Abstract
Background:
The objective of this study is to estimate the average diagnosis and treatment costs of chronic hepatitis B and C, with respect to different therapeutic strategies in Iran.
Methods:
This is a descriptive, analytical, and cross-sectional study carried out on patients with hepatitis B and C, who were referred to the Liver Disease Research Center for Prevention and Treatment of Hepatitis, Isfahan University of Medical Sciences, in 2011. We have estimated the direct medical costs including doctors′ fees, cost of para-clinical tests, medical treatments, and liver biopsy, in different treatment strategies.
Findings:
The results of this study showed that the total cost of diagnostic services for hepatitis B virus (HBV) and hepatitis C virus (HCV) patients, with state medical tariffs, was US$ 1499.07 and US$ 2084.89, respectively. The patients’ profiles showed that there were currently seven therapeutic strategies available to treat HBV patients. The total cost of treatment strategies varied significantly from US$ 73 to US$ 8256. There were also four main strategies for HCV patients, each of these could be applied in two periods of time. The total cost of these treatment strategies showed a high discrepancy from US$ 242 to US$ 8256.
Conclusion:
The results confirmed that the total direct medical cost for an HBV patient in Iran exceeded US$ 5.5 Milliard in 2011. The results implied that the market price of direct medical cost of HBV and HCV patients in Iran is much higher than the estimated state costs. These costs would likely be saved or reduced by effective disease management and early prevention.
Keywords: Direct medical costs, hepatitis B, hepatitis C
INTRODUCTION
Health expenditures have considerably increased worldwide in the recent decades. The Iranian healthcare system has also faced considerable challenges because of increasing its health expenditures.[1] These expenditures may have originated directly from the cost of diagnosis and treatment services of the disease or indirectly from loss of productivity and quality of life. Considering the limited resources for delivery of healthcare services, effective healthcare delivery is one of the general concerns of healthcare systems over the world.[2] As treatment costs are the major portion of health expenditure in many countries, recognizing and analyzing the direct treatment costs of diseases may provide health care managers with a better understanding of their financing issues and strategies.[3]
Hepatitis B Virus infection is the most widespread cause of chronic hepatitis across the world. A ten-year study of the histological variation in the liver, in 100 inactive hepatitis B cases, showed that many of inactive HBV patients would significantly transfer to chronic hepatitis,[4] that is, inactive hepatitis, may potentially deliver the risk of disease to the patients themselves, their relatives, and also other healthy people. The Hepatitis C virus affects 100 to 300 million people worldwide. Chronic HCV infection is one of the leading causes of chronic liver disease and related complications such as cirrhosis and hepatocellular carcinoma.[5,6] These two diseases, like many other chronic diseases, can impose various kinds of costs on patients and health systems worldwide.
Hepatitis B and C are two autoimmune liver diseases, which impose a high economic burden on individuals and the society in all countries.[7,8] A considerable number of studies have been published in literature, which aim to measure the economic burden of hepatitis on societies.[9,10]
The treatment cost of patients with hepatitis B was evaluated and determined in different studies. As these studies were carried out in different areas, times, and under different conditions, the results were widely different. Nevertheless, all of them indicated that the treatment costs of chronic diseases including hepatitis B and C were very high and could impose a great socioeconomic burden on individuals and their societies.
One of the elements that affects the cost of treatment significantly is the selected treatment regimen for patients. Thus, it is necessary to estimate the cost of each treatment procedure separately. In contrast to the importance of the economic costs of these treatments in Iran, to the best of our knowledge, the cost analysis of these treatments in Iran has not yet been published. The aim of this study is therefore to estimate the direct economic costs of hepatitis treatments in Iran.
METHODS
This is a descriptive, analytical, and cross-sectional study carried out on patients with chronic hepatitis B and C, who were referred to the Liver Disease Research Center for Prevention and Treatment of Hepatitis, in the Isfahan University of Medical Sciences in 2011. First, a review of the literature was undertaken to recognize the latest treatments available for patients with chronic HBV and chronic HCV, in Iran. Subsequently, the process of chronic HBV and chronic HCV management, including para-clinical tests, physician visits, and drug treatments was reviewed using patients′ profiles in the Liver Disease Research Center for the Prevention and Treatment of Hepatitis in Isfahan.
