Abstract
Although ovarian cancer is the most lethal gynecologic malignancy in women, little is known about how the cancer initiates and metastasizes. In the last decade, new evidence has challenged the dogma that the ovary is the main source of this cancer. The fallopian tube has been proposed instead as the primary origin of high-grade serous ovarian cancer, the subtype causing 70% of ovarian cancer deaths. By conditionally deleting Dicer, an essential gene for microRNA synthesis, and Pten, a key negative regulator of the PI3K pathway, we show that high-grade serous carcinomas arise from the fallopian tube in mice. In these Dicer-Pten double-knockout mice, primary fallopian tube tumors spread to engulf the ovary and then aggressively metastasize throughout the abdominal cavity, causing ascites and killing 100% of the mice by 13 mo. Besides the clinical resemblance to human serous cancers, these fallopian tube cancers highly express genes that are known to be up-regulated in human serous ovarian cancers, also demonstrating molecular similarities. Although ovariectomized mice continue to develop high-grade serous cancers, removal of the fallopian tube at an early age prevents cancer formation—confirming the fallopian tube origin of the cancer. Intriguingly, the primary carcinomas are first observed in the stroma of the fallopian tube, suggesting that these epithelial cancers have a mesenchymal origin. Thus, this mouse model demonstrates a paradigm for the origin and initiation of high-grade serous ovarian carcinomas, the most common and deadliest ovarian cancer.
Keywords: epithelial ovarian cancer, oviduct, mesenchymal-to-epithelial transition, carcinoma initiation
Epithelial ovarian cancer, accounting for 90% of all ovarian tumors, is grouped into four major histologic types: serous (70%), endometrioid (10–15%), clear-cell (10%), and mucinous (3%) carcinomas (1). The serous-type cancers are also overwhelmingly high-grade (90%)—the culprit of 70% of ovarian-cancer deaths and a key contributor to an overall ovarian cancer 5-yr survival rate of 31% (2–4). Most cases of high-grade serous ovarian cancers are diagnosed at advanced stages, when the tumors have already metastasized. Despite the steady improvement of surgery and chemotherapy, >90% of women with advanced ovarian cancers die after the cancer relapses (5). Early detection of these high-grade serous carcinomas is thus key to reducing ovarian cancer deaths (6). However, the origin and molecular pathogenesis of these high-grade serous ovarian cancers are largely unknown (1, 6).
Despite widespread peritoneal metastasis commonly seen in ovarian cancer at diagnosis, the ovary has long been considered the primary origin of this cancer—hence the name ovarian cancer. However, precursor lesions have not been identified in the ovary (1, 7). Over the past decade, new evidence has emerged to propose a different source of ovarian cancer: the fallopian tube (7, 8). After women with hereditary breast and ovarian cancer-susceptibility gene (BRCA1, BRCA2) mutations have their ovaries and fallopian tubes prophylactically removed to prevent ovarian cancer, early serous carcinomas have been found in the fallopian tube—not in the ovary (8). Further studies demonstrated early serous lesions of fallopian tube origin in 64–71% of nonhereditary high-grade ovarian serous carcinomas (9, 10). These studies have spawned a notion that the fallopian tube is a potential primary site of origin of high-grade serous carcinomas (7, 8). Intriguing as this theory is, the direct evidence is still lacking that the fallopian tube not only can initiate but, beyond that, can also advance de novo to the full-spectrum metastatic malignancy of high-grade serous carcinomas.
In the present study, we provide direct evidence to this “fallopian tube hypothesis.” When Dicer and Pten are conditionally disabled with Amhr2-Cre in mice, high-grade serous carcinomas arise from the fallopian tube. These primary fallopian tube cancers subsequently spread to the ovary and then aggressively metastasize throughout the abdominal cavity, leading to ascites and 100% lethality. In addition to these clinical similarities to the human cancer, gene expression analyses also affirm that these fallopian tube tumors resemble human serous ovarian cancer at the molecular level. Moreover, in these Dicer-Pten double-knockout (DKO) mice, the primary epithelial cancers originate in the stroma of the fallopian tube, suggesting that the cancers arise from cells of a mesenchymal lineage (i.e., cell of a nonepithelial lineage). Our study thus presents a paradigm for the origin and initiation of deadly high-grade serous ovarian cancer.
