Previous cardiac valvular surgery, vascular prosthesis, aortic aneurysm, renal insufficiency, and older age increased risk.
Keywords: Q fever, Coxiella burnetii, chronic Q fever, risk factors, case–control study, acute Q fever, cardiac valvular surgery, vascular prosthesis, aortic aneurysm, renal insufficiency, older age, bacteria, the Netherlands
Keywords: Suggested citation for this article: Kampschreur LM, Dekker S, Hagenaars JCJP, Lestrade PJ, Renders NHM, de Jager-Leclercq MGL, et al. Identification of risk factors for chronic Q fever, the Netherlands. Emerg Infect Dis [serial on the internet]. 2012 Apr [date cited]. http://dx.doi.org/10.3201/eid1804.111478
Abstract
Since 2007, the Netherlands has experienced a large Q fever outbreak. To identify and quantify risk factors for development of chronic Q fever after Coxiella burnetii infection, we performed a case–control study. Comorbidity, cardiovascular risk factors, medications, and demographic characteristics from 105 patients with proven (n = 44), probable (n = 28), or possible (n = 33) chronic Q fever were compared with 201 patients who had acute Q fever in 2009 but in whom chronic Q fever did not develop (controls). Independent risk factors for development of proven chronic Q fever were valvular surgery, vascular prosthesis, aneurysm, renal insufficiency, and older age.
Q fever, a zoonosis caused by the intracellular gram-negative bacterium Coxiella burnetii, is prevalent worldwide (1,2) and has various acute and chronic clinical manifestations. Acute Q fever is mostly a self-limiting, mild, influenza-like disease, sometimes complicated by severe pneumonia or hepatitis. Asymptomatic acute infection occurs in 50%–60% of patients (3–5). Among patients infected by C. burnetii, infection progresses to chronic Q fever in 1%–5%, months to years after primary infection (2,4,6). Previous data, mainly from France, show that endocarditis is the most common clinical manifestation (±75%), followed by infections of aortic aneurysms and vascular prostheses (±10%) (5,7–9). In the Netherlands, however, an equal distribution of endocarditis and vascular infections has been seen (10).
Chronic Q fever leads to high illness and death rates if untreated, which makes early case finding and preventive measures critical for patients at high risk. Treatment for Q fever consists of long-term antimicrobial drug therapy, preferably a combination of doxycycline and hydroxychloroquine for 18–24 months. Previously identified risk factors for chronic Q fever are preexisting cardiac valvulopathy, vascular grafts and aneurysms, immunosuppression, and pregnancy; however, most published studies have been descriptive, lacked statistical quantification, or included specific high-risk groups only (6–9,11,12).
Since 2007, a large Q fever outbreak has been ongoing in the Netherlands, with >4,000 acute Q fever cases reported (13). Because of asymptomatic disease and overlap with other febrile diseases, however, the actual number of Q fever infections is probably much higher. Although the acute Q fever epidemic in the Netherlands has subsided, the number of patients with chronic Q fever is rising (10,14). In this unique population, we conducted a case–control study to identify and quantify risk factors for development of chronic Q fever after C. burnetii infection.
Methods
Study Design and Setting
Case-patients and controls were recruited from Jeroen Bosch Hospital in ’s-Hertogenbosch and Bernhoven Hospital in Oss and Veghel; both are regional hospitals located in the center of the Q fever epidemic area in the Netherlands. The study design was approved by the Medical Research Ethics Committee of the University Medical Centre Utrecht.
Patient Selection
We used existing datasets and spontaneous notifications from the 2 hospitals to identify all chronic Q fever diagnoses among patients >18 years of age during January 1, 2007–May 1, 2011. In the past, diagnosis of chronic Q fever has relied on results of serologic testing and PCR. Chronic Q fever is considered proven if C. burnetii is detected by PCR in blood or tissue in the absence of acute infection, but sensitivity of this technique is only ≈50% (15,16). Persisting high levels of IgG to phase I antigens (phase I IgG) and, to a lesser extent, phase II antigens (phase II IgG) are also indicative of chronic Q fever (1). The optimal immunofluorescence assay (IFA) cutoff value for phase I IgG titer is still matter of debate and is dependent on the test used but is probably within the range of 800–1,600 (7,17–19).
Recently, the Dutch Q Fever Consensus Group proposed a new diagnostic approach that combines PCR, serologic testing, and clinical data and categorizes cases into proven, probable, or possible chronic Q fever (20). Proven cases are those among patients with positive PCR results for C. burnetii in blood or tissue or a phase I IgG titer of >1,024 in combination with a vascular infection proven by positron emission tomography (PET), computed tomography (CT), magnetic resonance imaging (MRI), or endocardial involvement according to the major criteria of the modified Duke criteria on echocardiogram (21). Probable cases are those among patients with phase I IgG titers of >1,024 and known risk factors: nonmajor valvulopathy according to the modified Duke criteria (21), suspected nonvascular or noncardial localization of chronic Q fever infection, or aspecific signs of chronic infection. Possible cases are those among patients with phase I IgG titers of >1,024 without other risk factors as listed for probable or proven chronic Q fever. In contrast to the other 2 subgroups, in general, possible chronic Q fever patients do not receive long-term antimicrobial drug treatment but instead enter a follow-up program; many demonstrate spontaneous decline in phase I IgG titers. We defined cases according to these definitions (20) (Table 1).
Table 1. Classification of chronic Q fever according to Dutch Q Fever Consensus Group guidelines*.
| Classification | Definition |
|---|---|
| Proven | Any of the following: |
| Positive PCR for Coxiella burnetii in serum, plasma, or tissue in the absence of acute Q fever | |
| IFA phase I titer ≥1,024 with definite endocarditis according to the revised Duke criteria (21) | |
| Indication of vascular infection on PET/CT, CT, MRI, or ultrasound testing | |
| Probable | IFA phase I IgG titer >1,024 and any of the following clinical manifestations: |
| Valvulopathy not meeting the criteria of endocardial involvement of the major modified Duke criteria (22) | |
| Aneurysm, vascular prosthesis or prosthetic valve without signs of infection on PET/CT, CT, MRI, or ultrasound testing | |
| Signs of possible chronic Q fever infection of noncardiac or vascular origin on PET/CT, CT, or ultrasound testing | |
| Pregnancy | |
| Clinical symptoms of chronic infection (i.e., fever, night sweats, weight loss, hepatosplenomegaly) | |
| Histopathologic proven granulomatous inflammation | |
| Immune disorder | |
| Possible | IFA phase I IgG titer >1,024 without clinical manifestations as described above |
*Described in (20). IFA, immunofluorescence assay; PET, positron emission tomography; CT, computed tomography; MRI, magnetic resonance imaging.
