Abstract
The present work describes the synthesis and spectral analysis of some new 3(Z)-{4-[4-(arylsulfonyl)piperazin-1-ylbenzylidene)-1,3-dihydro-2H-indol-2-one (5a-j). Ten of the synthesized compounds were screened in vitro against six species of microorganisms, Staphylococcus aureus, Streptococcus pyogenes, Escherichia coli, Pseudomonas aeruginosa, Asperigellus niger and Asperigellus clavatus. Most of the compounds exhibited significant antimicrobial activity. All of these compounds were also screened in vitro for the antioxidant activity using DPPH assay. Most of them have shown very significant antioxidant activity.
Keywords: Antibacterial, antifungal and antioxidant activity, oxindole
Oxindole and other related ring systems, have several interesting biological activities[1–4]. According to the literature survey, 1-substituted aminomethyl-3-cyclohexylthiosemicarbazone-2-indolinones have shown significant antifungal, antibacterial and antiviral activities both in vivo and in vitro[5]. The new 1,3-dihydro-3-hydroxy-3-[2-hydroxyimino-2-(substituted phenyl)ethyl]-2H-indol-2-ones were synthesized and tested for antimicrobial activity and majority of the compounds were found to exhibit promising antibacterial and antifungal activities[6]. 3-amino-1-hydroxy-oxindole and related compounds have found to show significant antimicrobial activity[7]. Oxindole and related indole derivatives have also been found to show very good antioxidant activity[8,9].
Sulfonamide based drugs are known for their antimicrobial activities[10]. Arylsulfonamide-oxindole hybrid[11] has been explored for their anticancer activity, but few attempts have been made to explore the antimicrobial and antioxidant activity of aryl sulfonamide-oxindole hybrid. We herein, report the synthesis and biological testing of some 3(Z)-{4-[4-(arylsulfonyl)piperazin-1-ylbenzylidene)-1,3-dihydro-2H-indol-2-ones. These sulfonamide based oxindole derivatives were tested for antibacterial, antifungal and antioxidant activity.
All the recorded melting points were determined in open capillary and are uncorrected. IR spectra were recorded on Perkin-Elmer FTIR spectrophotometer in KBr disc. 1H NMR and 13C NMR spectra were recorded on 400 MHz spectrophotometer in DMSO-d6 as a solvent and TMS as an internal standard. Peak values are shown in δ ppm. Mass spectra were obtained using a Waters mass spectrometer.
General procedure for the synthesis of t-butyl-4-{4[(Z)-(2-oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]phenyl}piperazine-1-carboxylate (3) used was as follows; a mixture of 1 (0.01 mol) and 2 (0.01 mol) was dissolved in toluene and ammonium acetate (0.03 mol) was added to it. The reaction mixture stirred at 80° for 6 h. The reaction mixture was cooled to room temperature and poured into Hexane. Solid obtained was separated by filtration and crystallized from alcohol.
Compound (3): m.p.186°, Anal. Calcd. for C24H27N3O3: C, 71.09; H, 6.71; N, 10.36. Found: C, 71.00; H, 6.69; N, 10.33. IR (KBr)νmax (cm-1): 3621, 3380, 2887, 2336, 1693, 1590, 1340, 1078, 1030, 951, 1H NMR (400 MHz, DMSO-d6) δ (ppm): 1.40 (9H, s), 3.31 (4H, s), 3.45 (4H, s), 6.82 -6.98 (4H, m), 7.16 (1H, dt), 7.52 (1H, s, alkene H), 7.63 (1H, m), 7.76 (1H, d), 8.44 (1H, d), 10.48 (1H, s, NH). 13C NMR (400 MHz, DMSO-d6) δ (ppm): 169.38, 167.71, 137.51, 136.79, 134.59, 127.69, 125.97, 124.48, 123.95, 122.16, 121.70, 120.88, 118.92, 114.45, 110.19, 79.26, 46.93 and 28.24. MS m/z: 405 (M+) with all isotopic and other peaks.