The direct medical costs of the services[11] offered to patients were obtained from the official medical tariff for state-owned centers. The direct nonmedical and indirect costs of HBV and HCV were not included, because of unavailability of the data on the patients′ profiles.
RESULTS
The review of the literature, as well as patient's profiles showed that there were some diagnostic and treatment services, which were applicable for all patients with HBV and also for patients with HCV. We have summarized these services and their costs in Tables 1 and 2 separately. As it is clear from Table 1, the total cost of diagnostic services for HBV patients was US$ 1499.07 annually. The test for detection of HBV-DNA (deoxyribonucleic acid) was the most expensive service among the diagnostic and treatment services for HBV patients.
Table 1.
Common diagnostic and treatment services and their costs for patients with chronic hepatitis B
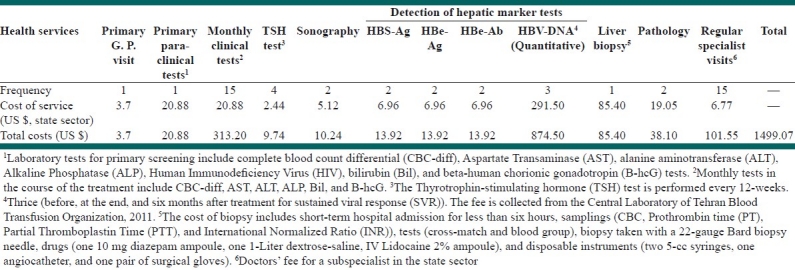
Table 2.
Common diagnostic and treatment services and their costs for patients with chronic hepatitis C
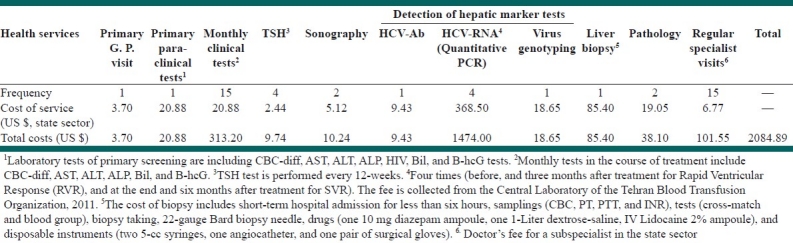
The average total cost of diagnostic and treatment services for each HCV patient was estimated to be US$ 2084.89 per year [Table 2], which is nearly 40% higher than the estimated cost for HBV patients. Similar to HBV patients, the most expensive service for HCV patients was the test for the detection of HCV-RNA (Ribonucleic Acid).
The patient's profiles showed that there are currently seven therapeutic strategies available in Iran to treat HBV patients. The total cost of treatment strategies listed in Table 3 is related to a single patient with hepatitis B, which is based on the duration of treatment and the amount of medications used. Each of these strategies can be used as first-line treatments for HBV.[12]
Table 3.
The summary of therapeutic procedures and its associated costs for treatment of HBV patients
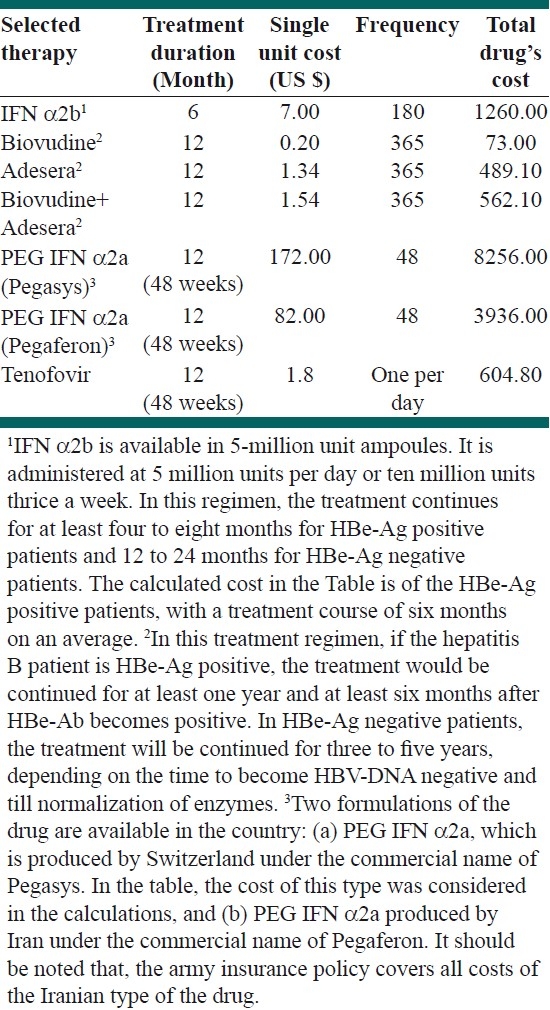
There are also various therapeutic regimens available for treating HCV patients. These regimens are summarized in Table 4. As it is clear from the table, there are four main strategies for HCV patients, each of these could be applied in two periods of time, 24 or 48 weeks.