Controls were selected from an existing cohort of patients with acute Q fever, seen by general practitioners in 2009, who had positive PCR results for C. burnetii in serum samples. Controls were included if they were >18 years of age at the time of acute Q fever and if the serologic profile was not suggestive of chronic Q fever during >1 year of follow-up (i.e., decreasing antibody titers and phase I IgG titer <1,024). Patients with serologic follow-up of <1 year after the episode of acute Q fever were excluded from analysis. All case-patients except 1 and all controls lived in the same postal code area (5000–5400) in the Netherlands.
Microbiological Analyses
Microbiological diagnostics for chronic Q fever case-patients consisted of IFA (Focus Diagnostics, Inc., Cypress, CA, USA) of serum samples and PCR for C. burnetii DNA in serum, plasma, and tissue samples. The diagnostic workup to evaluate C. burnetii infection in control patients with documented acute Q fever had been performed according to a diagnostic algorithm for acute Q fever introduced in May 2009. In brief, serum samples were screened with ELISA for IgM against C. burnetii phase II antigens (MII-screen; Institut Virion Serion GmbH, Würzburg, Germany). Depending on date of onset of disease and inpatient or outpatient setting, PCR for C. burnetii DNA was performed if the MII-screen result was negative (23–25). In patients with confirmed acute Q fever, serologic follow-up was performed at 3, 6, and 12 months, consisting of IFA for IgM and IgG against C. burnetii phase I and phase II antigens.
Data Collection and Storage
We collected patient characteristics including demographic variables, medical history, medication, pathology and microbiology results, imaging records, therapy, and outcome for case-patients and controls. Case-patient information was already available in the hospital registration systems and was interpreted by 2 researchers (L.K. and S.D.). All controls were sent a questionnaire and an informed consent form that asked for permission to request patient’s data from the general practitioner and from the hospital registration system.
Although debatable, routine echocardiographic screening after diagnosis of acute Q fever is not the standard of care in the Netherlands because no benefit was found in an earlier evaluation (26,27). Therefore, for chronic Q fever case-patients and acute Q fever controls, details about cardiac valvulopathy were retrieved by review of medical records. The obtained information was processed and stored anonymously with the use of coded data. SPSS version 18.0 was used for storage and analysis of the collected data (SPSS Inc., Chicago, IL, USA).
Statistical Analysis
Within this study, we conducted 3 analyses: 1) an overall analysis of all chronic Q fever cases (i.e., proven, probable, and possible); 2) an analysis of proven and probable chronic Q fever cases; and 3) an analysis of proven chronic Q fever cases only. We performed these analyses to determine whether exclusion of possible chronic Q fever and, to a lesser extent, probable chronic Q fever (the groups in which disease status is doubtful) influenced the overall results. Univariate and subsequent multivariate logistic regression analyses were performed to calculate odds ratios (ORs), corresponding 95% CIs, and p values for the development of chronic Q fever. In univariate analysis, missing values were excluded. Variables with no observations among case-patients and <2 observations in the control group (or vice versa) were excluded (i.e., hematologic malignancies, bone marrow transplantation, dialysis, renal transplant, nonrenal organ transplant, congenital cardiac deviation, pulmonary diseases, and autoimmune disorder). For potential dichotomous risk factors, i.e., those that had 0 observations among either the case-patients or controls but >2 observations in the other, we applied a Fisher exact test to calculate p values. Variables with >1 observations and <25% missing values in case-patients and controls, a p value of <0.10 in univariate analysis, or known association in previous reports with the development of chronic Q fever were subsequently analyzed in a multivariate model.
The variables vascular history and valvulopathy were not included in multivariate analysis because they were included in variables that were listed separately (i.e., vascular prosthesis, aneurysm, other vascular surgery, peripheral arterial disease, cerebrovascular disease, valvular surgery, and nonsurgical valvular disease). Eighteen case-patients and 0 controls had a history of valvular surgery. Because of the expected importance of this risk factor and the high incidence among case-patients, we considered its inclusion in the multivariate analysis critical; moreover, the logistic regression model could not be fitted with this variable excluded. Therefore, we randomly changed one of the observations of the control group from 0 to 1, which artificially reduced the association but enabled us to fit the regression model.
The variables age, vascular history, vascular prosthesis, aneurysm, other vascular surgery, cerebrovascular disease, peripheral vascular disease, valvulopathy, valvular surgery, valvular deviation, ischemic heart disease, other cardiovascular diseases, hypertension, dyslipidemia, diabetes, nonhematologic malignancy, and renal insufficiency could be included in multivariate analysis of all groups. The variable immune disorder was also included in multivariate analysis for the probable and proven and the proven subgroups. The variable pacemaker was also included in multivariate analysis for the proven group.
Differences between case-patients and controls were shown in use of statins, clopidogrel, acenocoumarol, and proton pump inhibitors and hospitalization and adequate treatment during acute Q fever (p<0.10). However, these variables could not be included in the multivariate analysis because >25% of values were missing, most among case-patients in whom an acute Q fever episode had gone unrecognized.
After selecting predictors for our final multivariate model, we evaluated their possible interactions by including 2-way interactions in consecutive models. Interactions were not significant and therefore not included in the model. To assess the goodness-of-fit of the final model, we plotted sensitivity and specificity by using a receiver operating characteristic curve and estimated the area under the curve (c-statistic). p<0.05 was considered significant.
