Abstract
A high proportion of triatomine insects, vectors for Trypanosoma cruzi trypanosomes, collected in Arizona and California and examined using a novel assay had fed on humans. Other triatomine insects were positive for T. cruzi parasite infection, which indicates that the potential exists for vector transmission of Chagas disease in the United States.
Keywords: Chagas disease, Triatominae, Triatoma, insect vectors, feeding behavior, United States, Trypanosoma cruzi, vector-borne infections, Arizona, California
Keywords: Suggested citation for this article: Stevens L, Dorn PL, Hobson J, de la Rua NM, Lucero DE, Klotz JH, et al. Vector blood meals and Chagas disease transmission potential, United States. Emerg Infect Dis [serial on the internet]. 2012 Apr [date cited]. http://dx.doi.org/10.3201/eid1804.111396
Chagas disease is a vector-borne disease caused by Trypanosoma cruzi trypanosomes. Although these parasites are rarely transmitted by insects in the United States, there is concern that vector transmission may increase (1). Chagas disease, endemic to most of Latin America, can be transmitted to mammals by >130 species of blood-feeding insect vectors (subfamily Triatominae). In the United States, the most common result of a triatomine bite is allergic reaction, including anaphylaxis, elicited in sensitized persons (2). Despite only 7 cases of vector transmission to humans reported in the United States (3,4), T. cruzi trypanosomes are present in >20 wildlife species. In Latin America, 8–10 million persons are infected with these parasites (5), and an estimated 300,000 of the ≈13 million persons from disease-endemic areas now living in the United States carry the parasite (6). Although vector transmission of T. cruzi trypanosomes is a minimal risk, 9 of the 11 triatomine species in the United States are potential vectors (1,7), and parasite transmission could increase because of climate change (1).
One critical aspect of transmission is parasite reservoirs; hence, the epidemiologic importance of identifying animal sources of the vectors’ blood meals, the likelihood of these vectors also feeding on humans, and their incidence of T. cruzi infection. Blood meals consumed by insect vectors have been detected by using several molecular techniques (8), but assays are challenging because of degradation of the blood in the vector’s gut, PCR inhibition, the often small size of a blood meal, and the difficulty of distinguishing multiple blood meals. We analyzed T. cruzi vectors collected in California and Arizona by using a novel technique— cloning following PCR amplification using universal vertebrate primers—to determine the source of blood meals and indicate the likelihood of parasite transmission to humans.
The Study
Insects were collected by using light traps at Redington Road, Tucson, Arizona, and Escondido, California, in 2007, and within the Arizona-Sonora Desert Museum, Tucson, in 2009 (Table 1). We examined blood meals (9) and T. cruzi parasite infection (10) of 13 insects from 2 species of kissing bugs, Triatoma rubida and T. protracta. A mouse-fed T. recurva bug served as a control (11). Using universal vertebrate primers for cytB and 12S (12,13), we identified as many blood meals as possible (Table 1). We cloned and sequenced the PCR products to isolate multiple blood meals within a single insect (Table 2). Blood meal sources were inferred by using BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Pearson χ2 tests compared the likelihood of feeding on humans between vector species and compared the cytB and 12S assays for differences in the number of blood meal taxa and blood meal haplotypes (i.e., unique DNA sequences) per insect (JMP Ver9; SAS, Cary, NC, USA).
Table 1. Blood meal sources of Trypanosoma cruzi insect vectors collected in Arizona and California, USA, 2007 and 2009, as determined by using cytB and 12S rDNA assays, and haplotypes identified*.