Table 4.
The summary of therapeutic procedures and its associated costs for treatment of HCV patients
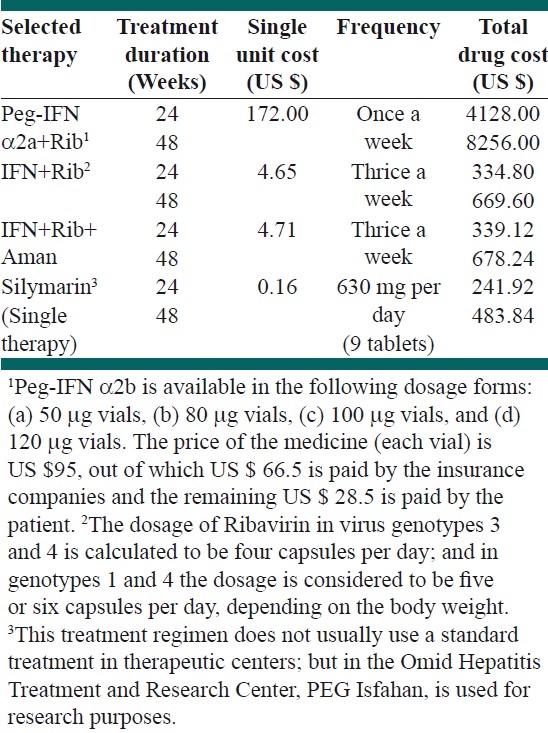
Although medical literature states that the first treatment choice for chronic hepatitis C is Rib+Peg IFN α2a, and the alternative regimen is Ribavirin+IFN α2b, the patients profiles show that the other therapeutic strategies used for HCV patients are Silymarin and Amantadin+Ribavirin+IFN α2b. Chronic hepatitis C is usually treated with multiple-drug regimens, but in patients with contraindications for Ribavirin, for instance those with hemolytic diseases such as thalassemia or severe anemia (Hb<10 mg/dl), Ribavirin should be omitted from the treatment regimens.
The total cost of treatment in Table 4 shows that the treatment regimens with Peg-IFN impose the highest economic burden on the society.
DISCUSSION
Hepatitis B and particularly C are significant diseases that could turn into chronic diseases.[4,5] Similar to other chronic diseases, these can impose great costs on the individuals, health insurance organizations, health systems, and as a final point, to the society.
The results of this study show that the direct medical costs of treating HBV and HCV are very high in Iran. It is particularly more important to bear in mind that these direct medical costs are estimated using state medical tariffs. However, as the price of medical services in the private sector is much higher than in the state sector, it is reasonable to conclude that the market price of treating HBV and HCV patients, and consequently the cost of treating these patients, are at least five times greater than the estimated cost using the state sector medical tariffs.
Summarizing the availability of therapeutic strategies for treating HBV patients confirmed that there are currently six active treatment protocols for these patients. Although this could be optimistically considered as freedom of choice for both patients and clinicians, this may also be regarded as a source of inefficient HBV management. This is particularly important considering the wide variation in costs of these strategies. As Table 3 shows, the lowest price therapeutic strategy is Biovudine treatment with US $73, whereas, the cost of the Pegasys strategy is almost 113 times higher than that of Biovudine [Table 3].