Results
We identified 105 case-patients with proven, probable, or possible chronic Q fever; 44 (42%) had proven, 28 (27%) probable, and 33 (31%) possible disease. Of the case-patients with proven chronic Q fever, 27 (61%) had positive PCR results for C. burnetii in blood only, 5 (11%) in tissue only, 8 (18%) in tissue and blood, and 4 (9%) in neither blood nor tissue. The focus of infection in cases of proven chronic Q fever was endocarditis for 12 case-patients (27%) and endovascular infection for 26 (59%); 6 (14%) had no clear infection focus. Of the case-patients with probable chronic Q fever, suspected foci were cardiac valves in 12 (43%), endovascular lesions in 1 (4%), and another focus (e.g., pregnancy or clinical symptoms of infection such as weight loss, night sweats, and fever) in 15 (54%).
Long-term antimicrobial drug treatment was started for 40/44 case-patients (91%) with proven chronic Q fever, 18/28 case-patients (64%) with probable chronic Q fever, and 5/32 case-patients (15%) with possible chronic Q fever. Three patients with proven chronic Q fever patients died before diagnosis of chronic Q fever; 1 refused therapy.
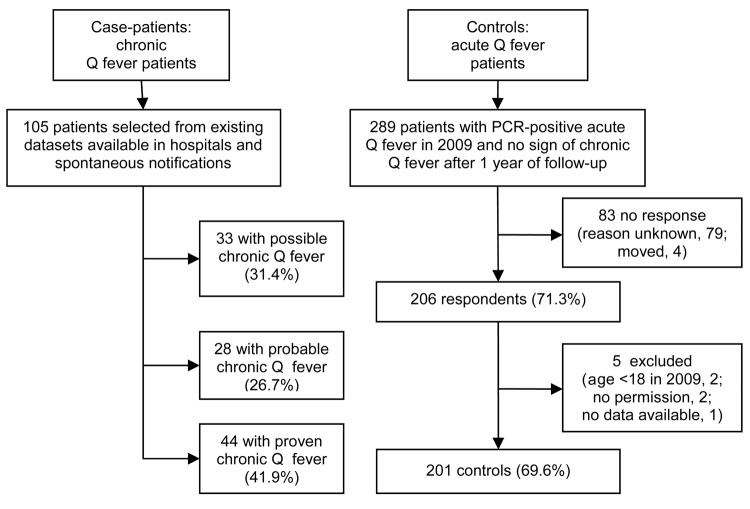
In all, 289 controls who had PCR-proven acute Q fever in 2009 were sent a questionnaire. Of these, 201 (69.6%) responded, signed the informed consent form, and fulfilled the inclusion criteria (Figure 1).
Figure 1.
Enrollment, selection, and inclusion criteria forcase-patients and controls for case–control study to identify risk factors for chronic Q fever, the Netherlands.
Results of the univariate analysis are listed in Table 2. Comparisons for age, vascular history, vascular prosthesis, aneurysm, other vascular surgery, cerebrovascular disease, peripheral vascular disease, valvulopathy, valvular surgery, valvular deviation, ischemic heart disease, other cardiovascular diseases, hypertension, dyslipidemia, diabetes, nonhematologic malignancy (defined as several kinds of solid tumors), renal insufficiency, and pregnancy showed significant differences between case-patients and controls.
Table 2. Results of univariate analysis of risk factors for chronic versus acute Q fever*.
| Risk factor | Acute Q fever, no. (%), n = 201 | All chronic Q fever, n = 105 |
|
Proven and probable chronic Q fever, n = 72 |
|
Proven chronic Q fever, n = 44 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. (%) | OR (95% CI) | p value | No. (%) | OR (95% CI) | p value | No. (%) | OR (95% CI) | p value | ||||
| Male | 129 (64.2) | 70 (66.7) | 1.12 (0.68–1.84) | 0.665 | 50 (69.4) | 1.27 (0.71–2.26) | 0.420 | 32 (72.7) | 1.49 (0.72–3.07) | 0.281 | ||
| Mean age, y (±SD) | 52.5 (±13.7) | 63.9 (±13.5) | 1.06 (1.04–1.09)† | 0.000 | 67.3 (±11.8) | 1.09 (1.07–1.12)† | 0.000 | 68.4 (±10.8) | 1.11 (1.07–1.15)† | 0.000 | ||
| Smoker | 85 (42.5) | 43 (44.3) | 1.08 (0.66–1.76) | 0.765 | 33 (49.3) | 1.31 (0.75–2.29) | 0.336 | 22 (55.0) | 1.65 (0.84–3.27) | 0.149 | ||
| Medical history | ||||||||||||
| Vascular history | 9 (4.5) | 33 (31.4) | 9.78 (4.46–21.4) | 0.000 | 29 (40.3) | 14.4 (6.35–32.6) | 0.000 | 23 (52.3) | 23.4 (9.57–57.1) | 0.000 | ||
| Vascular prosthesis | 2 (1.0) | 15 (14.3) | 16.5 (3.71–74.0) | 0.000 | 15 (20.8) | 26.2 (5.82–118) | 0.000 | 14 (31.8) | 46.4 (10.0–215) | 0.000 | ||
| Aneurysm | 2 (1.0) | 12 (11.4) | 12.8 (2.82–58.5) | 0.001 | 12 (16.7) | 19.9 (4.33–91.4) | 0.000 | 9 (20.5) | 25.6 (5.30–123) | 0.000 | ||
| Other vascular surgery | 3 (1.5) | 7 (6.7) | 4.71 (1.19–18.6) | 0.027 | 5 (6.9) | 4.93 (1.15–21.2) | 0.032 | 4 (9.1) | 6.60 (1.42–30.6) | 0.016 | ||
| Peripheral arterial disease | 6 (3.0) | 11 (10.5) | 3.80 (1.37–10.6) | 0.011 | 8 (11.1) | 4.06 (1.36–12.2) | 0.012 | 6 (13.6) | 5.13 (1.57–16.8) | 0.007 | ||
| Cerebrovascular disease‡ | 8 (4.0) | 11 (10.5) | 2.82 (1.10–7.25) | 0.031 | 9 (12.5) | 3.45 (1.28–9.31) | 0.015 | 5 (11.4) | 3.09 (0.96–9.96) | 0.058 | ||