General procedure for the synthesis of (3Z)-3-(4-piperazin-1-ylbenzylidene)-1, 3-dihydro-2H-indol-2-one (4) was as follows, compound 3 (0.01 mol) was dissolved in 10 ml methylene dichloride and 3 ml trifluoroacetic acid was added slowly to the reaction mixture (Scheme 1). The reaction mixture stirred at room temperature for 3 h and concentrated under vacuum. The reaction mixture was basified by liquor NH3 and extracted by ethyl acetate. Ethyl acetate layer separated, dried over Na2SO4 and concentrated under vacuum.
Scheme 1.

Synthetic route for the preparation compounds 5 (a-j)
Synthetic route for the preparation of the 3(Z)-{4-[4-(arylsulfonyl)piperazin-1-ylbenzylidene)-1,3-dihydro-2H-indol-2-one, 5 (a-j), which were tested for antimicrobial and antioxidant activity
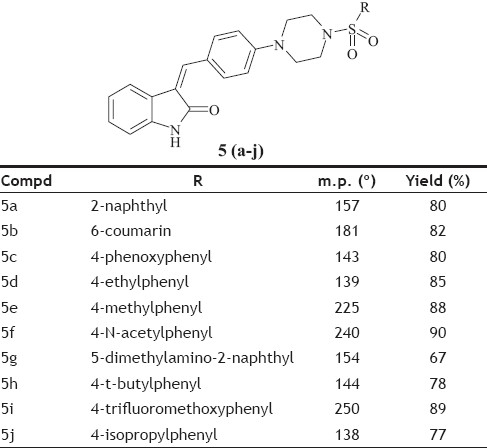
General procedure for the synthesis of 5 (a-j) was as follows; compound 4 (0.01 mol) and aromatic sulfonyl chloride (0.01 mol) were dissolved in tetrahydrofuran in presence of pyridine (0.03 mol) and catalytic amount of dimethyl aminopyridine. The reaction mixture was stirred at room temperature for 8 h and then poured into water. Aqueous layer extracted by ethyl acetate. Ethyl acetate layer separated, dried over Na2SO4 and concentrated under vacuum. The crude product obtained, was crystallized by alcohol. The Melting point and yield of the 5 (a-j) is given in Table 1.
TABLE 1.
STRUCTURAL DATA OF THE SYNTHESIZED OXINDOLES
Compound 5a, Anal. Calcd. for C29H25N3O3S: C, 70.28; H, 5.08; N, 8.48. Found: C, 70.21; H, 5.06; N, 8.45. IR (KBr)νmax (cm-1): 3621, 3380, 3277, 2887, 2336, 1693, 1590, 1340, 1078, 1030, 951, 1H NMR (400 MHz, DMSO-d6) δ (ppm): 3.01 (4H, s), 3.47 (4H, s), 6.53-8.76 (16H, m, aromatic protons), 10.52 (1H, s, NH). MS m/z: 495 (M+) with all isotopic and other peaks. Compound 5b, Calcd. for C28H23N3O5S: C, 65.48; H, 4.51; N, 8.18. Found: C, 65.35; H, 4.49; N, 8.15. IR (KBr)νmax (cm -1): 3583, 3342, 3277, 2887, 1735, 1693, 1592, 1355, 1236, 1055, 950, 1H NMR (400 MHz, DMSO-d6) δ (ppm): 3.08 (4H, s), 3.44 (4H, s), 6.67-8.39 (14H, m, aromatic protons), 10.52 (1H, s, NH). MS m/z: 513 (M+) with all isotopic and other peaks. Compound 5c, Calcd. for C31H27N3O4S: C, 69.26; H, 5.06; N, 7.82. Found: C, 69.20; H, 5.05; N, 7.80. IR (KBr)νmax (cm-1): 3342, 3255, 2877, 1693, 1592, 1350, 1238, 1055, 952, 1H NMR (400 MHz, DMSO-d6) δ (ppm): 3.02 (4H, s), 3.44 (4H, s), 6.86-8.40 (18H, m, aromatic protons), 10.52 (1H, s, NH). MS m/z: 537 (M+) with all isotopic and other peaks.Compound 5d, Calcd. for C27H27N3O3S: C, 68.48; H, 5.75; N, 8.87. Found: C, 68.39; H, 5.74; N, 8.84.IR (KBr)νmax (cm-1): 3779, 3552, 3340, 3180, 2929, 2624, 1697, 1590, 1461,1349, 1184, 948, 729, 1H NMR (400 MHz, DMSO-d6) δ (ppm): 1.16-1.22 (3H, t), 2.68-2.74 (2H, q), 3.01 (4H, s), 3.46 (4H, s), 6.80-8.40 (13H, m, aromatic protons), 10.52 (1H, s, NH). MS m/z: 473 (M+) with all isotopic and other peaks.