| Assay and Triatoma spp. | T. cruzi | Location† | No. vertebrate blood meal sources |
Haplotypes (no.) of vertebrate blood meal sources amplified in clones |
No. non–blood meal clones |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clones | Taxa | Haplo | Human | Rat | Chick | Dog | Pig | Mouse‡ | Vector§ | ND | |||||
| cytB | |||||||||||||||
| T. rubida | – | R | 8 | 1 | 1 | A | 6 | 1 | |||||||
| T. rubida | – | R | 10 | 1 | 2 | B, C | 7 | 1 | |||||||
| T. protracta | – | E | 8 | 1 | 1 | D | 5 | 2 | |||||||
| T. protracta | – | E | 11 | 1 | 1 | A | 9 | 1 | |||||||
| T. protracta | + | E | 9 | 0 | 0 | 7 | 1 | ||||||||
| T. protracta | + | E | 8 | 1 | 2 | A, B | 6 | ||||||||
| T. protracta | – | E | 9 | 0 | 0 | 9 | |||||||||
| T. protracta | – | E | 8 | 0 | 0 | 7 | 1 | ||||||||
| T. protracta | + | E | 8 | 0 | 0 | 8 | |||||||||
| T. recurva‡ | – | 8 | A (7), B | ||||||||||||
| 12S rRNA | |||||||||||||||
| T. protracta | + | M | 8 | 1 | 2 | A (7), B | |||||||||
| T. protracta | + | M | 8 | 2 | 4 | A (4) | A (2), B, C | ||||||||
| T. rubida | – | M | 6 | 2 | 3 | A (4), B | A | ||||||||
| T. rubida | – | M | 7 | 2 | 2 | A | A (6) | ||||||||
*Vector species, T. cruzi infection status, collection location, number of clones sequenced, number and identity of taxa, and number of haplotypes represented in the clone sequences are indicated. Blank cells indicate clones were not found. For the cytB assay, the number of clones that were Triatoma spp. vector DNA or had uninterpretable sequences are indicated. The mouse-fed control (cytB assay) had 2 mouse haplotypes. Haplo, haplotypes; rat, woodrat; chick, chicken; ND, not determined because of low quality sequence data; –, negative; +, positive. †Insects were collected by using light traps at Redington Road, Tucson, Arizona (R), and Escondido, CA (E), in 2007, and within the Arizona-Sonora Desert Museum, Tucson, AZ (M), in 2009. The light traps were in “wilderness” (museum) and “sylvatic” (Redington Road and Escondido) habitats and not in human habitations. ‡Control. §Triatoma spp.
Table 2. Assays used to determine the source of blood meals and Trypanosoma cruzi trypanosome infection in insects collected in Arizona and California, USA*.
| Assay and reference | Primers, 5′ → 3′ | PCR cycling† | Amplicon size |
|---|---|---|---|
| cytB (12) | cca tcc aac atc tca gca tga tga a | 95°C, 40 s; 44°C, 40 s; 72°C, 40 s | 358 bp |
| ccc ctc aga atg att att tgt cct ca | |||
| 12S (13) | ccc aaa ctg gga tta gat acc c | 95°C, 30 s; 57°C, 15 s; 72°C, 30 s | 215 bp |
| gtt tgc tga aga tgg cgg ta | |||
| TCZ‡ (11) | cga gct ctt gcc cac acg ggt gct | 94°C, 20 s; 57°C, 10 s; 72°C, 30 s | 188 bp |
| cct cca agc agc gga tag ttc agg |
*Insects were collected by using light traps in Tucson, Arizona, and Escondido, CA, in 2007, and within the Arizona-Sonora Desert Museum, Tucson, in 2009. For the blood meal assays, cloned PCR products (pGEM-T, Promega, Madison, WI), USA were sequenced by using the BigDye v3.1 Terminator Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA) and analyzed by using an ABI PRISM 3730xl (Beckman Coulter, Fullerton, CA, USA). †For all assays: initial denaturation of 95°C for 5 m; 35 cycles of PCR and final extension of 72°C for 10 m. ‡A negative control (lacking T. cruzi DNA template) was included with every assay. Samples that failed to amplify were spiked with 1 μL of T. cruzi parasites boiled in 1× PCR buffer and retested to ensure that the lack of product was not caused by PCR inhibition.
Five of the 13 bugs (38%) had positive test results for human blood (Table 1); T. rubida bugs were significantly more likely than T. protracta bugs to have fed on humans (χ2 9.24; p<0.01). T. rubida bugs had also fed on dogs and T. protracta bugs on woodrats (Neotoma spp.), chickens, dogs, and pigs. T. cruzi infection was found in 5/9 T. protracta and 0/4 T. rubdia bugs. No insect that had fed on humans was infected with T. cruzi trypanosomes.