Results and Discussion
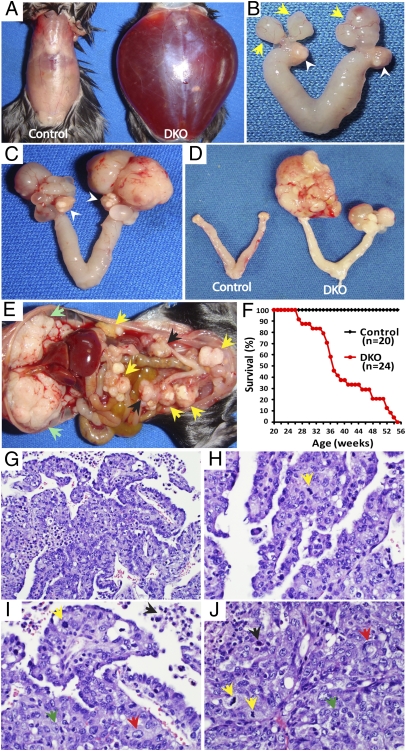
Using a mouse model, we show herein clear evidence that the fallopian tube is the origin of high-grade serous carcinoma. When Dicer, the RNase III essential for the conversion of premiRNAs to mature miRNAs, and Pten, a tumor suppressor inhibiting the PI3K pathway, were disabled in the female reproductive tract by using anti-Müllerian hormone receptor type 2-directed Cre (Amhr2-Cre), these Dicer-Pten DKO (Dicerflox/flox Ptenflox/flox Amhr2cre/+) mice universally develop early serous carcinomas in the fallopian tube (Fig. 1B). In contrast, the DKO ovaries are grossly distinguishable from these fallopian tube serous carcinomas and show no gross and histologic evidence of tumor (Fig. 1 B and C and Fig. S1). The fallopian tube tumors are unique to the DKO mice because Amhr2-Cre deletion of Dicer alone leads to diverticuli in the fallopian tube and no tumors (11), and disabling only Pten fails to cause a tumor phenotype in the ovary or fallopian tube (12). In DKO mice, these fallopian tube cancers subsequently spread to envelop the ovaries, and then aggressively metastasize throughout the abdominal cavity, including the mesentery and pancreas, with prominent cancer lesions on the diaphragm and peritoneal membrane (Fig. 1E), a membranous site analogous to the omentum—a common implantation environment for metastases in women with ovarian cancer. After developing ascites (Fig. 1A), 100% of the DKO females die from the metastatic cancers between 6.5 and 13 mo (Fig. 1F). These DKO mice thus present with similar cancer manifestations and progression as women with high-grade serous ovarian cancers.
Fig. 1.
Dicer-Pten DKO mice develop high-grade metastatic serous carcinoma. (A) Severe ascites in an 8.4-mo-old DKO mouse. (B) Early tumors form in the fallopian tube (yellow arrows) of a 5-mo-old DKO mouse with normal ovaries (white arrowheads). (C) Progression of the fallopian tube tumors in a DKO mouse at 8 mo. Ovaries are still intact (white arrowheads). (D) Bilateral fallopian tube/ovarian tumors are observed in a DKO mouse at 6 mo. (E) The DKO mouse described in A showing extensive peritoneal metastasis with clusters of tumor nodules (yellow arrows) and a massive accumulation on the diaphragm (green arrows), besides fallopian tube/ovarian tumors (black arrows). (F) Survival curve of DKO and control mice. (G–I) DKO fallopian tube/ovarian tumors showing papillary structure and irregular glands with slit-like spaces, characteristic of high-grade serous carcinoma (H&E) and representative of fallopian tube/ovarian tumors from 16 DKO mice. (H–J) The nuclear features of high-grade serous carcinomas including nuclear pleomorphism (red arrow), prominent nucleoli with irregular chromatin patterns (green arrow), apoptosis (black arrow), and brisk mitotic activity (yellow arrow) (H&E). (J) Fallopian tube/ovarian tumor with a solid growth pattern (H&E). Magnifications: G, 20×; H–J, 40×.)
Histologically, these primary and metastatic cancers in the DKO mice are confirmed as high-grade serous carcinomas—or as undifferentiated carcinomas in some cases. The tumors are characterized by complex papillae and glands forming slit-like spaces and solid sheets of tumor cells (Fig. 1 G–J) with pleomorphic nuclei, prominent nucleoli, and high mitotic activity—the cardinal features of high-grade serous ovarian cancer in women (Fig. 1 H–J and Fig. S2A). These high-grade serous carcinomas are reproducible in vivo. When cells isolated from primary tumors, ascites, or metastatic tumors were injected intraperitoneally into immunocompromised (NOD SCID) or immunocompetent mice, the injected mice (11 of 11 mice for NOD SCID; 9 of 13 for immunocompetent mice) developed histologically identical high-grade serous carcinomas (Fig. S2B). Thus, our Dicer-Pten DKO model develops high-grade metastatic serous carcinomas from the fallopian tube that phenotypically and histologically mirror high-grade serous ovarian cancer in women.
Moreover, these mouse serous carcinomas resemble human serous carcinomas at the molecular level. Many genes known to be up-regulated in human serous carcinomas are also highly expressed in the DKO mouse serous carcinomas. Analyzing microarray gene expression profiles between the mouse fallopian tube carcinomas and human ovarian serous cancers yields a list of known, up-regulated genes shared by these mouse and human cancers. Some of these up-regulated genes are secreted or transmembrane proteins (Table 1). The list shows several known important genes in serous ovarian cancer: secreted phosphoprotein 1 (Spp1), CA125 (Muc16), folate receptor 1 (Folr1), and chemokines such as Cxcl9, Cxcl10, and Ccl8. To further confirm molecular similarities between the mouse and human cancers, we performed gene set enrichment analysis (GSEA)—a robust analysis comparing independent gene-expression datasets (13)—using the genes up-regulated or down-regulated more than twofold from the mouse DKO serous carcinoma dataset and the human TCGA serous ovarian cancer dataset 1. In this global analysis, the gene expression profiles of mouse fallopian tube cancers shared widespread similarities to those of human serous ovarian cancers (GSEA; P < 0.001, Fig. S3). Thus, this molecular similarity of mouse serous cancers to the human cancer strengthens our conclusion that the fallopian tube is a site for the initiation and development of high-grade serous carcinomas.
