INTRODUCTION
The introduction of combination antiretroviral therapy (ARV) was followed by changes in fat distribution and metabolic abnormalities in HIV-infected individuals that may contribute to cardiovascular disease[1]. Both peripheral fat loss (lipoatrophy) and central fat gain (lipohypertrophy) have been reported in HIV infection. A major finding of the first study of Fat Redistribution and Metabolic Change in HIV infection (FRAM) was that HIV-infected participants differed from controls in terms of lipoatrophy, but not lipohypertrophy, as defined by self-report confirmed by exam, as well as by direct measurement using total body regional MRI[2, 3]. Participants with clinical lipoatrophy had less subcutaneous adipose tissue (SAT) in all depots than those without clinical lipoatrophy and controls. HIV-infected men without clinical lipoatrophy also had less SAT than healthy controls. Less SAT was associated with use of specific antiretroviral drugs. In contrast, the amount of visceral adipose tissue (VAT) was independent of SAT and not associated with specific antiretroviral drugs.
Little is known about what happens to AT over the long-term In HIV-infected patients. Previous studies in uninfected subjects have found that younger and middle-aged adults gain 0.5–1.0 kg per year[4]. Total body fat is known to increase with age (until age 55 in men and age 65 in women)[5].
Many studies have assessed the effects of switching antiretroviral drug regimens on AT in HIV-infected participants, but most lasted one year or less and those studies varied in results[6, 7]. When participants were switched off protease inhibitors (PI), loss of AT often continued. When participants were switched off stavudine or thymidine analogs, increases in leg or limb AT were usually small. In the few studies lasting up to 96–144 weeks, where participants were switched off nucleoside reverse transcriptase inhibitors (NRTI), gain in fat was more consistently found, ranging from 10% to 42%[8–13]. However, none of these studies compared changes in fat to the changes found in healthy controls.
Furthermore, these studies mostly used dual-energy x-ray absorptiometry (DEXA) or CT scans, hence were limited in the regional depots studied. In the large observational studies that studied fat changes in HIV infection and included controls, measures were limited to the use of anthropometry[14, 15]. Thus, no large study has compared changes over several years in whole body regional AT depots including VAT in a nationally representative, multi-ethnic cohort of both HIV-infected participants and controls. A primary aim of the second FRAM study was to determine the changes in SAT and VAT using whole body MRI in both HIV-infected and control participants after five years of follow up[16]. We hypothesized that a well-treated cohort of HIV-infected participants in the HAART era would not resolve their HIV-associated lipoatrophy over five years. We also sought to investigate the associations of ARV use and discontinuation with changes in fat.
METHODS
The FRAM study was designed to evaluate the prevalence and correlates of changes in fat distribution, insulin resistance, and dyslipidemia in a representative sample of HIV-infected participants and controls in the United States. The methods of the FRAM study have been described in detail previously[16].
Study Population
HIV-infected participants were recruited from 16 HIV or infectious disease clinics or cohorts in 1999. Control participants were recruited from two centers from the Coronary Artery Risk Development in Young Adults (CARDIA) study[17]. A follow-up FRAM exam was conducted approximately five years later. The institutional review boards at all sites approved the protocols for both FRAM exams.
Retention outcomes for participants enrolled in the first exam have been reported[18]. The second exam included 581 HIV-infected and 241 controls recruited from those seen at the first exam. We report here on the subset of 477 HIV-infected participants and 214 controls that had measurements of AT depots at both FRAM exams. The time between the two AT measurements averaged 4.9+0.76(SD) years. Because a greater percentage of HIV-infected participants did not have measured MRI at both exams, we adjusted analyses as described below to address the concern of selection bias.
Magnetic Resonance Imaging
Whole body MRI was performed to quantify regional and total AT[19]. Body composition was measured with participants in the supine position, arms extended over head, and analyzed as described in detail elsewhere[2, 3, 16, 19]. In brief, using the inter-vertebral space between the fourth and fifth lumbar vertebrae as origin, transverse images (10 mm slice thickness) were obtained every 40 mm from hand to foot. MRI scans were segmented using image analysis software (Tomovision Inc., Montreal, Canada). A single image reading center (IRC) was used to read all scans; imaging techniques and anatomical sites (based on bone landmarks) were identical between HIV-infected and control participants using a standardized acquisition protocol, with IRC performing site visits to ensure protocol adherence. Scans were sent to the IRC at the Obesity Research Center, St. Luke’s Roosevelt Hospital, New York, NY, which calculated tissue areas (cm2) by summing specific tissue pixels, then multiplying by individual pixel surface area. Volume per slice (cm3) of each tissue was calculated by multiplying area by thickness. Volume of each tissue for the space between two consecutive slices was calculated via a mathematical algorithm[20]. Volumes were normalized by dividing by height2 with summaries back-transformed to 1.75 m of height. We did not adjust to body mass index (BMI), as BMI is influenced by the phenomenon being studied: quantity of fat. Anatomic sites considered in this analysis were: leg, lower trunk (abdomen and back), upper trunk (chest and back), arm, and VAT. The difference in results on repeated measurements in the IRC average 0.7% for skeletal muscle and 1.1% for adipose tissue[19]. We calculated geometric mean relative change as log (year 5 measure / baseline measure / years * 5), where years denotes time between baseline and year 5; results were back-transformed to produce percentage effects.