The cytB and 12S assays differed in the specificity of primers for vertebrate DNA and number of blood meals per insect (Figure). More than 70% of DNA cloned in the cytB assay was from the insect (64/87 clones); ≈10% of clones did not produce interpretable sequences. In contrast, the 12S assay did not clone insect DNA, and all sequences were interpretable. The average number of blood meals per insect was not statistically different (cytB 0.56 taxa/insect, 12S 1.75 taxa/insect; χ2 8.31; p<0.10); however, the average number of haplotypes/insect was significantly higher for 12S (cytB 0.78, 12S 2.75; χ2 9.09; p<0.02).
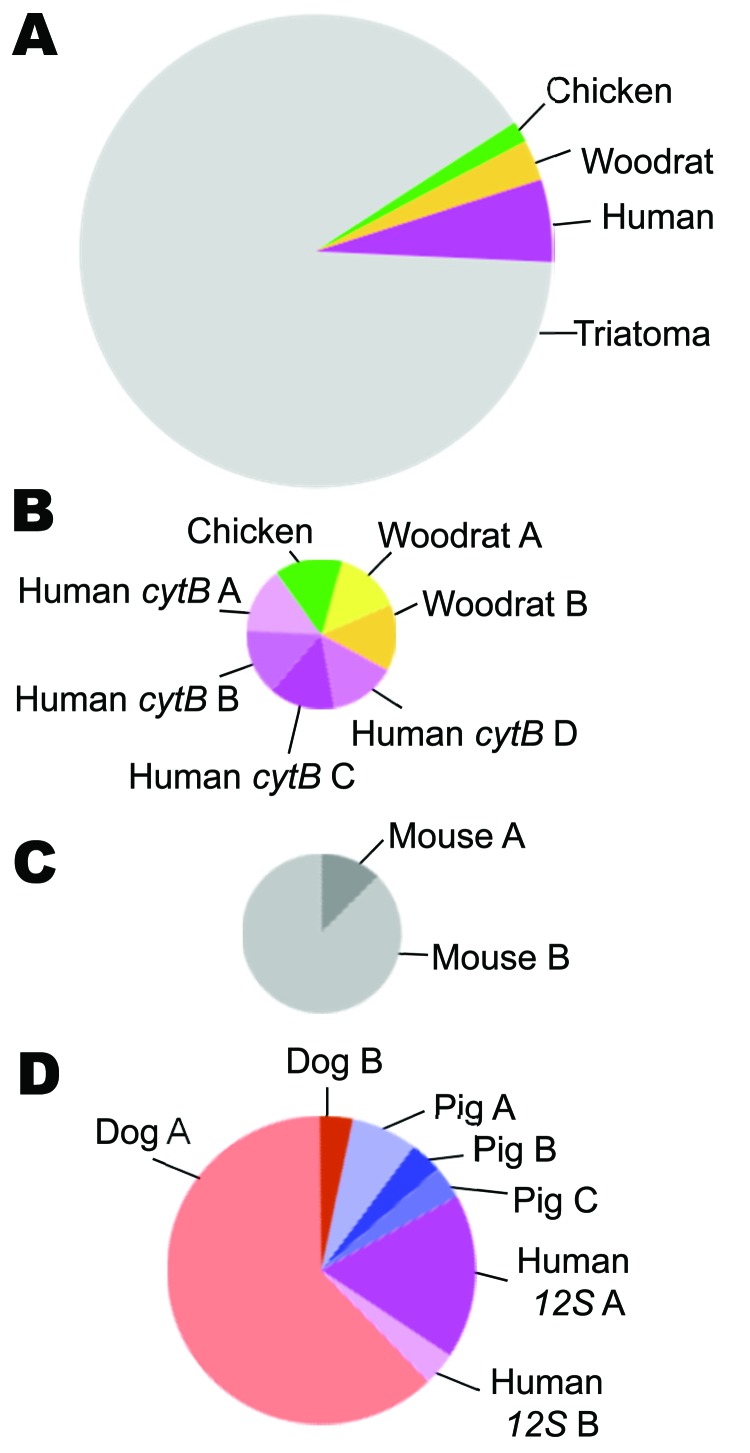
Figure.
