-Kalimanovska V, Topic A, et al. Association of oxidative stress and PON1 with LDL and HDL particle size in middle-aged subjects. Eur J Clin Invest. 2007;37:715–23. doi: 10.1111/j.1365-2362.2007.01849.x. [DOI] [PubMed] [Google Scholar]
- 9.Piconi L, Quagliaro L, Ceriello A. Oxidative stress in diabetes. Clin Chem Lab Med. 2003;41:1144–9. doi: 10.1515/CCLM.2003.177. [DOI] [PubMed] [Google Scholar]
- 10.Stephens JW, Khanolkar MP, Bain SC. The biological relevance and measurement of plasma markers of oxidative stress in diabetes and cardiovascular disease. Atherosclerosis. 2009;202:321–9. doi: 10.1016/j.atherosclerosis.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Iamele L, Fiocchi R, Vernocchi A. Evaluation of an automated spectrophotometric assay for reactive oxygen metabolites in serum. Clin Chem Lab Med. 2002;40:673–6. doi: 10.1515/CCLM.2002.115. [DOI] [PubMed] [Google Scholar]
- 12.Vassalle C. An easy and reliable automated method to estimate oxidative stress in the clinical setting. Methods Mol Biol. 2008;477:31–9. doi: 10.1007/978-1-60327-517-0_3. [DOI] [PubMed] [Google Scholar]
- 13.Kotani K, Sakane N, Tsuzaki K, Matsuoka Y, Sano Y, Hamada T, et al. Lifestyles and oxidative stress in type 2 diabetic patients. Scand J Clin Lab Invest. 2008;68:516–8. doi: 10.1080/00365510802023090. [DOI] [PubMed] [Google Scholar]
- 14.Hirose H, Kawabe H, Komiya N, Saito I. Relations between serum reactive oxygen metabolites (ROMs) and various inflammatory and metabolic parameters in a Japanese population. J Atheroscler Thromb. 2009;16:77–82. doi: 10.5551/jat.e265. [DOI] [PubMed] [Google Scholar]
- 15.Teramoto T, Sasaki J, Ueshima H, Egusa G, Kinoshita M, Shimamoto K, et al. Japan Atherosclerosis Society (JAS) Committee for Epidemiology and Clinical Management of Atherosclerosis. Diagnostic criteria for dyslipidemia. Executive summary of Japan Atherosclerosis Society (JAS) guideline for diagnosis and prevention of atherosclerotic cardiovascular diseases for Japanese. J Atheroscler Thromb. 2007;14:155–8. doi: 10.5551/jat.e537. [DOI] [PubMed] [Google Scholar]
- 16.Examination Committee of Criteria for ‘Obesity Disease’ in Japan; Japan Society for the Study of Obesity. New criteria for ‘obesity disease’ in Japan. Circ J. 2002;66:987–92. doi: 10.1253/circj.66.987. [DOI] [PubMed] [Google Scholar]
- 17.Tsuzaki K, Kotani K, Fujiwara S, Sano Y, Matsuoka Y, Domichi M, et al. The Trp64Arg polymorphism of the beta3-adrenergic receptor gene is associated with increased small dense low-density lipoprotein in a rural Japanese population: The Mima study. Metabolism. 2007;56:1689–93. doi: 10.1016/j.metabol.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 18.Howard BV. Insulin resistance and lipid metabolism. Am J Cardiol. 1999;84:J28–32. doi: 10.1016/s0002-9149(99)00355-0. [DOI] [PubMed] [Google Scholar]
- 19.Packard CJ, Shepherd J. Lipoprotein heterogeneity and apolipoprotein B metabolism. Arterioscler Thromb Vasc Biol. 1997;17:3542–56. doi: 10.1161/01.atv.17.12.3542. [DOI] [PubMed] [Google Scholar]
- 20.Madamanchi NR, Hakim ZS, Runge MS. Oxidative stress in atherogenesis and arterial thrombosis: The disconnect between cellular studies and clinical outcomes. J Thromb Haemost. 2005;3:254–67. doi: 10.1111/j.1538-7836.2004.01085.x. [DOI] [PubMed] [Google Scholar]
- 21.Nikkilä M, Pitkäjärvi T, Koivula T, Solakivi T, Lehtimäki T, Laippala P, et al. Women have a larger and less atherogenic low density lipoprotein particle size than men. Atherosclerosis. 1996;119:181–90. doi: 10.1016/0021-9150(95)05645-9. [DOI] [PubMed] [Google Scholar]
- 22.Bittner V. Perspectives on dyslipidemia and coronary heart disease in women: An update. Curr Opin Cardiol. 2006;21:602–7. doi: 10.1097/01.hco.0000245739.47712.0a. [DOI] [PubMed] [Google Scholar]
- 23.Stan S, Levy E, Delvin EE, Hanley JA, Lamarche B, O’Loughlin J, et al. Distribution of LDL particle size in a population-based sample of children and adolescents and relationship with other cardiovascular risk factors. Clin Chem. 2005;51:1192–200. doi: 10.1373/clinchem.2004.046771. [DOI] [PubMed] [Google Scholar]


