Abstract
The coxsackie and adenovirus receptor (CAR) plays key roles in epithelial barrier function at the tight junction, a localization guided in part by a tyrosine-based basolateral sorting signal, 318YNQV321. Sorting motifs of this type are known to route surface receptors into clathrin-mediated endocytosis through interaction with the medium subunit (μ2) of the clathrin adaptor AP-2, but how they guide new and recycling membrane proteins basolaterally is unknown. Here, we show that YNQV functions as a canonical YxxΦ motif, with both Y318 and V321 required for the correct basolateral localization and biosynthetic sorting of CAR, and for interaction with a highly conserved pocket in the medium subunits (μ1A and μ1B) of the clathrin adaptors AP-1A and AP-1B. Knock-down experiments demonstrate that AP-1A plays a role in the biosynthetic sorting of CAR, complementary to the role of AP-1B in basolateral recycling of this receptor. Our study illustrates how two clathrin adaptors direct basolateral trafficking of a plasma membrane protein through interaction with a canonical YxxΦ motif.
Keywords: trans-Golgi network, recycling endosomes, exocytosis, epithelial cells, protein sorting
The coxsackie and adenovirus receptor (CAR) forms a complex with junctional adhesion molecule L, which plays a key role in inflammatory response, immunity, and tissue homeostasis (1, 2). During embryo development, CAR is expressed predominantly in heart and brain, but after birth its expression is most abundant in epithelial cells and brain (3). Importantly, CAR is the primary receptor for group B coxsackievirus and most adenovirus serotypes, facilitating their entry into body epithelia and thus contributing in a major way to human diseases caused by these viruses (4). CAR is targeted to the basolateral plasma membrane (PM) by the signal 318YNQV321 (5), thus it belongs to a class of ∼12 basolateral PM proteins sorted by a YXXΦ motif (Table 1). We recently reported that the clathrin adaptor AP-1B is critically involved in the polarity and biology of CAR, as epithelia lacking AP-1B constitutively, such as retinal pigment epithelium, and Madin-Darby canine kidney (MDCK) cells knocked-down (KD) of μ1B (B-KD MDCK), express CAR at the apical surface, allowing efficient penetration of adenoviruses from the apical side (6). B-KD disrupts the postendocytic basolateral recycling of CAR, consistent with the localization of AP-1B at recycling endosomes (7, 8), eliciting transcytosis to the apical membrane, but does not impair the accurate biosynthetic delivery of CAR (6). Two important questions remain unanswered on the mechanisms regulating the basolateral localization of CAR: (i) how does AP-1B interact with CAR's basolateral signal, and (ii) what is the mechanism involved in its biosynthetic delivery to the PM?
Table 1.
Proteins with YxxΦ-based basolateral-sorting signals
| Basolateral sorting role of |
||||||
| Protein | YxxΦ | Y | Φ | Endocytic activity | Interacting adaptors | References |
| CAR | YNQV | Yes | Yes | ? | AP-1A AP-1B | 5, present work |
| Influenza HA-Y543 | YKSF | Yes | ? | Yes | ? | 17, 18 |
| VSV G protein | YTDI | Yes | ? | No | AP-1B AP-3 | 15, 40, 41 |
| p75 | YSSL | Yes | ? | Yes | ? | 19, 42 |
| LAP | YRHV | Yes | ? | Yes | AP-2 | 43 |
| TGN38 | YQRL | Yes | ? | Yes | AP-1A, AP-2, AP3 | 10, 44, 45 |
| AE1 | YVEL | Yes | Yes | No | ? | 46 |
| AGPR-H1 | YQDL | Yes | ? | Yes | ? | 47 |
| HIV gp41 | YSPL | Yes | ? | Yes | AP-1A, AP-3, AP-2 | 48, 49 |
| hPVRa | YSAV | Yes | ? | ? | AP-1B | 50 |
| LAMP-1 | YQTI | Yes | Yes | Yes | AP-1A, AP-2, AP-3 | 45, 51 |
Bold indicates residues presumed key for basolateral sorting function.