Types of blood meals found by using cytB and 12S assays in insect vector species that carry Trypanosoma cruzi, the pathogen that causes Chagas disease, Arizona and California, USA, 2007 and 2009. Circle size is proportional to the sample size for that comparison. A) Vertebrate taxa and vector DNA (n = 71 sequences), showing that the cytB assay amplified vector DNA more often than blood meal DNA. B) Four vertebrate taxa among the blood meals detected by the cytB assay (n = 7 sequences). Unique haplotypes (DNA sequences or alleles) of human and woodrat are indicated by letters. C) Two mouse haplotypes detected in the mouse-fed control insect (n = 8 sequences). D) Types of blood meal based on the 12S assay (n = 29 sequences).
The cytB assay detected more haplotypes from each blood meal taxon, indicating the bugs fed on unique individuals; 4 human and 2 woodrat sequences were all different from each other. In contrast, for 12S, 17/18 dog sequences were identical, as were 2/3 human and 3/4 pig sequences.
The mouse-fed control (11) was the only insect for which no vector DNA was cloned in the cytB assay. All 8 clones from the control were mouse; 7 were identical. Although unexpected, heteroplasmic mitochondrial DNA has been reported for inbred mice (14).
Conclusions
We found that 38% bugs of 2 species of T. cruzi vectors endemic to the United States, T. rubida and T. protracta, fed on humans. Infection with the Chagas parasite, T. cruzi, was high (55% for T. protracta bugs), but no insect was positive for human blood meals and the parasite. Both vectors are common in the foothills of Tucson, Arizona (2); although the T. rubida bugs in this study were uninfected, another study found that 67% of adult bugs collected around Tucson were infected with T. cruzi parasites (15).
The Arizona-Sonora Desert Museum in Tucson exhibits desert animals. All 4 insects collected from near the museum had fed on canids (dog/coyote/wolf; Canis spp.); 1 had fed on pigs and 2 on humans. Although canid samples are not distinguishable by 12S, there are no dogs at the museum, so the insects probably fed on coyotes or wolves. Although javelina (Tayassu tajacu), a species similar to pigs, are at the museum, sequences were 99% identical to pig (Sus scrofa) and only a 90% match with javelina. The source of the human blood meals is not clear. No one lives at the museum, but there is camping in the area.
Around Escondido, we found T. protracta bugs fed on humans, woodrats, and domestic chickens, according to the cytB assay. This assay amplified only vector DNA from 4/7 insects, which could mean it had been a long time since the last blood meal and thus the DNA was highly degraded. We detected only 1 blood meal source in the other 3 insects from California but found 2 woodrat sequences in a single insect. Both T. rubida bugs collected in California had fed only on humans; 2 unique human sequences from 2 clones from 1 insect suggest it had fed on 2 humans.
Compared with cytB, the 12S assay had better amplification and sequence quality and no recovery of insect vector DNA. Attempts to improve the cytB assay (e.g., higher annealing temperature) were unsuccessful, leading us to try the 12S assay, which had a smaller amplicon size and higher primer specificity for vertebrate DNA (13). Only mouse DNA was detected from the control bug that had fed on mouse in the laboratory (2), demonstrating the cytB assay worked well for fresh blood meals; however, for degraded DNA, 12S is a better assay.
Overall, cytB is more variable than 12S, producing more haplotypes, and thus can detect feeding on multiple individuals of the same taxon. Because identifying the source of blood meals depends on the availability of similar sequences in GenBank, another advantage of cytB is that GenBank contains 3–4× as many vertebrate sequences for comparison.
In conclusion, although allergic reaction from triatomine bites is well known (12), the high incidence of human blood meals in these bugs in our study suggests that the potential for human transmission of T. cruzi parasites might be greater than previously thought. Our assays using vertebrate primers and cloning PCR products may be especially useful for detecting unpredicted blood meal sources and multiple blood meals.
Acknowledgments
Part of this material is based on work supported by the National Science Foundation under core faculty funding from grant no. NSF EPS-0701410 (to L.S.) and National Institutes of Health grant 1R15 A1079672-01A1 (to P.L.D.). All procedures using mice were first approved by the Southwestern Biological Institute Animal Care and Use Committee.
Biography
Dr Stevens is professor of biology at the University of Vermont. Her research interests include feeding sources and population genetics of triatomine vectors in North, Central, and South America.