| Valvulopathy | 10 (5.0) | 25 (23.8) | 5.97 (2.47–13.0) | 0.000 | 23 (31.9) | 8.97 (4.00–20.1) | 0.000 | 13 (29.5) | 8.01 (3.23–19.8) | 0.000 | ||
| Valvular disease, NS§ | 10 (5.0) | 17 (16.2) | 3.69 (1.62–8.39) | 0.002 | 14 (19.4) | 4.61 (1.95–10.9) | 0.001 | 9 (20.5) | 4.91 (1.86–13.0) | 0.001 | ||
| Valvular surgery | 1 (0.5)¶ | 18 (17.1) | 41.4 (5.44–315) | 0.000 | 18 (25.0) | 66.7 (8.70–511) | 0.000 | 10 (22.7) | 58.8 (7.29–474) | 0.000 | ||
| Congenital cardiac disease | 1 (0.5) | 1 (1.0) | 1.92 (0.12–31.1) | 0.645 | NA | NA | NA | NA | NA | NA | ||
| Ischemic cardiac disease# | 17 (8.5) | 28 (26.7) | 3.94 (2.04–7.61) | 0.000 | 23 (31.9) | 5.08 (2.52–10.2) | 0.000 | 17 (38.6) | 6.82 (3.11–14.9) | 0.000 | ||
| Pacemaker | 2 (1.0) | 3 (2.9) | 2.93 (0.48–17.8) | 0.244 | 3 (4.2) | 4.33 (0.71–26.4) | 0.113 | 3 (6.8) | 7.28 (1.18–45.0) | 0.033 | ||
| Other cardiac history** | 12 (6.0) | 26 (24.8) | 5.18 (2.49–10.8) | 0.000 | 23 (31.9) | 7.39 (3.44–15.9) | 0.000 | 15 (34.1) | 8.15 (3.47–19.1) | 0.000 | ||
| Hypertension | 56 (27.9) | 44 (41.9) | 1.87 (1.14–3.07) | 0.013 | 35 (48.6) | 2.45 (1.41–4.27) | 0.002 | 24 (54.5) | 3.11 (1.59–6.06) | 0.001 | ||
| Dyslipidemia | 39 (19.4) | 32 (30.5) | 1.82 (1.06–3.13) | 0.031 | 23 (31.9) | 1.95 (1.06–3.58) | 0.031 | 16 (36.4) | 2.37 (1.17–4.81) | 0.017 | ||
| Diabetes mellitus, type 1 or 2 | 13 (6.5) | 15 (14.3) | 2.41 (1.10–5.28) | 0.028 | 10 (13.9) | 2.33 (0.97–5.58) | 0.057 | 7 (15.9) | 2.74 (1.02–7.32) | 0.045 | ||
| Nonhematologic malignancy | 6 (3.0) | 16 (15.2) | 5.84 (2.21–15.4) | 0.000 | 10 (13.9) | 5.24 (1.83–15.0) | 0.002 | 6 (13.6) | 5.13 (1.57–16.8) | 0.007 | ||
| Immune disorder†† | 2 (1.0) | 4 (3.8) | 3.94 (0.71–21.9) | 0.117 | 4 (5.6) | 5.85 (1.05–32.7) | 0.044 | 3 (6.8) | 7.28 (1.18–45.0) | 0.033 | ||
| COPD | 14 (7.0) | 13 (12.4) | 1.89 (0.85–4.18) | 0.117 | 9 (12.5) | 1.91 (0.79–4.62) | 0.152 | 6 (13.6) | 2.11 (0.76–5.84) | 0.151 | ||
| Other pulmonary disease‡‡ | 6 (3.0) | 3 (2.9) | 0.96 (0.23–3.90) | 0.950 | 2 (2.8) | 0.93 (0.18–4.71) | 0.929 | NA | NA | NA | ||
| Liver disease | 1 (0.5) | 3 (2.9) | 5.88 (0.60–57.3) | 0.127 | 2 (2.8) | 5.71 (0.51–64.0) | 0.157 | 1 (2.3) | 4.65 (0.29–75.8) | 0.280 | ||
| Renal insufficiency | 2 (1.0) | 12 (11.4) | 12.8 (2.82–58.5) | 0.001 | 12 (16.7) | 19.9 (4.33–91.4) | 0.000 | 9 (20.5) | 25.6 (5.30–123) | 0.000 | ||
| Autoimmune disease§§ | 2 (1.0) | 1 (1.0) | 0.96 (0.09–10.7) | 0.971 | 1 (1.4) | 1.40 (0.13–15.7) | 0.784 | NA | NA | NA | ||
| Pregnancy¶¶ | 0 (0.0) | 3 (2.9) | NA | 0.040 | 2 (2.8) | NA | 0.069 | 1 (2.3) | NA | 0.180 | ||
| Medication at time of acute Q fever | ||||||||||||
| Proton pump inhibitors¶¶ | 15 (7.5) | 7 (11.7) | 1.63 (0.63–4.20) | 0.313 | 5 (14.7) | 2.13 (0.72–6.29) | 0.173 | 5 (23.8) | 3.85 (1.24–12.0) | 0.020 | ||
| Statin¶¶ | 29 (14.5) | 19 (31.7) | 2.73 (1.40–5.35) | 0.003 | 15 (44.1) | 4.66 (2.13–10.2) | 0.000 | 13 (61.9) | 9.58 (3.65–25.1) | 0.000 | ||
| Carbasalate calcium¶¶ | 6 (3.0) | 2 (3.3) | 1.12 (0.22–5.67) | 0.896 | 2 (5.9) | 2.02 (0.39–10.5) | 0.401 | 2 (9.5) | 3.40 (0.64–18.0) | 0.150 | ||
| Acenocoumarol¶¶ | 6 (3.0) | 7 (11.7) | 4.27 (1.38–13.3) | 0.012 | 5 (14.7) | 5.58 (1.60–19.4) | 0.007 | 2 (9.5) | 3.40 (0.64–18.0) | 0.150 | ||
| Clopidogrel¶¶ | 2 (1.0) | 3 (5.0) | 5.21 (0.85–31.9) | 0.074 | 2 (5.9) | 6.19 (0.84–45.5) | 0.073 | 2 (9.5) | 10.4 (1.39–78.2) | 0.023 | ||
| Acute Q fever | ||||||||||||
| Adequate treatment## | 157 (89.7) | 37 (84.1) | 0.61 (0.24–1.56) | 0.298 | 22 (78.6) | 0.42 (0.15–1.17) | 0.098 | 12 (70.6) | 0.28 (0.09–0.87) | 0.028 | ||
| Hospitalization | 36 (18.0) | 26 (35.1) | 2.47 (1.36–4.49) | 0.003 | 16 (38.1) | 2.80 (1.37–5.76) | 0.005 | 9 (34.6) | 2.41 (0.99–5.84) | 0.051 | ||