AP-1B belongs to a group of clathrin-associated sorting proteins (CLASPs) with characteristic ability to bind different types of sorting signals and to coordinate with clathrin the sorting along endocytic and exocytic routes of various classes of membrane proteins (9). The best-studied CLASP is the tetrameric adaptor protein complex AP-2 (α, β2, μ2, σ2), which facilitates endocytosis of a variety of surface receptors at the PM. Crystallography and yeast two-hybrid (Y2H) assays have shown that AP-2 interacts with YXXΦ motifs via a hydrophobic pocket in the C-terminal portion of the medium (μ2) subunit (10, 11). AP-1 (γ, β1, μ1, σ1), the second best-studied CLASP, exists as the epithelial-specific form AP-1B, a major regulator of basolateral trafficking in epithelial cells (12, 13), and the ubiquitous form AP-1A (9). Both adaptors are known to direct basolateral proteins with sorting signals based on YXXΦ motifs along exocytic routes; however, their interactions with these signals are far less well characterized, both functionally and structurally, than those of AP-2. AP-3 and AP-4 (AP-4 is not strictly a CLASP, as it does not bind clathrin) have been reported to sort or interact with basolateral proteins (14, 15), but the basolateral sorting roles of these adaptors are still unclear.
Tyrosine motifs were identified as basolateral sorting signals in epithelial cells years before their ability to interact with clathrin adaptors was first reported (16). Initial evidence for this role was provided by mutagenesis experiments that created a YXXΦ motif in the cytoplasmic tail of apical PM protein influenza hemagglutinin (17, 18) and p75 nerve growth-factor receptor (19), resulting in basolateral localization and increased endocytic rates in MDCK cells, and by mutagenesis of tyrosine residues in low density lipoprotein receptor, which resulted in loss of its normal basolateral localization (20). Subsequently, many other basolateral proteins have been shown to depend on tyrosine motifs for basolateral localization (16, 21). However, none of these studies has shown how a tyrosine motif contributes, through interaction with specific clathrin adaptors, to the sorting of a membrane protein along biosynthetic and recycling routes to the PM.
Here, we show that the basolateral signal of CAR behaves as a canonical YXXΦ motif, with Y and Φ residues being critical for basolateral sorting and interaction with clathrin adaptors AP-1A and AP-1B, and demonstrate that AP-1A sorts CAR in its biosynthetic pathway in cooperation with AP-1B. These experiments, in combination with our previous study demonstrating a role of AP-1B in postendocytic recycling of CAR (6), are unique in illustrating how interactions between YXXΦ motifs and clathrin adaptors direct a basolateral PM protein along different exocytic routes to the PM.
Results
Characterization of a YXXΦ Motif That Drives Basolateral Localization of CAR.
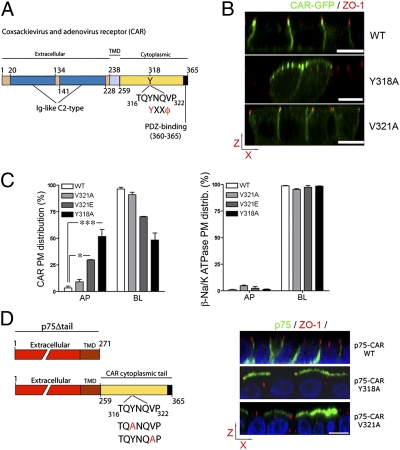
CAR is a 46-kDa type I transmembrane protein with an N-terminal signal peptide, an extracellular domain with two Ig-like C2 loops, and a cytoplasmic tail that exhibits its basolateral sorting signal (318YNQV321) and a PDZ-binding domain that binds zonula occludens-1 protein (ZO-1) (Fig. 1A) (5). Bergelson and colleagues have shown that ablation of the cytoplasmic tail and site-directed mutation of Y318 result in apical localization of CAR in polarized MDCK cells (5); however, their study did not explore the role of V321 in basolateral sorting and, consequently, did not demonstrate that CAR's basolateral signal is a canonical YXXΦ motif. A review of a dozen basolateral proteins that use YXXΦ motifs as basolateral sorting signals (Table 1) indicates that only two of these studies characterized the basolateral sorting role of the hydrophobic residue in the position Y+3, known to be very important for interaction of YXXΦ motifs with AP-2 and clathrin-mediated endocytosis. Furthermore, none of these studies fully characterized how the interaction of the basolateral sorting signal with specific clathrin adaptors mediates basolateral sorting.