Table 1.
Genes encoding secreted and/or transmembrane proteins up-regulated in mouse fallopian tube carcinomas and human serous carcinomas versus respective fallopian tubes
| Expression level |
Fold change |
||||
| Symbol | Gene name | Mouse FT | Mouse FT cancer | Mouse cancer: FT | Human cancer: FT |
| Spp1 | Secreted phosphoprotein 1 | 169 | 15,370 | 102.7 | 39.5 |
| Cxcl9 | Chemokine (C-X-C motif) ligand 9 | 56 | 2,268 | 33.8 | 4.1 |
| Cxcl10 | Chemokine (C-X-C motif) ligand 10 | 56 | 1,951 | 25.8 | 1.9 |
| Cd72 | CD72 antigen | 216 | 2,955 | 13.7 | 1.9 |
| Slc15a3 | Solute carrier family 15, member 3 | 84 | 1,168 | 13.0 | 4.6 |
| Cd84 | CD84 antigen | 101 | 1,275 | 12.5 | 1.7 |
| C1qb | Complement component 1qB | 1,368 | 13,752 | 10.0 | 6.5 |
| Plau | Plasminogen activator, urokinase | 75 | 747 | 9.8 | 4.8 |
| Ly86 | Lymphocyte antigen 86 | 566 | 4,479 | 7.8 | 3.1 |
| Muc16 | Mucin 16 (CA125) | 435 | 3,508 | 7.6 | 26.1 |
| Folr1 | Folate receptor 1 | 204 | 1,590 | 7.3 | 77.6 |
| Slc11a1 | Solute carrier family 11, member 1 | 154 | 1,122 | 7.2 | 2.1 |
| Slc12a8 | Solute carrier family 12, member 8 | 71 | 531 | 7.2 | 4.8 |
| Cd40 | CD40 antigen | 100 | 684 | 6.8 | 2.5 |
| Igsf9 | Ig superfamily, member 9 | 602 | 3311 | 5.4 | 9.1 |
| Il10ra | Interleukin 10 receptor, alpha | 85 | 450 | 5.3 | 2.0 |
| Tnfrsf12a | Tumor necrosis factor receptor, member 12a | 265 | 1351 | 5.3 | 2.7 |
| Apoe | Apolipoprotein E | 2,573 | 13,702 | 5.1 | 1.7 |
| Tlr7 | Toll-like receptor 7 | 81 | 429 | 5.1 | 2.2 |
| Tmem48 | Transmembrane protein 48 | 152 | 705 | 4.7 | 5.2 |
| Il1r2 | Interleukin 1 receptor, type II | 44 | 195 | 4.4 | 1.8 |
| Lair1 | Leukocyte-associated Ig-like receptor 1 | 76 | 319 | 4.1 | 20.1 |
| Ly6e | Lymphocyte antigen 6 complex, locus E | 1,580 | 6,370 | 4.1 | 33.3 |
| Adam17 | A disintegrin and metallopeptidase domain 17 | 488 | 1,975 | 4.0 | 2.0 |
| Ptn | Pleiotrophin | 1,202 | 4,648 | 3.8 | 1.9 |
| Cd83 | CD83 antigen | 173 | 603 | 3.6 | 5.0 |
| Ccl8 | Chemokine (C-C motif) ligand 8 | 1,106 | 3,961 | 3.6 | 12.0 |
| Tmc6 | Transmembrane channel-like gene family 6 | 231 | 730 | 3.2 | 5.2 |
| Tmem49 | Transmembrane protein 49 | 1,169 | 3,369 | 2.9 | 8.3 |
| Esm1 | Endothelial cell-specific molecule 1 | 106 | 288 | 2.7 | 4.6 |
| Amhr2 | Anti-Mullerian hormone type 2 receptor | 184 | 475 | 2.6 | 5.4 |
| Mdk | Midkine | 1,551 | 4,092 | 2.6 | 1.8 |
| Tmem173 | Transmembrane protein 173 | 89 | 233 | 2.6 | 3.7 |
| Tnfrsf21 | Tumor necrosis factor receptor, member 21 | 260 | 642 | 2.6 | 14.6 |
| Cfb | Complement factor B | 184 | 439 | 2.3 | 9.1 |
| Scamp5 | Secretory carrier membrane protein 5 | 523 | 1,114 | 2.1 | 2.6 |
Mean expression levels of independent samples of mouse fallopian tubes (n = 3) and fallopian tube serous cancers (n = 3) are shown. Fold changes in gene expression are compared between mouse fallopian tube cancer and human serous ovarian cancer with their respective fallopian tubes as controls. FT, fallopian tube.
As expected in these Pten-Dicer DKO mice, Pten absence also disrupts the tight regulatory loop comprising PTEN (phosphatase) and PI3K (kinase). The resulting activation of the PI3K pathway, the signaling pathway known to be altered in 45% of high-grade ovarian carcinomas (14), leads to aberrantly activated AKT and increased phosphorylation of AKT downstream proteins known to be highly expressed in ovarian cancer including STMN1 (stathmin) and BIRC5 (survivin) (Fig. 2A).