*No. (%) case patients. n indicates no. patients wth information available for that category. OR, odds ratio; NS, nonsurgical; NA, not applicable; COPD, chronic obstructive pulmonary disease. †OR per year of increasing age. ‡Cerebrovascular disease and transient ischemic attack. §Case-patients: aortic valve defects, 10 (no bicuspid valves); mitral valve defects, 9 (no prolapse); tricuspid valve defects, 4. Controls: aortic valve defects, 6 (no bicuspid valves); mitral valve defects, 3 (1 prolapse). ¶n = 0 in reality. #Angina pectoris and myocardial infarction. **Atrial fibrillation, congestive heart failure, pericarditis, bradycardia, ischemic cardiomyopathy, and left ventricular hypertrophy. ††Prednisone cumulative dose >750 mg; use of tumor necrosis factor α–blocker, methotrexate, mycofenolate mofetil; splenectomy. §§Asthma, recurrent pneumonia, rheumatoid arthritis. ¶¶>25% missing in case groups. ##Defined as 10–14 d of doxycycline treatment
Results of the multivariate analyses are shown in Table 3. Valvular surgery (OR 31.5, 95% CI 3.99–249), vascular prosthesis (OR 10.4, 95% CI 2.17–50.0), aneurysm (OR 8.65, 95% CI 1.74–42.9), nonhematologic malignancy (OR 3.90, 95% CI 1.33–11.5), and age (OR 1.03, 95% CI 1.01–1.06) were independently associated with the development of chronic Q fever. The final discriminative performance was good, with a c-statistic of 0.71 (95% CI 0.71–0.83) (Figure 2).
Table 3. Results of multivariate analyses of risk factors for development of chronic Q fever, the Netherlands*.
| Risk factor*† | All chronic Q fever |
|
Proven and probable chronic Q fever |
|
Proven chronic Q fever |
|||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | |||
| Valvular surgery†‡ | 31.5 (3.99–249) | 0.001 | 47.7 (5.87–387) | 0.000 | 43.6 (4.70–405) | 0.001 | ||
| Vascular prosthesis‡§ | 10.4 (2.17–50.0) | 0.003 | 14.9 (2.96–75.2) | 0.001 | 26.8 (4.88–147) | 0.000 | ||
| Aneurysm§¶ | 8.65 (1.74–42.9) | 0.008 | 13.5 (2.60–70.4) | 0.002 | 25.9 (4.55–147) | 0.000 | ||
| Renal insufficiency¶# | – | – | 9.08 (1.44–57.2) | 0.019 | 16.0 (2.06–123) | 0.008 | ||
| Nonhematologic malignancy | 3.90 (1.33–11.5) | 0.013 | – | – | – | – | ||
| Age, continuous | 1.03 (1.01–1.06)# | 0.005 | 1.06 (1.03–1.09)# | 0.000 | 1.06 (1.02–1.11)# | 0.005 | ||
*OR, odds ratio. †Possible risk factors entered in all analyses: age, vascular prosthesis, aortic aneurysm, other vascular surgeries, peripheral arterial disease, cerebrovascular disease, valvular surgery, valvular disease (nonsurgical), ischemic cardiac disease, other cardiac history, hypertension, dyslipidemia, diabetes, nonhematologic malignancy, renal insufficiency. Immune disorder was also entered in the analyses of proven and probable chronic Q fever and of proven chronic Q fever. Pacemaker was also entered in the analysis of proven chronic Q fever. ‡Valvular surgeries in the proven group are subdivided into biological valve (n = 6), prosthetic valve (n = 3), and valve repair (n = 1) all located in the aortic valve (n = 10). Within the controls there were no patients with history of valvular surgery. §Locations of vascular prostheses in proven group were infrarenal and iliac (n = 6), infrarenal (n = 4), thoracic (n = 2), and unknown (n = 2). Types of vascular prosthesis were Y-prosthesis (n = 7), endovascular aneurysm repair (n = 2), stent graft (n = 2), Bentall (n = 1), and unknown (n = 2). For the 2 control patients, specifications of the prostheses were unknown. ¶Locations of aneurysms in proven group were infrarenal (n = 6), infrarenal and iliac (n = 2), and suprarenal, infrarenal, and iliac (n = 1). Within the control group, aneurysms were infrarenal and iliac (n = 2). #Observed stages of chronic kidney disease according to the Kidney Disease Outcome Quality Initiative guidelines (28) in the proven group were stage 3 (n = 6), stage 4 (n = 2), and stage 5 (n = 1) and in the controls solely stage 3 (n = 2). #OR per year of increasing age.
Figure 2.