References
- 1.Click Lambert R, Kolivras KN, Resler LM, Brewster CC, Paulson SL. The potential for emergence of Chagas disease in the United States. Geospat Health. 2008;2:227–39. [DOI] [PubMed] [Google Scholar]
- 2.Klotz JH, Dorn PL, Logan JL, Stevens L, Pinnas JL, Schmidt JO, et al. “Kissing bugs”: potential disease vectors and cause of anaphylaxis. Clin Infect Dis. 2010;50:1629–34. 10.1086/652769 [DOI] [PubMed] [Google Scholar]
- 3.Kjos SA, Snowden KF, Olson JK. Biogeography and Trypanosoma cruzi infection prevalence of Chagas disease vectors in Texas, USA. Vector Borne Zoonotic Dis. 2009;9:41–50. 10.1089/vbz.2008.0026 [DOI] [PubMed] [Google Scholar]
- 4.Dorn PL, Perniciaro L, Yabsley MJ, Roellig DM, Balsamo G, Diaz J. Autochthonous transmission of Trypanosoma cruzi, Louisiana. Emerg Infect Dis. 2007;13:605–7. 10.3201/eid1304.061002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hotez PJ, Bottazzi ME, Franco-Paredes C, Ault SK, Periago MR. The neglected tropical diseases of Latin America and the Caribbean: a review of disease burden and distribution and a roadmap for control and elimination. PLoS Negl Trop Dis. 2008;2:e300. 10.1371/journal.pntd.0000300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bern C, Montgomery SP. An estimate of the burden of Chagas disease in the United States. Clin Infect Dis. 2009;49:e52–4. 10.1086/605091 [DOI] [PubMed] [Google Scholar]
- 7.Stevens L, Dorn PL, Schmidt JO, Klotz JH, Lucero D, Klotz SA. Kissing bugs. The vectors of Chagas. Adv Parasitol. 2011;75:169–92. 10.1016/B978-0-12-385863-4.00008-3 [DOI] [PubMed] [Google Scholar]
- 8.Kent RJ. Molecular methods for arthropod blood meal identification and applications to ecological and vector-borne disease studies. Mol Ecol Resour. 2009;9:4–18. 10.1111/j.1755-0998.2008.02469.x [DOI] [PubMed] [Google Scholar]
- 9.Pizarro JC, Stevens L. A new method for forensic DNA analysis of the blood meal in Chagas disease vectors demonstrated using Triatoma infestans from Chuquisaca, Bolivia. PLoS ONE. 2008;3:e3585. 10.1371/journal.pone.0003585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pizarro JC, Lucero DE, Stevens L. PCR reveals significantly higher rates of Trypanosoma cruzi infection than microscopy in the Chagas vector, Triatoma infestans: high rates found in Chuquisaca, Bolivia. BMC Infect Dis. 2007;7:66–73. 10.1186/1471-2334-7-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klotz SA, Dorn PL, Klotz JH, Pinnas JL, Weirauch C, Kurtz JR, et al. Feeding behavior of triatomines from the southwestern United States: an update on potential risk for transmission of Chagas disease. Acta Trop. 2009;111:114–8. 10.1016/j.actatropica.2009.03.003 [DOI] [PubMed] [Google Scholar]
- 12.Kocher TD, Thomas WK, Meyer A, Edwards SV, Paabo S, Villablanca FX, et al. Dynamics of mitochondrial DNA evolution in animals: amplification and sequencing with conserved primers. Proc Natl Acad Sci U S A. 1989;86:6196–200. 10.1073/pnas.86.16.6196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitano T, Umetsu K, Tian W, Osawa M. Two universal primer sets for species identification among vertebrates. Int J Legal Med. 2007;121:423–7. 10.1007/s00414-006-0113-y [DOI] [PubMed] [Google Scholar]
- 14.Sachadyn P, Zhang XM, Clark LD, Naviaux RK, Heber-Katz E. Naturally occurring mitochondrial DNA heteroplasmy in the MRL mouse. Mitochondrion. 2008;8:358–66. 10.1016/j.mito.2008.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reisenman CE, Gregory T, Guerenstein PG, Hildebrand JG. Feeding and defecation behavior of Triatoma rubida (Uhler, 1894) (Hemiptera: Reduviidae) under laboratory conditions, and its potential role as a vector of Chagas disease in Arizona, USA. Am J Trop Med Hyg. 2011;85:648–56. 10.4269/ajtmh.2011.11-0137 [DOI] [PMC free article] [PubMed] [Google Scholar]