Fig. 2.
Molecular alterations in Dicer-Pten DKO mice. (A) Western blot analysis of DKO fallopian tube tumors showing activation of PI3K signaling compared with control fallopian tubes as indicated by the enhanced expression of phosphorylated (P)-AKT, P-PRAS40 (AKT1S1), P-4EBP1, survivin, and stathmin. On the right side, mRNA enrichment (fold change) in the mouse fallopian tube cancers versus control fallopian tubes is presented. (B) DNA copy number changes in the PTEN and DICER1 alleles in 481 high-grade serous ovarian tumors from The Cancer Genome Atlas (TCGA): yellow, gain; blue, loss. Values are from Affymetrix SNP 1M array dataset.
To further understand the relevance of PTEN and DICER in human high-grade serous ovarian cancers, we analyzed the copy number changes of PTEN and DICER in the 481 cancers in the TCGA database (14). Both PTEN and DICER demonstrate frequent allele loss in the human cancers (Fig. 2B)—results that are consistent with frequent mutations in the PI3K pathway in high-grade serous ovarian cancer (14) and the association of low DICER levels with advanced ovarian cancer and poor patient survival (15). Fittingly, as shown in our mouse model, a combined deletion of Dicer and Pten produces highly aggressive metastatic serous carcinomas that closely resemble the human serous cancers.
To confirm the fallopian tube origin of these serous carcinomas, we unilaterally or bilaterally removed either ovary or fallopian tube from DKO mice (Table 2). When ovaries are removed unilaterally from DKO mice at postnatal weeks 6–11, tumors continue to form in the fallopian tube in 10 of 11 mice with the same metastatic potential as tumors from DKO mice with intact ovaries (Fig. 3A and Table 2). In contrast, upon unilateral removal of the fallopian tube, cancers fail to form—despite the presence of the ovary, whereas the other side with both ovary and fallopian tube intact still develops cancers (9 of 10 mice) (Fig. 3B and Table 2). Even when both ovaries are removed from DKO mice, metastatic serous carcinomas initiate and develop in the fallopian tubes in the absence of ovarian steroids, similar to the postmenopausal development of most serous cancers in women (Fig. 3C and Table 2; 8 of 11 mice). When both fallopian tubes are removed with both ovaries left intact, however, none of the DKO mice develop cancer (Fig. 3D and Table 2; 17 mice; 14–16 mo to date). Together, these results further confirm that high-grade serous carcinomas in DKO mice arise from the fallopian tube.
Table 2.
Fallopian tube origin of Dicer-Pten DKO serous carcinomas
| Ovary removal |
Fallopian tube removal |
|||
| Unilateral | Bilateral | Unilateral | Bilateral | |
| Total no. of DKO mice | 11 | 11 | 10 | 17 |
| No. of DKO mice with tumor | 10* | 8† | 9‡ | 0 |
| % of mice with tumor | 90.9a | 72.7a | 90a | 0b |
| Age at death, mo | 6–13 | 7–13 | 6–13 | — |
| Current age of live mice, mo | — | 15 | 16 | 14–16 |
Ovaries or fallopian tubes were surgically removed unilaterally or bilaterally from Dicer-Pten DKO mice at 6–11 wk of age and examined for tumor development. Fisher's exact test was used to analyze the statistical significance in different tumor occurrences between groups. Different letters indicate statistically significant difference between groups (P < 0.0001); same letters no significant difference.
*One other mouse also died after developing tumor, but no tumor was found from the side where the ovary was removed; thus, it was not counted as tumor development.
†Three live mice were sacrificed at 15 mo. All three mice had fallopian tube tumors; one of these mice also had peritoneal metastases with accumulating ascites.
‡Despite tumor development from the side with intact ovary and fallopian tube, no tumor was observed on the other side after fallopian tube removal—with ovary alone.
Fig. 3.
Fallopian tube is the origin of high-grade serous carcinomas in Dicer-Pten DKO mice. (A) Tumor (black arrowhead) in an 8-mo-old DKO mouse after unilateral removal of the ovary. (B) No tumors in the ovary (white arrowhead) in an 8-mo-old DKO mouse with the fallopian tube removed unilaterally. (C) After removal of both ovaries, massive tumors still form from the fallopian tubes in a 10.5-mo-old DKO mouse. (D) No tumors with both fallopian tubes removed in an 11.5-mo DKO mouse. (E) Histology of an early fallopian tube lesion displaying high-grade serous carcinomas (representative of early tumors from seven DKO mice), in which tumor cells extensively infiltrate and expand the stroma of the fallopian tube (H&E). (F and G) Proliferating tumor cells show strong and abundant immunohistochemical staining of cytokeratin 14 (KRT14) and cytokeratin 8 (KRT8). (H) Tumor cells are primarily located within the stroma with preservation (arrow) and focal attenuation (arrowhead) of overlying benign-looking tubal epithelium (H&E, 20× magnification of the dotted region from E). (I) KRT14-positive tumor cells focally invade and erode the fallopian tube epithelium (20× magnification of the dotted region from F). (J) Proliferating tumor cells show abundant Ki67 expression with no significant expression in fallopian tube epithelium (arrowheads). (K) Abundant CDH1 expression in tumor cells of fallopian tube stroma (arrow) and in the epithelial layer (arrowheads). (L and M) An early fallopian tube lesion. A small nest (long arrow) and a few single tumor cells (short arrow) show strong KRT14 (L) and KRT17 (M) expression, compared with fallopian tube epithelium (arrowhead) and uninvolved stroma that are KRT14-negative (L). (N) Specific CA125 staining in these early tumor clusters (long arrow) in the fallopian tube stroma. (O and P) Histology of deeper sections obtained from the early tubal lesion corresponding to L–N. High-grade carcinoma cells form nests and ill-defined glands (dotted circles), which are primarily expanding the stroma. There is focal involvement of the serosal surface (O, arrow) with essentially uninvolved tubal epithelium in early lesions (P, arrowhead). (Scale bars: 0.5 cm.) (Magnification: E–G, 4×; H and I, 20×; J–L, 10×; M–P, 20×.)