The presented results in Table 4 also illustrate a wide range of variations in the therapeutic protocols for treating HCV infections. The difference between the lowest price protocol, 24 weeks Silymarin utilization, and the highest price protocol, 48 weeks combination therapy with Peg-IFN α2a+Rib, is more than 34 times [Table 4].
Taking into account the fact that the cost of diagnostic services is identical for all therapeutic strategies, it is certainly reasonable to evaluate whether the clinical outcomes associated with these protocols are worth their costs.
With regards to both diagnostic and therapeutic costs, the average direct medical costs for each HBV patient per year is estimated to be US $3668 with the state medical tariffs. Although there is no accurate figure of HBV patients in Iran, some studies suggest that the population with HBV in Iran was around 1.5 million, in 2008.[13] Therefore, the total direct medical cost of HBV patients in Iran could be expected to be higher than US $5.5 Milliard, in 2011, considering the state medical tariffs. Likewise, the average direct medical cost for each HCV patient, per year, is estimated to be US $3976. Regarding the fact that the population with HCV in Iran is estimated to have been 120 thousand in 2008,[14] the total direct medical cost of HCV patients in Iran is nearly US $0.5 Milliard in 2010; with state medical tariffs.
The results of this study may encourage clinicians and health policy makers to think more about the most cost-effective therapeutic strategies for managing HBV and HCV patients and also to consider preventive strategies, which may possibly provide better value for money for managing hepatitis in Iran.
Hajarizadeh et al., carried out a study to determine the costs of a hepatitis B immunization program in juveniles, in 2006-2007. The cost of each vaccine inoculation was determined to be almost $2.10.[15] Comparing the costs of HBV and HCV management, the immunization cost was considered small. However, as vaccination must cover the whole population and not only the patients, it was recommended to undertake a cost effectiveness analysis of public vaccination against HBV and HCV in Iran.
Although there are several studies evaluating the cost-effectiveness of different HBV immunization strategies worldwide,[16–19] many of them used uncertain HBV-associated costs.[20] Thus, it seems necessary to undertake a further study, to collect a more comprehensive and detailed cost analysis of the HBV and HCV patients in Iran. The results of such studies could include direct and indirect costs of medical and nonmedical services for hepatitis patients. These studies may help health policy makers to undertake a more accurate and evidence-based policy regarding different HBV and HCV immunization strategies in Iran.
CONCLUSION
This study provides useful information on the cost of various treatment protocols for HBV and HCV patients in Iran. The results implied that the market price of the direct medical cost of HBV and HCV patients in Iran is much higher than the estimated state costs. The study also showed that there are considerable variations in the costs of various therapeutic strategies, which encourage the evaluation of clinical outcomes, as well as economic costs of these protocols. The results can also be useful in the future cost-effectiveness analysis of these different treatment protocols as well as preventive strategies for hepatitis in Iran.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Davari M, Walley T, Haycox A. Pharmaceutical policy and market in Iran: past experiences and future challenges. J Pharm Health Serv Res. 2011;2:47–52. [Google Scholar]
- 2.Palfrey Colin, Paul Thomas, Phillips Ceri. Effective Healthcare Management: An Evaluative Approach. Oxford: Blackwell; 2004. [Google Scholar]