Other Measurements
Height and weight were measured by standardized protocols. Standardized questionnaires were used to determine demographic characteristics; medical history; risk factors for HIV; adequate food intake; physical activity; and use of alcohol, tobacco, and illicit drugs[21, 22]. Total physical activity score was calculated using the validated CARDIA activity questionnaire and was quartiled for analysis. Alcohol was categorized as drinks per week. Tobacco and illicit drug use were categorized as current, past, or never use. Research associates interviewed participants and reviewed medical charts regarding ARV medication use. A diagnosis of AIDS was made by history of opportunistic infection or CD4 count <200 cells/mm3.
HCV RNA testing was performed on frozen sera using the Bayer Versant 3.0 branched DNA (bDNA) assay (Leverkusen, Germany) in the entire cohort. CD4 lymphocyte count and percent, HIV RNA level in HIV-infected participants, and other blood specimens were analyzed in a single centralized laboratory (Covance, Indianapolis, IN).
Statistical Methods
For each adipose tissue depot, baseline and five year follow-up exam were compared using a paired t-test within HIV-infected and control groups separately. A two sample t-test was used for comparisons of changes from baseline to follow-up in HIV-infected versus control participants. Analyses that compared characteristics of HIV-infected participants with controls excluded HIV-infected individuals with recent opportunistic infection(OI), and were restricted to those between the ages of 33 and 45 at baseline (n=294), because the control population did not include participants outside this age range.
We analyzed changes in MRI-measured adipose tissue using multivariable linear regression with the five-year change in adipose tissue as the dependent variable. Interactions of HIV status with gender, ethnicity and age were assessed and included if they reached statistical significance.
Models were constructed for each outcome using HIV status, demographics (age, gender, and race), and lifestyle factors as predictor variables. Age, gender, race, and time between MRI exams were forced to be included in every model. The linearity assumption was tested for continuous measures by adding quadratic terms to the models and by examining generalized additive models[23]. Confidence intervals were determined using the bias-corrected accelerated bootstrap method[24], with p-values defined as one minus the highest confidence level that still excluded zero; this was necessary because the error residuals appeared to be non-Gaussian.
Candidate lifestyle factors included physical activity, smoking, alcohol use, adequate food intake, and illicit drug use. Candidate HIV-related factors (tested only for analyses that did not include controls) included AIDS diagnosis (by CD4 or OI), reported HIV duration, HIV RNA level (log10), current and nadir CD4 count (log2), hepatitis C infection (by virus detection), days since last OI, recent OI status (last 100 days), and HIV risk factors. In multivariable models controlling for the above factors, we evaluated ever use, duration on, and duration off each individual ARV drug and ARV class: NRTI, PI, non-nucleoside reverse transcriptase inhibitor (NNRTI), and highly active antiretroviral therapy (HAART) as previously defined[2].
Multiple imputation utilizing the MCMC method for arbitrary missing data was used to impute missing covariate values[25]. Because HIV-infected participants were missing MRI more often than controls, we adjusted estimates using an inverse probability weighting (IPW) approach[26] by modeling the participant’s probability of having non-missing AT using logistic regression analysis. The inverse of this probability was then used as a weight (applied to persons with known AT) in multivariable regression analyses.
We defined lipoatrophy as leg SAT below the 10th percentile of the control subjects at each exam, with men and women done separately, as in previous analyses[27, 28]. As an alternative, we defined lipoatrophy using the clinical definition, which is concordance of report of change in fat with abnormal looking fat on exam[2, 3]. The prevalence of lipoatrophy at baseline and follow-up was compared by using McNemar’s test.
All analyses were conducted using the SAS system, version 9.2 (SAS Institute, Inc., Cary, NC).
RESULTS
Participants
Body composition by MRI at baseline and follow-up were available on 691 participants whose characteristics at the follow up exam are presented in Table 1. HIV-infected and control participants were similar in age, height, and percentage of Caucasians and African Americans, but HIV-infected participants were more often male (68% vs. 53%) because of the design of the control study. Control participants weighed more and accordingly had higher BMI.
TABLE 1.
Baseline subject characteristics by HIV Status
| HIV+ (AR, OI) | Control | HIV+ (all) | |
|---|---|---|---|
| n | 294 | 214 | 477 |
| Baseline Age (y) | 41.0 (38.0–45.0) | 41.0 (37.0–43.0) | 43.0 (37.0–48.0) |
| Gender | |||
| Female | 93 (32%) | 100 (47%) | 139 (29%) |
| Male | 201 (68%) | 114 (53%) | 337 (71%) |
| Transgendered | 0 | 0 | 1 (1%) |
| Ethnicity | |||
| Caucasian | 142 (48%) | 123 (57%) | 232 (49%) |
| African-American | 127 (43%) | 91 (43%) | 204 (43%) |
| Hispanic | 21 (7%) | 0 | 32 (7%) |
| Other | 4 (1%) | 0 | 9 (2%) |
| Height (cm) | 172.5 (165.4–178.6) | 173.4 (165.4–179.0) | 172.4 (165.5–178.0) |
| Weight (kg) | 74.5 (64.5–82.8) | 81.5 (70.9–91.6) | 74.0 (64.4–82.4) |
| BMI (kg/m2) | 24.4 (22.0–27.4) | 26.8 (23.6–30.4) | 24.5 (22.1–27.2) |
| Current CD4 (cells/uL) | 384 (214–534) | 376 (218–554) | |
| HIV RNA (1000/mL) | 0.4 (0.4–6.4) | 0.4 (0.4–6.8) | |
| Detectable HIV RNA | 125 (43%) | 206 (44%) | |
| History of AIDS by OI/CD4 | 212 (73%) | 336 (71%) | |
| Hepatitis C infection | 70 (24%) | 106 (23%) | |
Data are presented as Median (IQR).