Our model also suggests a unique mechanism of cancer initiation. To define the cellular origin of these serous carcinomas, we analyzed Dicer-Pten DKO mice at earlier time points before development of ascites and metastasis. Although the serous tumors in DKO mice are clearly epithelial cancers, histological analysis of the fallopian tubes from the DKO mice at earlier ages shows that the abnormal proliferation begins in the stromal compartment—not in the epithelial layer—of the fallopian tube (Fig. 3 E–P). During early-tumor formation (Fig. 1B), the mitotically active cancer cells gradually fill the stromal compartment of the fallopian tube and compress the lumen (Fig. 3 E and J). The appearance of these cancer cells in the stromal compartment is consistent with the Cre activity in the mesenchymal-derived stroma of the fallopian tube and uterus where Amhr2 is expressed (16). Besides being highly proliferative (Fig. 3J), these tumor cells in the stroma abundantly express several epithelial markers: cytokeratin 14 (KRT14; 198.3-fold increased), cytokeratin 17 (KRT17; 14.3-fold increased), and cytokeratin 8 (KRT8; 1.5-fold increased) that are up-regulated in the microarrays of fallopian tube serous carcinomas from DKO mice (Fig. 3 F, G, I, L, and M). The tumors also express E-cadherin (CDH1), another epithelial marker (Fig. 3K). In the stromal regions of the fallopian tube that lacked grossly obvious cancer (Fig. 3 O and P), clusters of histologically confirmed high-grade cancer cells express KRT14 and KRT17 (Fig. 3 L and M). These early tumor cells show high expression of CA125 (MUC16) (Fig. 3N)—a biomarker known to be elevated in the serum of >80% of patients with ovarian cancer (6). Collectively, these results suggest an interesting mechanism of tumor initiation in which stromal cells in the fallopian tube undergo a transition to an epithelial cell type during serous carcinoma formation.
During the menstrual cycle in women, a large part of the endometrium is shed, and the remaining endometrium then undergoes extensive regeneration. Mesenchymal stem cells found in the stroma and epithelial progenitor cells are thought to drive this dynamic regenerative capacity of the endometrium (17, 18). Unlike the epithelial progenitor cells that appear to be locked into an epithelial cell fate, mesenchymal stem cells can give rise to diverse cell types such as smooth muscle cells, adipocytes, chondrocytes, and osteoblasts (18). In the uterus, mesenchymal stem cells in the stroma appear to differentiate into epithelial cells during endometrial regeneration (19). Like in the uterus, the Müllerian duct epithelium and mesenchyme likely give rise to the fallopian tube epithelium and stroma, respectively (16). It is thus plausible that the fallopian tube stroma has mesenchymal stem cells that can differentiate into epithelial cells. However, determining the precise cell of origin of the DKO tumors—whether they arise from a mesenchymal-to-epithelial transition or from mesenchymal stem cell differentiation—will require detailed lineage-tracing experiments.
In addition to defining the tumor origin, this Dicer-Pten DKO mouse model will help to understand the early progression and metastasis of high-grade serous carcinomas. Although mouse models for serous ovarian carcinomas have been reported before (20–22), the detailed molecular map underlying this deadly cancer has yet to emerge (7). Our DKO model shows a clear progression of the cancers from the fallopian tube stroma to the ovary and eventual metastasis to peritoneal tissues. This mouse model therefore offers a rare and invaluable opportunity to uncover the molecular mechanisms of this most common and lethal ovarian cancer. A glimpse of this potential is presented in the list of the genes that we identified from the early tumors of DKO mice (Table 1)—prospective biomarkers for early detection and screening of serous carcinoma. Beyond these early markers, understanding the molecular mechanisms underlying the tumor progression and metastasis will also allow us to discover novel drug targets and pathways for more targeted and effective treatment of advanced ovarian cancers.