- 3.Kanwal F, Farid M, Martin P, Chen G, Gralnek IM, Dulai GS, et al. Treatment alternatives for hepatitis B cirrhosis: A cost-effectiveness analysis. Am J Gastroenterol. 2006;101:2076–89. doi: 10.1111/j.1572-0241.2006.00769.x. [DOI] [PubMed] [Google Scholar]
- 4.Kalantari H, Jalali M. Ten year study of histological variations of liver and laboratory findings in 100 healthy hepatitis B Carriers. Journal of Isfahan Medical School. 2003;67:32–4. [Google Scholar]
- 5.Kalantari H, Kazemi F, Minakari M. Efficacy of triple therapy with interferon alpha-2b ribavirin and amantadine in the treatment of naïve patients with chronic hepatitis C. Journal of Research in Medical Sciences. 2007;12:178–85. [Google Scholar]
- 6.Kalantari H, Shahshahan Z, Hejazi SM, Ghafghazi T, Sebghatolahi V. Effects of silybum marianum on patients with chronic hepatitis C. Journal of Research in Medical Sciences. 2011;16:287–90. [PMC free article] [PubMed] [Google Scholar]
- 7.Arnot R. The evolving efforts to control hepatitis B virus. Pediatr Infect Dis J. 1998;17(7 Suppl):S26–9. doi: 10.1097/00006454-199807001-00002. [DOI] [PubMed] [Google Scholar]
- 8.Bréchot C, Jaffredo F, Lagorce D, Gerken G, Meyer zum Büschenfelde K, Papakonstontinou A, et al. Impact of HBV, HCV and GBV-C/HGV on hepatocellular carcinomas in Europe: Results of a European concerted action. J Hepatol. 1998;29:173–83. doi: 10.1016/s0168-8278(98)80001-9. [DOI] [PubMed] [Google Scholar]
- 9.Kanwal F, Farid M, Martin P, Chen G, Gralnek IM, Dulai GS, et al. Treatment alternatives for hepatitis B cirrhosis: A cost-effectiveness analysis. Am J Gastroenterol. 2006;101:2076–89. doi: 10.1111/j.1572-0241.2006.00769.x. [DOI] [PubMed] [Google Scholar]
- 10.Kanwal F, Gralnek IM, Martin P, Dulai GS, Farid M, Spiegel BM. Treatment alternatives for chronic hepatitis B virus infection: A cost-effectiveness analysis. Ann Intern Med. 2005;142:821–31. doi: 10.7326/0003-4819-142-10-200505170-00007. [DOI] [PubMed] [Google Scholar]
- 11.Drummond M, Sculpher M, Torrance G, O’Brien B, Stoddart G. Methods for Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005. [Google Scholar]
- 12.Longo D, Fauci A, Kasper DL, Hauser S, Jameson J, Loscalzo J, editors. Harrison's principles of internal medicine. 18th ed. New York: McGraw Hill; 2011. [Google Scholar]
- 13.Alavian SM, Hajarizade B, et al. Hepatitis B virus infection in Iran: A systematic review. J Hepat Mon. 2008;8:281–94. [Google Scholar]
- 14.Alavian SM, Ahmadzad-Asl M, et al. Hepatitis C infection in general population of Iran: A systematic review. J Hepat Mon. 2009;9:211–23. [Google Scholar]
- 15.Hajarizadeh B, Rashidian A, Haghdoost AA, Alavian SM. Estimating the costs of the mass vaccination campaign against hepatitis B in Iranian adolescents. GOVARESH. 2009;14:27–34. [Google Scholar]
- 16.Lahaye D, Strauss P, Baleux C, Van Ganse W. Cost-benefit analysis of hepatitis-B vaccination. Lancet. 1987;2:441–3. doi: 10.1016/s0140-6736(87)90971-8. [DOI] [PubMed] [Google Scholar]
- 17.Williams JR, Nokes DJ, Medley GF, Anderson RM. The transmission dynamics of hepatitis B in the UK; A mathematical model for evaluating costs and effectiveness of immunization programmes. Epidemiol Infect. 1996;116:71–89. doi: 10.1017/s0950268800058970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Damme P, Kane M, Meheus A. Integration of hepatitis B vaccination into national immunization programmes. Br Med J. 1997;314:1033–6. doi: 10.1136/bmj.314.7086.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szucs TD, Smala A, Berger K, Windorfer A. Economic evaluation of various hepatitis B immunisation strategies in children and adolescents. Med Klin. 1998;93:468–77. doi: 10.1007/BF03042596. [DOI] [PubMed] [Google Scholar]
- 20.Jefferson T, Demicheli V. Is vaccination against hepatitis B efficient.A review of world literature? Health Econ. 1994;3:25–37. doi: 10.1002/hec.4730030105. [DOI] [PubMed] [Google Scholar]