Abbreviations: IQR, interquartile range; AR, age-restricted; OI, OI-excluded; BMI, body mass index. Note: Only subjects with MRI measured at both FRAM1 and FRAM2 are included.
Comparison of Fat Gains in HIV-infected and Control Participants
Mean AT volumes at baseline and follow up exam for each regional depot in HIV-infected and control men, restricted to the age range of controls (age 33–45 at baseline), are shown in Figure 1A. Mean change in regional AT volumes between exams is shown in Figure 1B. Corresponding values for women are in Figure 2A and 2B. Statistically significant gains were seen in nearly every depot in both HIV-infected and control men and women (with most p<.0001, Figures 1B and 2B).
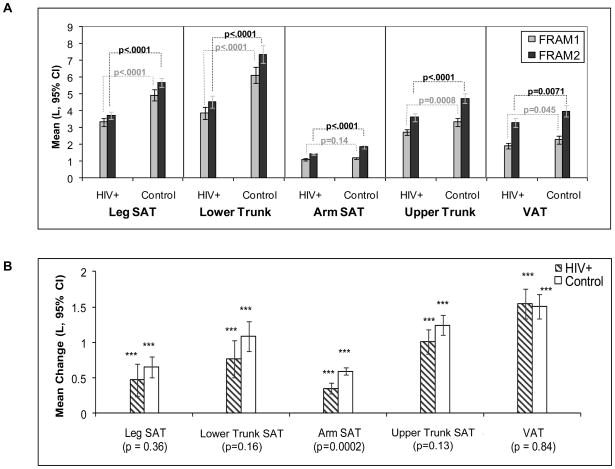
Figure 1.
Comparison of Subcutaneous Adipose Tissue (SAT) and Visceral Adipose Tissue (VAT) Depots at Baseline and Follow-up by HIV Status in Men. (A) Mean levels at FRAM1 and FRAM2 exams: Statistics are t-test of HIV-infected vs. control participants at baseline (FRAM1) and follow-up (FRAM2). (B) Mean changes from FRAM1 to FRAM 2 adjusted for time between exams: Asterisks represent paired t-test for within-group change from baseline; p-values below bars represent t-test of change in HIV vs. change in Control.
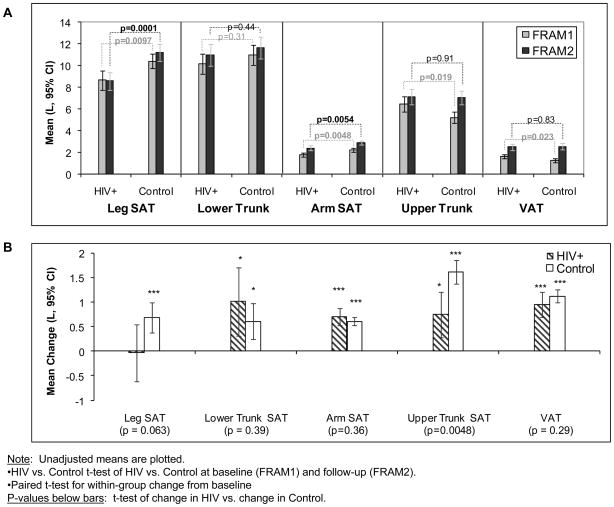
Figure 2.
Comparison of Subcutaneous Adipose Tissue (SAT) and Visceral Adipose Tissue (VAT) at Baseline and Follow-up by HIV Status in Women. (A) Mean levels at FRAM1 and FRAM2 exams: Statistics are t-test of HIV-infected vs. control participants at baseline (FRAM1) and follow-up (FRAM2). (B) Mean changes from FRAM1 to FRAM 2 adjusted for time between exams: Asterisks represent paired t-test for within-group change from baseline; p-values below bars represent t-test of change in HIV vs. change in Control.
HIV-infected men had lower mean AT volumes in all depots (SAT and VAT) at the first exam than control men (Figure 1A), although the difference in arm SAT did not reach statistical significance (p=0.14). Mean five-year gains were somewhat smaller in all SAT depots for HIV-infected men than control men (Figure 1B); the difference only reached statistical significance for arm SAT (p=0.0002). Hence at the FRAM 2 exam, AT volumes remained lower in HIV-infected men relative to controls in a pattern similar to the first exam. For example, mean baseline leg SAT in HIV-infected men was 3.3L and increased to 3.7L, while baseline leg SAT in control men was 4.9L and increased to 5.7L. Thus mean leg SAT in these HIV-infected men was 67% that of control men at the first FRAM exam and 65% of control men at the second exam (Figure 1A). Similar results were found for each of the other SAT depots. Average VAT gain was similar in HIV-infected and control men. As a consequence, mean VAT also remained lower in HIV-infected men at the FRAM 2 exam.
HIV-infected women also had lower leg and arm SAT than control women at the first exam (Figure 2A). Five year gains in leg SAT were somewhat smaller and in arm SAT were slightly larger in HIV-infected compared with control women, although the differences did not reach statistical significance (Figure 2B); HIV women continued to have lower leg and arm SAT at exam 2 than control women (Figure 2A). For example, mean baseline leg SAT was 8.6L at both exams in HIV-infected women, while leg SAT in control women was 10.4L at baseline exam and 11.2L at the second exam (Figure 2A). Thus mean leg SAT in HIV-infected women was 83% of controls at the first exam and decreased to 77% at the second exam.