There are some unresolved issues in the DKO model in relation to human serous ovarian cancers. Unlike DKO mouse fallopian tube tumors, which arise from cells in the stroma with seeming epithelial characteristics, early carcinoma lesions in humans appear to initiate in the epithelial layer of the fallopian tube (7, 8). This understanding of the cell origin in the human serous cancer is, however, based primarily on the observation of early carcinomas in the fallopian tubes from women positive for hereditary BRCA1/2 mutations, a genetic alteration found in 10% of ovarian cancer (23) and studies of early tubal serous carcinomas from a limited number of nonhereditary ovarian cancers (9, 10). As mentioned earlier, it has yet to be established that these early fallopian tube carcinomas found in humans could progress into full-blown metastatic serous carcinomas. In light of our finding that cells in the fallopian tube stroma could initiate high-grade serous carcinomas, it would be worth looking into the possibility that nonepithelial cells in the fallopian tube could be a source for initiating serous tumors in nonhereditary human ovarian cancers.
Despite their functional similarity, the human and mouse fallopian tubes respond differently to hormonal changes during the respective menstrual cycle and estrous cycle. The human fallopian tube shows proliferative activity in the epithelium—likely induced by estrogen—during the menstrual cycle (24). In rhesus monkeys, estrogen stimulates the proliferation of the fallopian tube epithelium (25). These data may explain why a high number of ovulations correlate with an elevated risk of ovarian cancer in women (26). The mouse fallopian tube, however, does not proliferate during gonadotropin-induced ovulation (27), which may account for the relative lack of sporadic epithelial “ovarian” cancer in nonprimate mammals (28). However, as shown in our study, genetically deleting Dicer and Pten in the fallopian tube causes mice to develop high-grade serous cancer that arises from the fallopian tube. Because cancer is a genetic disorder driven by mutations, this Dicer-Pten DKO mouse model will be useful to understand the detailed molecular mechanisms underlying the origin and progression of human high-grade serous ovarian cancer.
If the fallopian tube is confirmed as the primary source of high-grade human serous carcinoma, as shown in our mouse model, this finding will certainly benefit clinical practice. In the United States, 55% (300,000) of all women undergoing a hysterectomy annually for benign uterine disease also choose to have their ovaries and fallopian tubes removed to lower the risk of ovarian cancer. However, hysterectomy with bilateral salpingo-oophorectomy elevates overall mortality, likely owing to loss of ovarian function (29). Thus, removing only the fallopian tubes while preserving the ovaries is likely to benefit both premenopausal and menopausal women (30).
Another puzzling aspect of our Dicer-Pten DKO mouse model is the role of the p53 tumor suppressor. In human high-grade serous ovarian cancer, the p53 tumor suppressor gene (TP53) is frequently mutated (14), leading to accumulation of the mutant p53 protein (31). In our model, however, the expression of p53 (Trp53) is low in the mouse serous carcinomas. Because p53 was reported to act upstream of DICER in mediating miRNA function (32), it is possible that Dicer deletion in our DKO model may substitute for loss of p53 function.
However, although p53 mutations are believed to play a critical role in ovarian cancer (14), there is little direct evidence that p53 mutations drive tumor initiation in ovarian cancer. Human genetics studies show that there is no increased incidence of ovarian cancers in women with germ-line p53 mutations, which lead to Li-Fraumeni Syndrome, a hereditary condition characterized by a wide spectrum of tumors (33). In a study of 501 individuals from 28 families prone to Li-Fraumeni Syndrome, ovarian carcinoma was found in only 1 case of the 148 tumors (0.7%) (34). Similarly, another extensive study of 185 people carrying germ-line p53 mutations identified ovarian tumors in a mere 8 cases of 738 tumors (1.1%) (35). Likewise, in mouse models, serous ovarian cancers have not been reported in mice harboring p53 mutations that model Li-Fraumeni syndrome or mice bearing p53-null mutations despite a wide range of tumors reported in both of these models (36, 37). Moreover, an ovarian cancer mouse model—in which both Trp53 and Rb genes were conditionally deleted only in the ovarian surface epithelium—was initially reported to have developed serous adenocarcinomas (38). However, an independent study failed to reproduce this finding; instead, deletion of Trp53 and Rb produced ovarian leiomyosarcomas (39). Another mouse study conditionally deleting Trp53 and Brca1 also resulted in leiomyosarcomas or sarcomas of the ovary (40). Thus, contrary to the common notion of p53 importance in ovarian cancer, it still remains to be clarified whether p53 mutations are drivers for serous cancers.
In conclusion, our study opens another chapter to better understand the molecular origins and progression of deadly high-grade serous ovarian carcinomas. We have genetically uncovered an in vivo progression of high-grade serous epithelial cancer, which begins from lesions in the fallopian tube and then spreads to the ovaries, ultimately leading to widespread peritoneal metastases and ending in death. Besides identifying the fallopian tube as the origin of this cancer, our study also suggests a rather intriguing possibility of serous carcinoma initiation—that epithelial cancers derive from cells in the stroma. Furthermore, because high-grade serous cancers are typically diagnosed at advanced stages with resulting high mortality, our mouse model will help identify biomarkers for early detection and screening and also discover new, effective drug targets in treating advanced high-grade serous cancers. Our model will thus be important for translational inroads, fundamentally changing the way we screen, diagnose, and treat the most common and deadliest form of “ovarian” cancer.