Compound 5e, Calcd. for C26H25N3O3S: C, 67.95; H, 5.48; N, 9.14. Found: C, 67.90; H, 5.46; N, 9.11. IR (KBr)νmax (cm-1): 3770, 3551, 3348, 2920, 2634, 1698, 1590, 1469, 1349, 1184, 950, 729, 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.42 (3H, s), 3.01 (4H, s), 3.45 (4H, s), 6.77-8.40 (13H, m, aromatic protons), 10.52 (1H, s, NH). MS m/z: 459 (M+) with all isotopic and other peaks. Compound 5f, Calcd. for C27H26N4O4S: C, 64.53; H, 5.21; N, 11.15. Found: C, 64.48; H, 5.20; N, 11.12. IR (KBr)νmax (cm-1): 3547, 3355, 2938, 1690, 1590, 1469, 1348, 1180, 946, 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.08 (3H, s), 2.99 (4H, s), 3.41 (4H, s), 6.66-8.10 (13H, m, aromatic protons), 10.52 (1H, s, NH). MS m/z: 502 (M+) with all isotopic and other peaks. Compound 5g, Calcd. for C31H30N4O3S: C, 69.12; H, 5.61; N, 10.40. Found: C, 69.00; H, 5.59; N, 10.36. IR (KBr)νmax (cm-1): 3867, 3554, 3488, 2947,2834, 2496, 2302, 1694, 1589, 1460, 1326, 1184, 947, 746; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 2.83 (6H, s), 3.23 (4H, s), 3.34 (4H, s), 6.78-8.56 (15H, m, aromatic protons), 10.52 (1H, s, NH). MS m/z: 538 (M+) with all isotopic and other peaks. Compound 5h, Calcd. for C29H31N3O3S: C, 69.44; H, 6.23; N, 8.38. Found: C, 69.32; H, 6.21; N, 8.33. IR (KBr)νmax (cm-1): 3778, 3550, 3276, 2968, 2624, 1697, 1590, 1461, 1349, 1184, 948, 786, 1H NMR (400 MHz, DMSO-d6) δ (ppm): 1.31 (9H, s), 3.03 (4H, s), 3.73 (4H, s), 6.67-7.74 (13H, m, aromatic protons), 10.52 (1H, s, NH). MS m/z: 501 (M+) with all isotopic and other peaks. Compound 5i, Calcd. for C26H22F3N3O4S: C, 58.97; H, 4.19; N, 7.94. Found: C, 58.75; H, 4.18; N, 7.91. IR (KBr)νmax (cm-1): 3342, 3255, 2877, 1693, 1592, 1350, 1238, 1055, 952, 1H NMR (400 MHz, DMSO-d6) δ (ppm): 3.06 (4H, s), 4.03 (4H, s), 6.78– 8.39 (13H, m, aromatic protons), 10.52 (1H, s, NH). MS m/z: 529 (M+) with all isotopic and other peaks. Compound 5j, Calcd. For C28H29N3O3S: C, 68.97; H, 5.99; N, 8.62. Found: C, 68.85; H, 5.97; N, 8.60.IR (KBr)νmax (cm-1): 3779, 3552, 3340, 3180, 2929, 2624, 1697, 1590, 1461, 1349, 1184, 948, 729, 1H NMR (400 MHz, DMSO-d6) δ (ppm): 1.15-1.21 (6H, m), 2.16 (1H,s), 2.98 (4H, s), 3.48 (4H, s), 6.64-8.44 (13H, m, aromatic protons), 10.52 (1H, s, NH). MS m/z: 487 (M+) with all isotopic and other peaks.