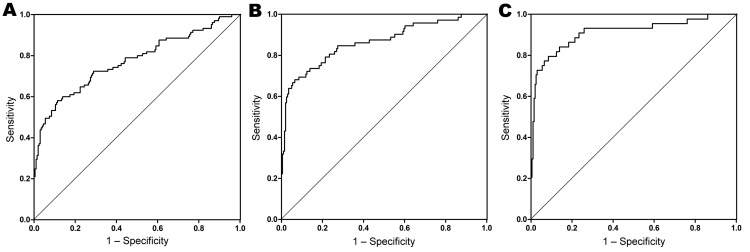
Goodness-of-fit models for case–control study to identify risk factors for chronic Q fever, the Netherlands. A) All chronic Q fever cases (n = 105); area under the curve (c-statistic) 0.77 (95% CI 0.71–0.83); p<0.001. B) Proven and probable chronic Q fever cases (n = 72); c-statistic 0.86 (95% CI 0.81–0.92); p<0.001. C) Proven chronic Q fever cases (n = 44); c-statistic 0.91 (95% CI 0.85–0.97); p<0.001. Patient risk factors included in the model (no. observations): A) valvular surgery (18); vascular prosthesis (15); aneurysm (12); nonhematologic malignancy (16); age, continuous, mean 63.9 y; B) valvular surgery (18); vascular prosthesis (15); aneurysm (12); renal insufficiency (12); age, continuous, mean 67.3 y; C) valvular surgery (10); vascular prosthesis (14); aneurysm (9); renal insufficiency (9); age, continuous, mean 68.4.
Patient risk factors identified in the analysis of the proven cases, representing the most definite chronic Q fever cases, were valvular surgery (OR 43.6, 95% CI 4.70–405), vascular prosthesis (OR 26.8, 95% CI 4.88–147), aneurysm (OR 25.9, 95% CI 4.55–147), renal insufficiency (OR 16.0, 95% CI 2.06–123), and age (OR 1.06, 95% CI 1.02–1.11). The final discriminative performance was good, with a c-statistic of 0.91 (95% CI 0.85–0.97) (Figure 2).
Discussion
To our knowledge, this is the first study that analyzed a large number of potential risk factors for chronic Q fever in a large number of patients. Most former studies have been limited by a low number of cases and evaluation of few risk factors. Moreover, quantification of these risk factors was lacking (6–9,11,12).
In our study, we focused mainly on case-patients with proven chronic Q fever becausethis group included patients with the most definite form of chronic Q fever. Proven chronic Q fever also showed the strongest correlation with the identified risk factors. In multivariate analysis, valvular surgery, vascular prosthesis, aneurysms, renal insufficiency, and age were significant risk factors for the development of chronic Q fever in patients with proven cases. In the analysis of all patients with chronic Q fever cases, nonhematologic malignancy also seemed to be a risk factor; however, this could not be reproduced in the subanalyses of the more definite cases (e.g., proven and probable cases). Hence, nonhematologic malignancy as a risk factor remains uncertain. Valvular surgery, vascular prostheses, and aneurysms were the strongest predictors in this study, which confirms observational findings from earlier studies. Explanation lies in the association with the preferred localization of chronic Q fever infection.
A novel finding is the association between mild renal insufficiency and chronic Q fever. The majority of patients with chronic Q fever and renal disease in our study had stage 3 renal insufficiency according to Kidney Disease Outcome Quality Initiative guidelines (28). Although terminal renal insufficiency can decrease the immune response, this association was not found for mild renal disease (29). Renal insufficiency is associated with vascular disease, which may explain the elevated incidence of chronic Q fever in these patients (30).
Increasing age also predisposes for the development of chronic Q fever; this predisposition was also illustrated in a recent report of van der Hoek et al. (24). The explanation probably lies in the increased prevalence of cardiovascular diseases and the decreased cellular immunity during aging (31,32). Age >60 years appeared the best cutoff above which the risk for chronic Q fever increases significantly.
Preexisting cardiac valvulopathy has been found to give an estimated risk of 39% for the development of chronic Q fever after infection with C. burnetii (6,33,34). In contrast, recent reports showed no elevated risk for patients with mild valvulopathy in the ongoing outbreak in the Netherlands (26,27). Although our univariate analyses showed that nonsurgical cardiac valvulopathy increased the risk for the development of chronic Q fever, this finding was not confirmed in the multivariate analysis. This finding can be explained by the fact that 9/17 (53%) case-patients with nonsurgical valvulopathy also had a history of valvular surgery of one of the other valves. The location and type of valvular defects did not differ significantly between case-patients and controls (Table 2). A possible explanation for the discrepancy with previous observations lies in the fact that our study was conducted 4 years after start of the Q fever epidemic, but chronic Q fever endocarditis in patients with nonsurgical cardiac valvulopathy might become evident later (6,8). Furthermore, strain-specific differences in clinical signs and symptoms might also be of importance (26). Presence of valvulopathy in case-patients and controls could have been missed becausethis was assessed only through review of medical records. However, echocardiography, which was standard care for all patients with suspected cases of chronic Q fever, revealed no additional congenital or bicuspid valve defects, in comparison to assessment of valvulopathy through review of medical records. From other than the above-mentioned defects, it could not be determined by these echocardiograms if these were preexisting or caused by chronic Q fever.
Immunosuppression, although not well defined, has been indicated as a risk factor in former reports, but clear definition and statistical empowerment is lacking (8). Although our univariate analysis did show an elevated risk for immunosuppression, especially for patients with proven chronic Q fever cases, this elevated risk was not confirmed in multivariate analysis. Immunocompromised patients may be underrepresented in our study becauseit was conducted in a peripheral hospital setting. Further evaluation of this risk factor should be performed in future studies.
Pregnancy, another formerly reported risk factor, showed an association with the development of chronic Q fever in univariate analysis. However, because there were no pregnant women in the control group and only 3 pregnant women in all case groups, evaluation of pregnancy in multivariate analyses was not possible. A study specifically designed to evaluate associations between pregnancy and Q fever is ongoing in the Netherlands (35).
In our opinion, our data were representative for this large Q fever outbreak because they were well documented data and patients were willing to participate. The fact that case-patients and controls were living in the same area increases the comparability of these groups and strengthens the results. However, our study does have potential weaknesses.
First, all controls had an acute episode in 2009, but information about their signs and symptoms was obtained in 2011, introducing possible recall bias. We tried to reduce this bias by requesting additional information from the general practitioners and by reviewing clinical test results and physicians’ reports in the hospital registration systems. Because information bias could also have been introduced by the subjective interpretation of physicians’ reports for case-patients and controls, 2 of our researchers interpreted the results independently.