Materials and Methods
Dicer-Pten DKO mice were generated by breeding of three genotypes: Dicerflox/flox, Ptenflox/flox, and Amhr2cre/+. Tumor-forming ability of the high-grade serous cancers in Dicer-Pten DKO was tested in severe combined immunodeficiency (NOD SCID) or immunocompetent (C57BL/129Sv) mice. The high-grade mouse serous carcinomas were histologically examined and confirmed by H&E staining of formalin-fixed paraffin sections. These tumor sections were further analyzed by immunohistochemistry in which critical protein markers of high-grade serous carcinomas were localized in the early tumor lesions by using specific antibodies. Microarray analysis was used to identify significant early gene-expression changes in the DKO fallopian tube tumors (41). GSEA was executed by using public software from the Broad Institute (http://www.broad.mit.edu/gsea/) (13). Western blot analyses of whole-cell extracts of these tumors were performed to confirm the overactivation of the PI3K signaling. Experimental details are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Richard Behringer for his comments on the manuscript and generous gift of the Amhr2-Cre transgenic mice; Dr. Jeffrey Rosen for sharing his key insights on this work; Dr. Robert Bast, Jr. for the CA125 antibody; Lang Ma and Felicia Cao for technical assistance; and Drs. Matthew Anderson, Preethi Gunaratne, and Thuy Phung for helpful suggestions and support of this research. This work was supported by the National Cancer Institute, the Ovarian Cancer Research Fund, and the Baylor College of Medicine Partnership. J.K. is supported by National Institute of Health Ruth L. Kirschstein National Research Service Award 1F32 CA159523.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 3608.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1117135109/-/DCSupplemental.
References
- 1.Cho KR, Shih IeM. Ovarian cancer. Annu Rev Pathol. 2009;4:287–313. doi: 10.1146/annurev.pathol.4.110807.092246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koonings PP, Campbell K, Mishell DR, Jr, Grimes DA. Relative frequency of primary ovarian neoplasms: A 10-year review. Obstet Gynecol. 1989;74:921–926. [PubMed] [Google Scholar]
- 3.Seidman JD, et al. The histologic type and stage distribution of ovarian carcinomas of surface epithelial origin. Int J Gynecol Pathol. 2004;23:41–44. doi: 10.1097/01.pgp.0000101080.35393.16. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 5.Bukowski RM, Ozols RF, Markman M. The management of recurrent ovarian cancer. Semin Oncol. 2007;34(Suppl 2):S1–S15. doi: 10.1053/j.seminoncol.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 6.Bast RC, Jr, Hennessy B, Mills GB. The biology of ovarian cancer: New opportunities for translation. Nat Rev Cancer. 2009;9:415–428. doi: 10.1038/nrc2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurman RJ, Shih IeM. The origin and pathogenesis of epithelial ovarian cancer: A proposed unifying theory. Am J Surg Pathol. 2010;34:433–443. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crum CP, et al. The distal fallopian tube: A new model for pelvic serous carcinogenesis. Curr Opin Obstet Gynecol. 2007;19:3–9. doi: 10.1097/GCO.0b013e328011a21f. [DOI] [PubMed] [Google Scholar]
- 9.Kindelberger DW, et al. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: Evidence for a causal relationship. Am J Surg Pathol. 2007;31:161–169. doi: 10.1097/01.pas.0000213335.40358.47. [DOI] [PubMed] [Google Scholar]
- 10.Przybycin CG, Kurman RJ, Ronnett BM, Shih IeM, Vang R. Are all pelvic (nonuterine) serous carcinomas of tubal origin? Am J Surg Pathol. 2010;34:1407–1416. doi: 10.1097/PAS.0b013e3181ef7b16. [DOI] [PubMed] [Google Scholar]
- 11.Nagaraja AK, et al. Deletion of Dicer in somatic cells of the female reproductive tract causes sterility. Mol Endocrinol. 2008;22:2336–2352. doi: 10.1210/me.2008-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan HY, et al. Cell type-specific targeted mutations of Kras and Pten document proliferation arrest in granulosa cells versus oncogenic insult to ovarian surface epithelial cells. Cancer Res. 2009;69:6463–6472. doi: 10.1158/0008-5472.CAN-08-3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Subramanian A, et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cancer Genome Atlas Research Network Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merritt WM, et al. Dicer, Drosha, and outcomes in patients with ovarian cancer. N Engl J Med. 2008;359:2641–2650. doi: 10.1056/NEJMoa0803785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arango NA, et al. A mesenchymal perspective of Müllerian duct differentiation and regression in Amhr2-lacZ mice. Mol Reprod Dev. 2008;75:1154–1162. doi: 10.1002/mrd.20858. [DOI] [PubMed] [Google Scholar]
- 17.Dimitrov R, et al. Characterization of clonogenic stromal cells isolated from human endometrium. Reproduction. 2008;135:551–558. doi: 10.1530/REP-07-0428. [DOI] [PubMed] [Google Scholar]