In the present investigation, the condensation reaction of oxindole 1 with aldehyde 2. gave the compound t-Butyl-4-{4[(Z)-(2-oxo-1,2-dihydro-3H-indol-3-ylidene)methy l]phenyl}piperazine-1-carboxylate (3) The compound 3 was treated with trifluoroacetic acid in methylene dichloride to give (3Z)-3-(4-piperazin-1-ylbenzylidene)-1,3-dihydro-2H-indol-2-one (4). The compound 4 was treated with substituted aromatic sulfonyl chloride in presence of pyridine in tetrahydrofuran to get 3(Z)-{4-[4-(arylsulfonyl) piperazin-1-ylbenzylidene)-1,3-dihydro-2H-indol-2-one, 5 (a-j). The synthetic scheme for the same is given in Scheme 1. The structural data of the synthesized oxindole derivatives 5 (a-j) is listed in Table 1. The compounds 5 (a-j) were characterized by IR, 1H NMR and mass spectroscopy. The compound 3 was characterized by 1H NMR and 13C NMR to determine geometrical isomerism of exocyclic double bond of indol-2-one derivative. The characteristic singlet of proton of alkene at δ 7.52 in 1H NMR and δ 124.48 in 13C NMR of carbon of alkene establish exclusive Z isomer formation.
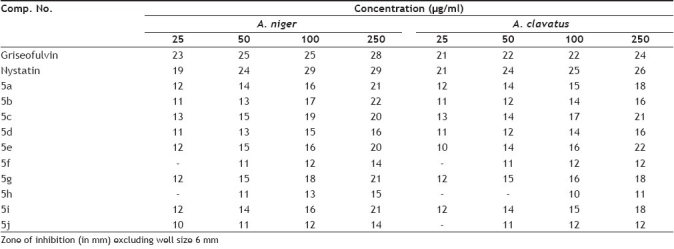
The in vitro antimicrobial activity of test compounds was assessed against 24 h cultures of several selected bacteria and fungi. The gram positive and gram-negative bacteria used were E. coli, P. aeruginosa, S. pyogenes and S. aureus and the fungi used were A. niger and A. clavatus. Antimicrobial activity of all the compounds was tested using Muller Hinton broth (Hi Media M 391) medium. The activity is reported by measuring the diameter of inhibition in millimeter (mm). The tested compounds show significant antifungal and antibacterial activity as shown in Table 2 and 3. Compounds 5a, 5c, 5e and 5g have shown very good antifungal as well as antibacterial activity, while 5f, 5h and 5j show very low antifungal activity. Except 5e, all other compounds with alkyl substituent viz. 5d, 5h and 5j show very low antifungal activity. Almost all the compounds show moderate activity against bacterial strains at 250 μg/ml concentration.
TABLE 2.
ANTIFUNGAL ACTIVITY OF THE OXINDOLES

TABLE 3.
ANTIBACTERIAL ACTIVITY OF THE OXINDOLES
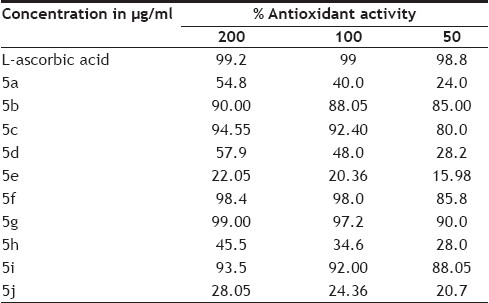
The in vitro antioxidant activity of the test compounds was determined by DPPH method by using L-ascorbic acid (an antioxidant agent) as a positive control. The compounds were tested for antioxidant activity at 200, 100 and 50 μg/ml concentrations. Amongst the compounds screened for antioxidant activity, 5b, 5c, 5f, 5g and 5i showed very good antioxidant activities as shown in Table 4. Compounds 5a, 5d, 5e, 5h and 5j do not show significant antioxidant activity. The compounds with alkyl substituent do not show any radical scavenging activity while the compounds with heterocyclic ring system like coumarin or the one with substituents like N(CH3)2, OCF3 and N-acetyl show very significant antioxidant activity. It suggests the significant role played by these substituents as radical scavengers.