At the baseline exam, upper trunk SAT and VAT were higher in HIV-infected women than in controls (mean upper trunk SAT = 6.5L vs. 5.2L, p=0.019; VAT = 1.6L vs. 1.3L, p = 0.023). However, the gains in upper trunk SAT and VAT were somewhat greater in control women than HIV-infected women with the differential gain in upper trunk SAT reaching statistical significance (Figure 2B). As a consequence, at the follow up exam (Figure 2A), HIV-infected women had similar mean upper trunk SAT and VAT to control women, (upper trunk SAT: HIV 7.1L, Control 7.0L, p=0.91; VAT: HIV 2.5L, control 2.5L; p=0.83).
We examined pooled models to determine whether the differences in five- year change in AT between HIV-infected and control participants remained after multivariable adjustment for demographic and lifestyle factors. After adjustment, HIV-infected participants showed smaller increases than controls in all regional SAT depots and VAT (Table 2), with differences reaching statistical significance for leg SAT (−0.81L, p=0.009), arm SAT (−0.33L, p=0.0002), and upper trunk SAT (−0.85L, p=0.0005). We observed statistically significant HIV by gender interactions for lower trunk (p=0.028) and arm SAT (p=0.014). In fully adjusted models, HIV infection in men was associated with smaller increases for lower trunk and arm SAT relative to controls (−0.98L, p=0.016; −0.45L, p<0.0001, respectively), while the changes in HIV-infected women relative to controls were small and did not reach statistical significance (0.16, p=0.77; −0.15, p=0.25).
TABLE 2.
Analysis of Change in Visceral Adipose Tissue (VAT) and Regional Subcutaneous Adipose Tissue (SAT) by HIV status (men and women pooled)
| 5 Year Change in: | HIV+ (n = 294) | Controls (n = 214) | P value |
|---|---|---|---|
|
Leg Subcutaneous Adipose Tissue (L)
| |||
| Mean ± SD | 0.31 (3.4) | 0.65 (1.7) | |
| Unadjusted Mean Difference (95% CI) † | −0.34 (−0.68, −0.009) | 0.044 | |
| Adjusted Mean Difference (95% CI) †† | −0.81 (−1.4, −0.20) | 0.009 | |
|
| |||
|
Lower Trunk Subcutaneous Adipose Tissue (L)
| |||
| Mean ± SD | 0.79 (4.3) | 0.84 (2.1) | |
| Unadjusted Mean Difference (95% CI) † | −0.048 (−0.46, 0.37) | 0.82 | |
| Adjusted Mean Difference (95% CI) †† | −0.54 (−1.3, 0.22)* | 0.16 | |
|
| |||
|
Arm Subcutaneous Adipose Tissue (L)
| |||
| Mean ± SD | 0.48 (1.04) | 0.60 (0.47) | |
| Unadjusted Mean Difference (95% CI) † | −0.12 (−0.22, −0.024) | 0.014 | |
| Adjusted Mean Difference (95% CI) †† | −0.33 (−0.50, −0.15)* | 0.0002 | |
|
| |||
|
Upper Trunk Subcutaneous Adipose Tissue (L)
| |||
| Mean ± SD | 0.93 (2.8) | 1.41 (1.4) | |
| Unadjusted Mean Difference (95% CI) † | −0.47 (−0.74, −0.20) | 0.0006 | |
| Adjusted Mean Difference (95% CI) †† | −0.85 (−1.3, −0.37) | 0.0005 | |
|
| |||
|
Visceral Adipose Tissue (L)
| |||
| Mean ± SD | 1.33 (2.5) | 1.30 (1.2) | |
| Unadjusted Mean Difference (95% CI) † | 0.028 (−0.22, 0.28) | 0.82 | |
| Adjusted Mean Difference (95% CI) †† | −0.30 (−0.72, 0.12) | 0.17 | |
Note: Results above are bootstrapped; m=5 imputations for missing data; all estimates are IPW-adjusted.
Unadjusted analyses control only for elapsed time.
Adjusted analyses control for elapsed time, demographics, and lifestyle factors.
Statistically significant HIV by gender interaction for Lower Trunk (p = 0.028) and Arm (p=0.014). HIV by gender interactions p-values for other depots were: Leg (p=0.46), Upper Trunk (p=0.36), VAT (p=0.86).
While HIV-infected and control participants gained AT on average, we found that the prevalence of any leg SAT loss was higher in HIV than controls (35% vs. 27%, p=0.0013). Additionally, we found that HIV-infected participants were more likely than controls to lose VAT (17% vs. 5.1%, p<.0001).
Change in AT Relative to Baseline AT
In these participants, 48% of the HIV-infected had lipoatrophy at the baseline exam, defined as having leg SAT below the cutoff of the lowest 10% of controls. The HIV-infected participants in that category gained an average of 0.96L between exams while the respective controls gained 1.23L (p=0.16). At the second exam, 53% of HIV-infected had leg SAT below the 10% cutoff of second exam control values. Among those HIV-infected who had lipoatrophy by this cutoff at the baseline exam, 82% had lipoatrophy at follow-up. When we instead defined lipoatrophy using the clinical definition[2, 3], 33% of the HIV-infected had lipoatrophy at the baseline exam, defined as concordance between participant report of fat loss and examination finding of less fat than a normal healthy person. The HIV-infected participants in that category gained even less, an average of 0.86L between exams.