- 18.Gargett CE, Schwab KE, Zillwood RM, Nguyen HP, Wu D. Isolation and culture of epithelial progenitors and mesenchymal stem cells from human endometrium. Biol Reprod. 2009;80:1136–1145. doi: 10.1095/biolreprod.108.075226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garry R, Hart R, Karthigasu KA, Burke C. Structural changes in endometrial basal glands during menstruation. BJOG. 2010;117:1175–1185. doi: 10.1111/j.1471-0528.2010.02630.x. [DOI] [PubMed] [Google Scholar]
- 20.Orsulic S, et al. Induction of ovarian cancer by defined multiple genetic changes in a mouse model system. Cancer Cell. 2002;1:53–62. doi: 10.1016/s1535-6108(01)00002-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Connolly DC, et al. Female mice chimeric for expression of the simian virus 40 TAg under control of the MISIIR promoter develop epithelial ovarian cancer. Cancer Res. 2003;63:1389–1397. [PubMed] [Google Scholar]
- 22.Mullany LK, et al. Molecular and functional characteristics of ovarian surface epithelial cells transformed by KrasG12D and loss of Pten in a mouse model in vivo. Oncogene. 2011;30:3522–3536. doi: 10.1038/onc.2011.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crum CP, et al. Lessons from BRCA: The tubal fimbria emerges as an origin for pelvic serous cancer. Clin Med Res. 2007;5:35–44. doi: 10.3121/cmr.2007.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donnez J, Casanas-Roux F, Caprasse J, Ferin J, Thomas K. Cyclic changes in ciliation, cell height, and mitotic activity in human tubal epithelium during reproductive life. Fertil Steril. 1985;43:554–559. doi: 10.1016/s0015-0282(16)48496-7. [DOI] [PubMed] [Google Scholar]
- 25.Slayden OD, Hirst JJ, Brenner RM. Estrogen action in the reproductive tract of rhesus monkeys during antiprogestin treatment. Endocrinology. 1993;132:1845–1856. doi: 10.1210/endo.132.4.8462480. [DOI] [PubMed] [Google Scholar]
- 26.Fleming JS, Beaugié CR, Haviv I, Chenevix-Trench G, Tan OL. Incessant ovulation, inflammation and epithelial ovarian carcinogenesis: Revisiting old hypotheses. Mol Cell Endocrinol. 2006;247:4–21. doi: 10.1016/j.mce.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 27.King SM, et al. The impact of ovulation on fallopian tube epithelial cells: Evaluating three hypotheses connecting ovulation and serous ovarian cancer. Endocr Relat Cancer. 2011;18:627–642. doi: 10.1530/ERC-11-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Land JA. Ovulation, ovulation induction and ovarian carcinoma. Baillieres Clin Obstet Gynaecol. 1993;7:455–472. doi: 10.1016/s0950-3552(05)80140-3. [DOI] [PubMed] [Google Scholar]
- 29.Parker WH, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the nurses’ health study. Obstet Gynecol. 2009;113:1027–1037. doi: 10.1097/AOG.0b013e3181a11c64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dietl J, Wischhusen J, Häusler SF. The post-reproductive Fallopian tube: Better removed? Hum Reprod. 2011;26:2918–2924. doi: 10.1093/humrep/der274. [DOI] [PubMed] [Google Scholar]
- 31.Zweemer RP, et al. Accumulation of p53 protein is frequent in ovarian cancers associated with BRCA1 and BRCA2 germline mutations. J Clin Pathol. 1999;52:372–375. doi: 10.1136/jcp.52.5.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuki HI, et al. Modulation of microRNA processing by p53. Nature. 2009;460:529–533. doi: 10.1038/nature08199. [DOI] [PubMed] [Google Scholar]
- 33.Varley JM. Germline TP53 mutations and Li-Fraumeni syndrome. Hum Mutat. 2003;21:313–320. doi: 10.1002/humu.10185. [DOI] [PubMed] [Google Scholar]
- 34.Birch JM, et al. Relative frequency and morphology of cancers in carriers of germline TP53 mutations. Oncogene. 2001;20:4621–4628. doi: 10.1038/sj.onc.1204621. [DOI] [PubMed] [Google Scholar]
- 35.Nichols KE, Malkin D, Garber JE, Fraumeni JF, Jr, Li FP. Germ-line p53 mutations predispose to a wide spectrum of early-onset cancers. Cancer Epidemiol Biomarkers Prev. 2001;10:83–87. [PubMed] [Google Scholar]
- 36.Olive KP, et al. Mutant p53 gain of function in two mouse models of Li-Fraumeni syndrome. Cell. 2004;119:847–860. doi: 10.1016/j.cell.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 37.Donehower LA. The p53-deficient mouse: A model for basic and applied cancer studies. Semin Cancer Biol. 1996;7:269–278. doi: 10.1006/scbi.1996.0035. [DOI] [PubMed] [Google Scholar]
- 38.Flesken-Nikitin A, Choi KC, Eng JP, Shmidt EN, Nikitin AY. Induction of carcinogenesis by concurrent inactivation of p53 and Rb1 in the mouse ovarian surface epithelium. Cancer Res. 2003;63:3459–3463. [PubMed] [Google Scholar]
- 39.Clark-Knowles KV, Senterman MK, Collins O, Vanderhyden BC. Conditional inactivation of Brca1, p53 and Rb in mouse ovaries results in the development of leiomyosarcomas. PLoS ONE. 2009;4:e8534. doi: 10.1371/journal.pone.0008534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quinn BA, et al. Induction of ovarian leiomyosarcomas in mice by conditional inactivation of Brca1 and p53. PLoS ONE. 2009;4:e8404. doi: 10.1371/journal.pone.0008404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci USA. 2003;100:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.