Serologic follow-up of the controls after the acute Q fever episode lasted only 1 year, which is the normal follow-up period in the Netherlands. However, because chronic Q fever can become manifest years after initial infection, development of chronic Q fever after this follow-up period is still possible (6,22). Still, 75% of chronic Q fever cases develop within 6 months after primary infection (22). Moreover, according to the observed decrease in antibody titers of these patients, progression to chronic Q fever is not likely. In addition, as a consequence of the inclusion of patients with symptomatic acute Q fever as a control group, the results can only be generalized to patients with symptomatic acute Q fever, although the results probably provide an adequate indication of risks factors for patients with mild or asymptomatic primary Q fever.
Notably, almost all controls received antimicrobial drug treatment at time of acute Q fever, in contrast to the case-patients, among which only a minority had symptomatic acute Q fever. Thus, antimicrobial drug treatment might influence the chance of chronic Q fever development, although there is no quantitative evidence that treatment for acute Q fever reduces the chance for chronic Q fever (4).
Chronic Q fever cases were selected and classified according to the definitions of the Dutch Q Fever Consensus Group (20), which still need confirmation. The definition of probable chronic Q fever contains several patient criteria that we also included as potential risk factors in our study (e.g., valvular disease, vascular prosthesis, aneurysm, and immunosuppressive state). Nevertheless, proven chronic Q fever, for which these criteria are not part of the definition, was also predicted with the identified risk factors in multivariate analysis, thereby confirming the independent risk association of these variables.
Some chronic Q fever cases were identified during screening programs for patients who had valvular surgery, aneurysms, or vascular prostheses. Patients with these risk factors may therefore be overrepresented within our study, although all proven case-patients had symptomatic disease.
Last, the results of this study have to be considered in view of a predominant C. burnetii strain that is responsible for the majority of Q fever cases in humans in the Netherlands (36). Worldwide, Q fever manifestations differ geographically, which might result from differences in C. burnetii strains (4).
In conclusion, previous valvular surgery, vascular prosthesis, aneurysms, renal insufficiency, and age were identified as major risk factors for the development of chronic Q fever among persons infected with C. burnetii. Because untreated chronic Q fever comes with serious risk for illness and death, awareness is required in people with acute Q fever possessing the identified risk factors. This may require close follow-up or even prophylactic treatment in high-risk groups. Moreover, in case of large Q fever outbreaks, screening is advisable for patients with these identified risk factors.
Biography
Dr Kampschreur is a medical doctor and PhD student at the Division of Medicine, Department of Internal Medicine and Infectious Diseases, University Medical Center Utrecht, the Netherlands. Her primary research interest is chronic Q fever in the Netherlands.
References
- 1.Raoult D, Marrie T, Mege J. Natural history and pathophysiology of Q fever. Lancet Infect Dis. 2005;5:219–26. 10.1016/S1473-3099(05)70052-9 [DOI] [PubMed] [Google Scholar]
- 2.Parker NR, Barralet JH, Bell AM. Q fever. Lancet. 2006;367:679–88. 10.1016/S0140-6736(06)68266-4 [DOI] [PubMed] [Google Scholar]
- 3.Angelakis E, Raoult D. Q fever. Vet Microbiol. 2010;140:297–309. 10.1016/j.vetmic.2009.07.016 [DOI] [PubMed] [Google Scholar]
- 4.Maurin M, Raoult D. Q fever. Clin Microbiol Rev. 1999;12:518–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tissot-Dupont H, Raoult D. Q fever. [ix.]. Infect Dis Clin North Am. 2008;22:505–14. 10.1016/j.idc.2008.03.002 [DOI] [PubMed] [Google Scholar]
- 6.Fenollar F, Fournier PE, Carrieri MP, Habib G, Messana T, Raoult D. Risks factors and prevention of Q fever endocarditis. Clin Infect Dis. 2001;33:312–6. 10.1086/321889 [DOI] [PubMed] [Google Scholar]
- 7.Frankel D, Richet H, Renvoise A, Raoult D. Q fever in France, 1985–2009. Emerg Infect Dis. 2011;17:350–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raoult D, Tissot-Dupont H, Foucault C, Gouvernet J, Fournier PE, Bernit E, et al. Q fever 1985–1998. Clinical and epidemiologic features of 1,383 infections. Medicine (Baltimore). 2000;79:109–23. 10.1097/00005792-200003000-00005 [DOI] [PubMed] [Google Scholar]
- 9.Botelho-Nevers E, Fournier PE, Richet H, Fenollar F, Lepidi H, Foucault C, et al. Coxiella burnetii infection of aortic aneurysms or vascular grafts: report of 30 new cases and evaluation of outcome. Eur J Clin Microbiol Infect Dis. 2007;26:635–40. 10.1007/s10096-007-0357-6 [DOI] [PubMed] [Google Scholar]
- 10.Delsing CE, Kullberg BJ, Bleeker-Rovers CP. Q fever in the Netherlands from 2007 to 2010. Neth J Med. 2010;68:382–7. [PubMed] [Google Scholar]
- 11.Brouqui P, Dupont HT, Drancourt M, Berland Y, Etienne J, Leport C, et al. Chronic Q fever. Ninety-two cases from France, including 27 cases without endocarditis. Arch Intern Med. 1993;153:642–8. 10.1001/archinte.1993.00410050074010 [DOI] [PubMed] [Google Scholar]
- 12.Tissot-Dupont H, Vaillant V, Rey S, Raoult D. Role of sex, age, previous valve lesion, and pregnancy in the clinical expression and outcome of Q fever after a large outbreak. Clin Infect Dis. 2007;44:232–7. 10.1086/510389 [DOI] [PubMed] [Google Scholar]