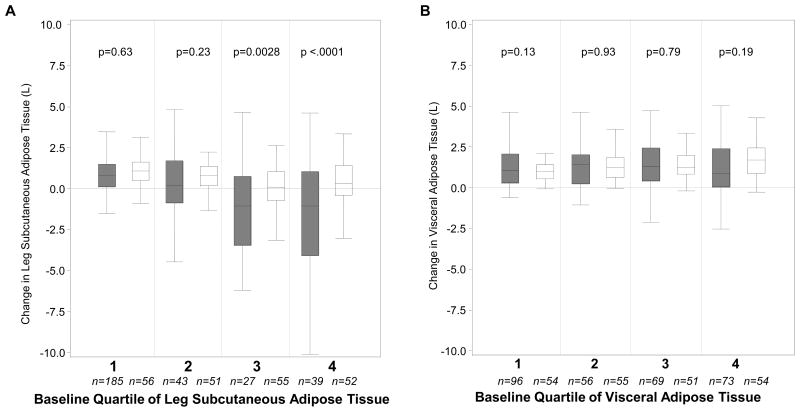
For leg SAT and VAT, we examined the relationship of quartiled baseline AT with change in AT, using quartile cut points based on control participants, to allow direct comparison of HIV-infected and control participants at similar baseline AT. For leg SAT (Figure 3A), in HIV-infected participants, the median change in those with the least baseline leg SAT was similar to that of controls in the same quartile and was significantly less than controls in the third and fourth baseline quartiles. By contrast, for VAT (Figure 3B), there was little difference between HIV-infected and control participants in change in VAT across baseline VAT quartiles.
Figure 3.
Five Year Change in Leg subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT) by HIV status (men and women pooled).
A: Leg SAT; B: VAT
Quartiles are those of controls (with men and women quartiled separately) to allow direct comparison of HIV-infected and control participants. Filled = HIV+; Open = control. Median is indicated by black center line, and the IQR (first and third quartiles) are the edges of the box. Whiskers denote Q1 – 1. 5x IQR and Q3 + 1.5 x IQR.
Discontinuation of Antiretroviral Drugs and Gain of Leg SAT in HIV-infected Participants
We also evaluated repeated measures models of amount of leg SAT at the two exams to determine whether any of the gains in leg SAT could be attributed to discontinuation of stavudine, the key antiretroviral drug responsible for leg SAT loss. 74% of the HIV-infected participants had a history of stavudine use, but only 9.4% were still taking stavudine at the time of the second exam (see Supplemental Table). Average duration of exposure to stavudine was 3.6+2.7(SD) years. Among those who had discontinued stavudine (65%), duration off stavudine was 4.3+2.6 years. Among the 9.4% of patients who were still on d4T at year 5, average gain in leg SAT was 0.53 ± 3.0L. Somewhat smaller gains were seen in past d4T users (0.34 ± 3.1L) and never d4T users (0.39 ± 3.3L), although the differences did not reach statistical significance.
In multivariable models, duration of stavudine use was strongly associated with smaller absolute amounts of leg SAT (−5.6% per year of exposure to d4T, p<.0001). However, time off stavudine was associated with an estimated increase of only 1.1% per year in leg SAT, which did not reach statistical significance (95%CI −0.65, 2.9; p = 0.22). Likewise, little gain was associated with discontinuation of zidovudine or other antiretroviral drugs (data not shown).
Discussion
In this five year follow-up study of regional adipose tissue volumes, we found that HIV-infected participants in the FRAM study gained less or similar AT on the average than control participants. Many HIV-infected participants started at lower levels due to HIV-associated lipoatrophy, and the results indicate that those with lipoatrophy had little recovery regardless of whether they discontinued the implicated antiretroviral drugs.
The diminished recovery was most obvious in leg SAT, the depot most affected by HIV-associated lipoatrophy. Over five years, control men and women gained on average 0.8L of SAT in the leg, whereas HIV-infected men gained only 0.4 L and HIV-infected women showed no average gain. Thus HIV-infected participants had even less leg SAT on average relative to controls at the year-five exam. In the Multicenter AIDS Cohort Study, thigh circumference increased more over four years in control than HIV-infected men[15]. In the Women’s Interagency HIV Study, over four years thigh circumference increased in control women and decreased in HIV-infected women[14].
Some studies of the change in AT after switching antiretroviral therapy report mean percent change, with longer-term switch studies finding 10–42% increases after switching off NRTI[8–13]. However, the absolute gains were typically small in these switch studies. In the study with the largest percent gain (42%), mean leg fat by DEXA increased from 1.6 to 2.2 kg[11]. Indeed, it may be that past papers reporting percentage increases led readers to believe there was more significant recovery. In our study, the geometric mean relative change in leg SAT was 28% in HIV-infected men compared to 3.9% in controls, which might suggest that substantial recovery was made in HIV-infected men. The percent changes should be contrasted with the baseline and absolute changes. HIV infected men started with a baseline average leg SAT of 3.3 L, while control men started with a baseline of 4.9 L. Despite starting lower, in HIV-infected men the actual mean gain in leg SAT was 0.47L vs. 0.65L in controls, which clearly conveys that HIV-infected men were not catching up. HIV-infected women started with less leg SAT than control women (8.7L vs. 10.4L). On average HIV-infected women did not gain leg SAT (−.03L), whereas control women did gain (0.69L). Furthermore, in the two AT depots that were higher in HIV-infected women than controls at baseline (upper trunk SAT and VAT), controls gained more AT; therefore, at five year follow-up these depots were virtually the same in HIV-infected and control women.
Many of the longer-term switch studies did not require the presence of lipoatrophy for entry[11–13]. When we examined HIV-infected subjects who had lipoatrophy defined as having leg SAT below the cutoff of the lowest control decile, the HIV-infected subjects had a 40% gain in leg SAT. However, the HIV-infected gained 0.96L, while the comparative controls gained 1.23L; the net result is that there is no relative recovery from lipoatrophy when HIV-infected participants are compared to controls.