TABLE 4.
% ANTIOXIDANT ACTIVITY OF THE OXINDOLES
In conclusion, a series of oxindole derivatives were synthesized and tested for antifungal, antibacterial and antioxidant activity. Most of the compounds have shown very good antimicrobial and antioxidant activity, which suggest a possible clinical significance of 3(Z)-{4-[4-(arylsulfonyl)piperazin-1-ylbenzylidene)-1,3-dihydro-2H-indol-2-ones.
Footnotes
Rindhe, et al.: Bioactive Oxindole Derivatives
REFERENCES
- 1.Tokunaga T, Ewan Hume W, Nagamine J, Nagata R. Structure-activity relationships of the oxindole growth hormone secretagogues. Bioorg Med Chem Lett. 2005;15:1789–92. doi: 10.1016/j.bmcl.2005.02.042. [DOI] [PubMed] [Google Scholar]
- 2.Tokunaga T, Ewan Hume W, Umezome T, Okazaki K, Ueki Y, Kumagai K, et al. Oxindole derivatives as orally active potent growth hormone secretagogues. J Med Chem. 2001;44:4641–9. doi: 10.1021/jm0103763. [DOI] [PubMed] [Google Scholar]
- 3.Strigacova J, Hudecova D, Mikulasova M, Varecka L, Lasikova A, Vegh D. Novel oxindole derivatives and their biological activity. Folia Microbiol (Praha) 2001;46:187–92. doi: 10.1007/BF02818531. [DOI] [PubMed] [Google Scholar]
- 4.Dickerson SH, Hunter RN, Kuyper LF, Lackey KL, Luzzio MJ, Wood ER. Substituted oxindole derivatives as tyrosine kinase inhibitors. Patent US 7,071,217 B22006 [Google Scholar]
- 5.Singh S SPnone, Jha Knone. Indolinone derivatives as potential antimicrobial agents. Zentralbl Mikrobiol. 1989;144:105–9. [PubMed] [Google Scholar]
- 6.El-GendyAAnone , Ahmedy AMnone. Synthesis and antimicrobial activity of some new 2-indolinone derived oximes and spiro-isoxazolines. Arch Pharm Res none. 2000;23:310–4. doi: 10.1007/BF02975439. [DOI] [PubMed] [Google Scholar]
- 7.Davis AL, Smith DR, McCord TJ. Synthesis and microbiological properties of 3-amino-1-hydroxy-2-indolinone and related compounds. J Med Chem. 1973;16:1043–5. doi: 10.1021/jm00267a020. [DOI] [PubMed] [Google Scholar]
- 8.Estevao MS, Carvalho LC, Ferreira LM, Fernandes E, Marques MM. Analysis of the antioxidant activity of an indole library: Cyclic voltammetry versus ROS scavenging activity. Tetrahedron Lett. 2011;52:101–6. [Google Scholar]
- 9.Aboul-Enein HY, Kladna A, Kruk I, Lichszteld K, Michalska T, Olgen S. Scavenging of reactive oxygen species by novel indolin-2-one and indoline-2-thione derivatives. Biopolymers. 2005;78:171–8. doi: 10.1002/bip.20268. [DOI] [PubMed] [Google Scholar]
- 10.Chio LC, Bolyard LA, Nasr M, Queener SF. Identification of a class of sulfonamides highly active against dihydropteroate synthase form Toxoplasma gondii, Pneumocystis carinii, and Mycobacterium avium. Antimicrob Agents Chemother. 1996;40:727–33. doi: 10.1128/aac.40.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Natarajan A, Guo Y, Harbinski F, Fan Y, Chen H, Luus L, et al. Novel aryl sulfoanilide-oxindole hybrid as an anticancer agent that inhibits translation initiation. J Med Chem. 2004;47:4979–82. doi: 10.1021/jm0496234. [DOI] [PubMed] [Google Scholar]