- 13.van der Hoek W, Dijkstra F, Schimmer B, Schneeberger PM, Vellema P, Wijkmans C, et al. Q fever in the Netherlands: an update on the epidemiology and control measures. Euro Surveill 2010;15:pii=19520 [cited 2011 May 20]. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19520 [PubMed]
- 14.Hogema BM, Slot E, Molier M, Schneeberger PM, Hermans MH, van Hannen EJ, et al. Coxiella burnetii infection among blood donors during the 2009 Q-fever outbreak in the Netherlands. Transfusion. Epub 2011 Jul 14. [DOI] [PubMed] [Google Scholar]
- 15.Fenollar F, Fournier PE, Raoult D. Molecular detection of Coxiella burnetii in the sera of patients with Q fever endocarditis or vascular infection. J Clin Microbiol. 2004;42:4919–24. 10.1128/JCM.42.11.4919-4924.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Musso D, Raoult D. Coxiella burnetii blood cultures from acute and chronic Q-fever patients. J Clin Microbiol. 1995;33:3129–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Healy B, van Woerden H, Raoult D, Graves S, Pitman J, Lloyd G, et al. Chronic Q fever: different serological results in three countries—results of a follow-up study 6 years after a point source outbreak. Clin Infect Dis. 2011;52:1013–9. 10.1093/cid/cir132 [DOI] [PubMed] [Google Scholar]
- 18.Dupont HT, Thirion X, Raoult D. Q fever serology: cutoff determination for microimmunofluorescence. Clin Diagn Lab Immunol. 1994;1:189–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rolain JM, Lecam C, Raoult D. Simplified serological diagnosis of endocarditis due to Coxiella burnetii and Bartonella. Clin Diagn Lab Immunol. 2003;10:1147–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wegdam-Blans MCA, Kampschreur LM, Nabuurs-Franssen MH, Renders NHM, Delsing CE, Bijlmer HA. Dutch consensus chronic Q fever [in Dutch]. Tijdschr Infect. 2011;6:71. [Google Scholar]
- 21.Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG Jr, Ryan T, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30:633–8. 10.1086/313753 [DOI] [PubMed] [Google Scholar]
- 22.Landais C, Fenollar F, Thuny F, Raoult D. From acute Q fever to endocarditis: serological follow-up strategy. Clin Infect Dis. 2007;44:1337–40. 10.1086/515401 [DOI] [PubMed] [Google Scholar]
- 23.Wegdam-Blans MC, Nabuurs-Franssen MN, Horrevorts AM, Peeters MF, Schneeberger PM, Bijlmer HA. Laboratory diagnosis of acute Q fever [in Dutch]. Ned Tijdschr Geneeskd. 2010;154:A2388. [PubMed] [Google Scholar]
- 24.van der Hoek W, Versteeg B, Meekelenkamp JC, Renders NH, Leenders AC, Weers-Pothoff I, et al. Follow-up of 686 patients with acute Q fever and detection of chronic infection. Clin Infect Dis. 2011;52:1431–6. 10.1093/cid/cir234 [DOI] [PubMed] [Google Scholar]
- 25.Jager MM, Weers-Pothoff G, Hermans MH, Meekelenkamp JC, Schellekens JJ, Renders NH, et al. Evaluation of a diagnostic algorithm for acute Q fever in an outbreak setting. Clin Vaccine Immunol. 2011;18:963–8. 10.1128/CVI.00009-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Limonard GJ, Nabuurs-Franssen MH, Weers-Pothoff G, Wijkmans C, Besselink R, Horrevorts AM, et al. One-year follow-up of patients of the ongoing Dutch Q fever outbreak: clinical, serological and echocardiographic findings. Infection. 2010;38:471–7. 10.1007/s15010-010-0052-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Limonard GJ, Nabuurs-Franssen MH, Dekhuijzen PN, Groot CA. Prevention of Q fever endocarditis. Lancet Infect Dis. 2011;11:82–3. 10.1016/S1473-3099(11)70016-0 [DOI] [PubMed] [Google Scholar]
- 28.Kidney Disease Outcome Quality Initiative clinical practice guidelines for chronic kidney disease: evaluation, classification and stratification. National Kidney Foundation. 2002. [cited 2011 June 13]. http://www.kidney.org/professionals/kdoqi/guidelines_ckd/p4_class_g1.htm
- 29.Betjes MG, Langerak AW, van der Spek A, de Wit EA, Litjens NH. Premature aging of circulating T cells in patients with end-stage renal disease. Kidney Int. Epub 2011 Apr 27. [DOI] [PubMed] [Google Scholar]
- 30.El Nahas M. Cardio-Kidney-Damage: a unifying concept. Kidney Int. 2010;78:14–8. 10.1038/ki.2010.123 [DOI] [PubMed] [Google Scholar]
- 31.Weiskopf D, Weinberger B, Grubeck-Loebenstein B. The aging of the immune system. Transpl Int. 2009;22:1041–50. 10.1111/j.1432-2277.2009.00927.x [DOI] [PubMed] [Google Scholar]
- 32.Lloyd-Jones DM, Leip EP, Larson MG, D’Agostino RB, Beiser A, Wilson PW, et al. Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age. Circulation. 2006;113:791–8. 10.1161/CIRCULATIONAHA.105.548206 [DOI] [PubMed] [Google Scholar]
- 33.Fenollar F, Thuny F, Xeridat B, Lepidi H, Raoult D. Endocarditis after acute Q fever in patients with previously undiagnosed valvulopathies. Clin Infect Dis. 2006;42:818–21. 10.1086/500402 [DOI] [PubMed] [Google Scholar]
- 34.Million M, Thuny F, Richet H, Raoult D. Long-term outcome of Q fever endocarditis: a 26-year personal survey. Lancet Infect Dis. 2010;10:527–35. 10.1016/S1473-3099(10)70135-3 [DOI] [PubMed] [Google Scholar]
- 35.Munster JM, Leenders AC, van der Hoek W, Schneeberger PM, Rietveld A, Riphagen-Dalhuisen J, et al. Cost-effectiveness of a screening strategy for Q fever among pregnant women in risk areas: a clustered randomized controlled trial. BMC Womens Health. 2010;10:32. 10.1186/1472-6874-10-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huijsmans CJ, Schellekens JJ, Wever PC, Toman R, Savelkoul PH, Janse I, et al. Single-nucleotide-polymorphism genotyping of Coxiella burnetii during a Q fever outbreak in the Netherlands. Appl Environ Microbiol. 2011;77:2051–7. 10.1128/AEM.02293-10 [DOI] [PMC free article] [PubMed] [Google Scholar]