In further support of this concept, multivariable modeling found that little gain could be attributed to years off stavudine or other antiretroviral drugs including zidovudine. Yet in the HIV-infected cohort, duration of exposure to stavudine was associated with lower leg SAT at both FRAM exams. In the WIHS study, women who discontinued stavudine had smaller annual decreases in thigh circumference than those who continued stavudine[14].
The results with leg SAT should be contrasted to those with VAT, where HIV-infected and control men saw similar gains, while HIV-infected women no longer had greater VAT than control women. These data, along with our previous studies of SAT and VAT in HIV-infection[2, 3], add additional support for the concept that these depots are independent and are not affected by the same factors.
There are several limitations to our study. We did not require lipoatrophy as an entry criterion, because our study was originally designed to be representative of the spectrum of HIV infection. However, we found little recovery in those HIV-infected who started with the lowest baseline SAT relative to controls. Likewise, several of the longer term switch studies did not require lipoatrophy as an entry criterion[11–13]. While our HIV-infected participants span a wide age range (19–76 years at baseline), comparisons with controls were restricted to a narrower age range (baseline 33–45 years), which limits our ability to generalize these results to older populations. Our study is observational. While a randomized study of discontinuation vs. continuation of relevant NRTI would be optimal, a five-year randomized study is neither feasible nor ethical. The longest NRTI switch study was 144 weeks. Finally, we cannot rule out accelerated gain of SAT after an even greater time off the drugs associated with lipoatrophy. However, in multivariable analyses the average 3.6 years of exposure to stavudine was associated with dramatically lower amounts of SAT, while in those who had been off of stavudine for an average of 4.3 years, little of the AT gain could be attributed to the time off drug.
A major strength of our study is the comparison of change in AT in HIV-infected persons over five years to that of controls in order to account for the normal changes in aging. Our controls come from the VIM substudy[29] of the CARDIA cohort, where the average BMI is similar to that of the nationally representative sample of NHANES.
In conclusion, five years after the first exam in the FRAM study, gain in AT was similar in HIV-infected and control participants. HIV-infected participants had significant subcutaneous lipoatrophy at the baseline exam and, five years later, relative lipoatrophy persisted compared to controls, even in those who discontinued antiretroviral drugs associated with lipoatrophy, such as stavudine, during this period. These data must be considered when studying the potential mechanisms underlying HIV-associated lipoatrophy. It remains to be determined whether there was destruction of adipose cells and precursors as proposed by some[30], or whether other factors continue to contribute to the persistence of lipoatrophy in HIV infection. Finally, as the presence of lipoatrophy has been associated with adverse metabolic effects[31, 32] and with depression[33], the long-term consequences and treatment of persistent lipoatrophy need additional study.
Supplementary Material
Acknowledgments
Supported by NIH grants RO1- DK57508, HL74814, and HL 53359, K23 AI66943, & UL1 RR024131; NIH GCRC grants M01- RR00036, RR00051, RR00052, RR00054, RR00083, RR0636, & RR00865; the Albert L. and Janet A. Schultz Supporting Foundation; and with resources and the use of facilities of the Veterans Affairs Medical Centers of, Atlanta, District of Columbia, New York and San Francisco. The funding agency had no role in the collection or analysis of the data.
Role of the Funder:
The funder played no role in the conduct of the study, collection of the data, management of the study, analysis of data, interpretation of the data or preparation of the manuscript. A representative of the funding agent participated in planning the protocol. As part of the standard operating procedures of CARDIA, the manuscript was reviewed at the NHLBI, but no revisions were requested.
Appendix
Sites and Investigators
University Hospitals of Cleveland (Barbara Gripshover, MD); Tufts University (Abby Shevitz, MD (deceased) and Christine Wanke, MD); Stanford University (Andrew Zolopa, MD); University of Alabama at Birmingham (Michael Saag, MD); John Hopkins University (Joseph Cofrancesco, MD and Adrian Dobs, MD); University of Colorado Heath Sciences Center (Lisa Kosmiski, MD and Constance Benson, MD); University of North Carolina at Chapel Hill (David Wohl, MD and Charles van der Horst, MD*); University of California at San Diego (Daniel Lee, MD and W. Christopher Mathews, MD*); Washington University (E. Turner Overton, MD and William Powderly, MD); VA Medical Center, Atlanta (David Rimland, MD); University of California at Los Angeles (Judith Currier, MD); VA Medical Center, New York (Michael Simberkoff, MD); VA Medical Center, Washington DC (Cynthia Gibert, MD); St Luke’s-Roosevelt Hospital Center (Donald Kotler, MD and Ellen Engelson, PhD); Kaiser Permanente, Oakland (Stephen Sidney, MD); University of Alabama at Birmingham (Cora E. Lewis, MD).
FRAM 2 Data Coordinating Center
University of Washington, Seattle (Richard A. Kronmal, PhD, Mary Louise Biggs, PhD, J. A. Christopher Delaney, Ph.D., and John Pearce).
Image Reading Centers
St Luke’s-Roosevelt Hospital Center: (Steven Heymsfield, MD, Jack Wang, MS and Mark Punyanitya). Tufts New England Medical Center, Boston: (Daniel H. O’Leary, MD, Joseph Polak, MD, Anita P. Harrington).
Office of the Principal Investigator
University of California, San Francisco, Veterans Affairs Medical Center and the Northern California Institute for Research and Development: (Carl Grunfeld, MD, PhD, Phyllis Tien, MD, Peter Bacchetti, PhD, Michael Shlipak, MD, Rebecca Scherzer, PhD, Mae Pang, RN, MSN, Heather Southwell, MS, RD)
Footnotes
Clinicaltrials.gov ID: NCT0033144
Conflicts of Interest:
All authors received funding from some of the supporting grants.
References
- 1.Grunfeld C, Kotler DP, Arnett DK, Falutz JM, Haffner SM, Hruz P, et al. Contribution of metabolic and anthropometric abnormalities to cardiovascular disease risk factors. Circulation. 2008;118:e20–28. doi: 10.1161/CIRCULATIONAHA.107.189623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Study of Fat Redistribution and Metabolic Change in HIV Infection (FRAM). Fat distribution in men with HIV infection. J Acquir Immune Defic Syndr. 2005;40:121–131. doi: 10.1097/01.qai.0000182230.47819.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The Study of Fat Redistribution and Metabolic Change in HIV Infection (FRAM). Fat distribution in women with HIV infection. J Acquir Immune Defic Syndr. 2006;42:562–571. doi: 10.1097/01.qai.0000229996.75116.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewis CE, Jacobs DR, Jr, McCreath H, Kiefe CI, Schreiner PJ, Smith DE, Williams OD. Weight gain continues in the 1990s: 10-year trends in weight and overweight from the CARDIA study. Coronary Artery Risk Development in Young Adults. Am J Epidemiol. 2000;151:1172–1181. doi: 10.1093/oxfordjournals.aje.a010167. [DOI] [PubMed] [Google Scholar]
- 5.Li C, Ford ES, Zhao G, Balluz LS, Giles WH. Estimates of body composition with dual-energy X-ray absorptiometry in adults. Am J Clin Nutr. 2009;90:1457–1465. doi: 10.3945/ajcn.2009.28141. [DOI] [PubMed] [Google Scholar]
- 6.Hansen BR, Haugaard SB, Iversen J, Nielsen JO, Andersen O. Impact of switching antiretroviral therapy on lipodystrophy and other metabolic complications: a review. Scand J Infect Dis. 2004;36:244–253. doi: 10.1080/00365540410019381. [DOI] [PubMed] [Google Scholar]
- 7.Barragan P, Fisac C, Podzamczer D. Switching strategies to improve lipid profile and morphologic changes. AIDS Rev. 2006;8:191–203. [PubMed] [Google Scholar]
- 8.Martin A, Smith DE, Carr A, Ringland C, Amin J, Emery S, et al. Reversibility of lipoatrophy in HIV-infected patients 2 years after switching from a thymidine analogue to abacavir: the MITOX Extension Study. Aids. 2004;18:1029–1036. doi: 10.1097/00002030-200404300-00011. [DOI] [PubMed] [Google Scholar]
- 9.Ribera E, Paradineiro JC, Curran A, Sauleda S, Garcia-Arumi E, Castella E, et al. Improvements in subcutaneous fat, lipid profile, and parameters of mitochondrial toxicity in patients with peripheral lipoatrophy when stavudine is switched to tenofovir (LIPOTEST study) HIV Clin Trials. 2008;9:407–417. doi: 10.1310/hct0906-407. [DOI] [PubMed] [Google Scholar]
- 10.Valantin MA, Lanoy E, Bentata M, Kalmykova O, Boutekadjirt A, Allavena C, et al. Recovery of fat following a switch to nucleoside reverse transcriptase inhibitor-sparing therapy in patients with lipoatrophy: results from the 96-week randomized ANRS 108 NoNuke Trial. HIV Med. 2008;9:625–635. doi: 10.1111/j.1468-1293.2008.00606.x. [DOI] [PubMed] [Google Scholar]
- 11.Tavassoli N, Bagheri H, Sommet A, Delpierre C, Marion-Latard F, Massip P, et al. Effects of discontinuing stavudine or protease inhibitor therapy on human immunodeficiency virus-related fat redistribution evaluated by dual-energy x-ray absorptiometry. Pharmacotherapy. 2006;26:154–161. doi: 10.1592/phco.26.2.154. [DOI] [PubMed] [Google Scholar]
- 12.Tebas P, Zhang J, Yarasheski K, Evans S, Fischl MA, Shevitz A, et al. Switching to a protease inhibitor-containing, nucleoside-sparing regimen (lopinavir/ritonavir plus efavirenz) increases limb fat but raises serum lipid levels: results of a prospective randomized trial (AIDS clinical trial group 5125s) J Acquir Immune Defic Syndr. 2007;45:193–200. doi: 10.1097/QAI.0b013e318042e204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madruga JR, Cassetti I, Suleiman JM, Etzel A, Zhong L, Holmes CB, et al. The safety and efficacy of switching stavudine to tenofovir df in combination with lamivudine and efavirenz in hiv-1-infected patients: three-year follow-up after switching therapy. HIV Clin Trials. 2007;8:381–390. doi: 10.1310/hct0806-381. [DOI] [PubMed] [Google Scholar]
- 14.Tien PC, Schneider MF, Cole SR, Justman JE, French AL, Young M, et al. Relation of stavudine discontinuation to anthropometric changes among HIV-infected women. J Acquir Immune Defic Syndr. 2007;44:43–48. doi: 10.1097/01.qai.0000248353.56125.43. [DOI] [PubMed] [Google Scholar]
- 15.Brown T, Wang Z, Chu H, Palella FJ, Kingsley L, Witt MD, Dobs AS. Longitudinal anthropometric changes in HIV-infected and HIV-uninfected men. J Acquir Immune Defic Syndr. 2006;43:356–362. doi: 10.1097/01.qai.0000243052.73321.8e. [DOI] [PubMed] [Google Scholar]
- 16.Tien PC, Benson C, Zolopa AR, Sidney S, Osmond D, Grunfeld C. The Study of Fat Redistribution and Metabolic Change in HIV Infection (FRAM): Methods, Design, and Sample Characteristics. Am J Epidemiol. 2006 doi: 10.1093/aje/kwj111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR, Jr, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 18.Cockerham L, Scherzer R, Zolopa A, Rimland D, Lewis CE, Bacchetti P, et al. Association of HIV Infection, Demographic and Cardiovascular Risk Factors With All-Cause Mortality in the Recent HAART Era. J Acquir Immune Defic Syndr. 2009 doi: 10.1097/QAI.0b013e3181b79d22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gallagher D, Belmonte D, Deurenberg P, Wang Z, Krasnow N, Pi-Sunyer FX, Heymsfield SB. Organ-tissue mass measurement allows modeling of REE and metabolically active tissue mass. Am J Physiol. 1998;275:E249–258. doi: 10.1152/ajpendo.1998.275.2.E249. [DOI] [PubMed] [Google Scholar]
- 20.Shen W, Wang Z, Tang H, Heshka S, Punyanitya M, Zhu S, et al. Volume estimates by imaging methods: model comparisons with visible woman as the reference. Obes Res. 2003;11:217–225. doi: 10.1038/oby.2003.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sidney S, Jacobs DR, Jr, Haskell WL, Armstrong MA, Dimicco A, Oberman A, et al. Comparison of two methods of assessing physical activity in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Epidemiol. 1991;133:1231–1245. doi: 10.1093/oxfordjournals.aje.a115835. [DOI] [PubMed] [Google Scholar]
- 22.Hoegerman GS, Lewis CE, Flack J, Raczynski JM, Caveny J, Gardin JM. Lack of association of recreational cocaine and alcohol use with left ventricular mass in young adults. The Coronary Artery Risk Development in Young Adults (CARDIA) study. J Am Coll Cardiol. 1995;25:895–900. doi: 10.1016/0735-1097(94)00469-7. [DOI] [PubMed] [Google Scholar]
- 23.Hastie T, Tibshirani R, editors. Generalized Additive Models. Chapman and Hall; 1990. [DOI] [PubMed] [Google Scholar]
- 24.Efron B, Tibshirani R. An Introduction to the Bootstrap. London: Chapman and Hall; 1993. pp. 178–188.pp. 214–178.pp. 398–403. [Google Scholar]
- 25.Schafer JL. Multiple imputation: a primer. Stat Methods Med Res. 1999;8:3–15. doi: 10.1177/096228029900800102. [DOI] [PubMed] [Google Scholar]
- 26.Robins JM, Finkelstein DM. Correcting for noncompliance and dependent censoring in an AIDS Clinical Trial with inverse probability of censoring weighted (IPCW) log-rank tests. Biometrics. 2000;56:779–788. doi: 10.1111/j.0006-341x.2000.00779.x. [DOI] [PubMed] [Google Scholar]
- 27.Kosmiski LA, Bacchetti P, Kotler DP, Heymsfield SB, Lewis CE, Shlipak MG, et al. Relationship of fat distribution with adipokines in human immunodeficiency virus infection. J Clin Endocrinol Metab. 2008;93:216–224. doi: 10.1210/jc.2007-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scherzer R, Shen W, Bacchetti P, Kotler D, Lewis CE, Shlipak MG, et al. Comparison of dual-energy X-ray absorptiometry and magnetic resonance imaging-measured adipose tissue depots in HIV-infected and control subjects. Am J Clin Nutr. 2008;88:1088–1096. doi: 10.1093/ajcn/88.4.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hill JO, Sidney S, Lewis CE, Tolan K, Scherzinger AL, Stamm ER. Racial differences in amounts of visceral adipose tissue in young adults: the CARDIA (Coronary Artery Risk Development in Young Adults) study. Am J Clin Nutr. 1999;69:381–387. doi: 10.1093/ajcn/69.3.381. [DOI] [PubMed] [Google Scholar]
- 30.Hooker DJ, Cherry CL. Apoptosis: a clinically useful measure of antiretroviral drug toxicity? Expert Opin Drug Metab Toxicol. 2009;5:1543–1553. doi: 10.1517/17425250903282781. [DOI] [PubMed] [Google Scholar]
- 31.Currier J, Scherzer R, Bacchetti P, Heymsfield S, Lee D, Sidney S, Tien PC. Regional Adipose Tissue and Lipid and Lipoprotein Levels in HIV-Infected Women. J Acquir Immune Defic Syndr. 2008 doi: 10.1097/QAI.0b013e318164227f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wohl D, Scherzer R, Heymsfield S, Simberkoff M, Sidney S, Bacchetti P, Grunfeld C. The Associations of Regional Adipose Tissue With Lipid and Lipoprotein Levels in HIV-Infected Men. J Acquir Immune Defic Syndr. 2008 doi: 10.1097/QAI.0b013e31816d9ba1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crane HM, Grunfeld C, Harrington RD, Uldall KK, Ciechanowski PS, Kitahata MM. Lipoatrophy among HIV-infected patients is associated with higher levels of depression than lipohypertrophy. HIV Med. 2008;9:780–786. doi: 10.1111/j.1468-1293.2008.00631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.