Fig. 1.
Mutation in CAR's YXXΦ motif alters its basolateral distribution in polarized MDCK cells. (A) Schematic representation of CAR indicating the position and amino acid composition of its YXXΦ motif. (B) Confocal images of MDCK cell lines expressing CAR-GFP WT or V321A, or Y318A (green). (Scale bar, 10 μm.) (C) Domain-selective biotinylation showed more than 90% of WT and V321A CAR at the basolateral PM, in contrast with only 69.9 ± 0.4% and 48.35 ± 6.6% of V321E and Y318A, respectively. The basolateral polarity of Na/K ATPase was conserved in the different cell lines. Histogram bars represent mean ± SEM. *P < 0.05, ***P < 0.001. (D) The extracellular and transmembrane domains of p75 were cloned in frame with CAR's cytoplasmic tail, either WT or carrying the mutations Y318A or V321A. Surface immunofluorescence using a monoclonal antibody against the ectodomain of p75 (green) shows basolateral localization of p75-CAR and apical localization of p75-CARY318A and p75-CARV321A. Nuclei were stained with DAPI (blue) and tight junctions with ZO-1 (red). (Scale bar, 10 μm.) See Table S1 for statistical analysis of all comparisons.
To test the hypothesis that 318YNQV321 is a canonical YxxΦ motif, with both 318Y and V321 required for basolateral localization of CAR, we established MDCK cell lines expressing full-length human CAR, either WT, or containing the mutations Y318A or V321A, all tagged C-terminally with GFP and analyzed their PM distributions by confocal microscopy (Fig. 1B) and domain-selective biotinylation (Fig. 1C). We observed that whereas CAR WT was 96.5% basolateral, CAR-Y318A was drastically depolarized (48.35% basolateral); however, CAR-V321A remained largely basolateral (91% basolateral) (for statistical analysis, see Table S1). The lack of effect of V321A on CAR polarity might be a result of the fact that it is a conservative substitution of a valine for another small and moderately hydrophobic amino acid; indeed, the nonconservative substitution V321E caused a significant depolarization of full-length CAR (69.9% basolateral; P < 0.05) (Fig. 1C and Table S1). All cell lines used for these experiments showed a conserved basolateral distribution of Na/K ATPase (P > 0.05) (Fig. 1C; see statistics in Table S1), indicating that the introduction of WT or mutant forms of CAR did not cause a generalized loss of epithelial polarity.
To study whether the basolateral sorting signal of CAR is transplantable, we tested its ability to target basolaterally a chimera of CAR's cytoplasmic tail and the transmembrane and extracellular domains of the apical marker p75 neurotrophin receptor (p75) (Fig. 1D, Left). In the absence of cytoplasmic sequences, p75 is targeted to the apical PM by signals in the ectodomain (Fig. S1). We have previously used this approach to study the basolateral signals of neural cell adhesion molecule and monocarboxylate transporters (22, 23). Using surface immunofluorescence with a monoclonal antibody against the extracellular domain of p75 in polarized MDCK cells, we observed that p75-CAR WT was localized preferentially to the basolateral PM, whereas p75-CAR Y318A and p75-CAR V321A were localized preferentially to the apical PM (Fig. 1D, Right). These experiments conclusively establish that: (i) the cytoplasmic tail of CAR is sufficient to direct p75 to the basolateral membrane of MDCK cells, and (ii) 318YNQV321 is a canonical YXXΦ motif because Y318 and Φ321 are essential for its function as a basolateral signal and the Φ321 position has plasticity to accommodate different hydrophobic amino acids.
YNQV Drives the Biosynthetic Delivery of CAR.
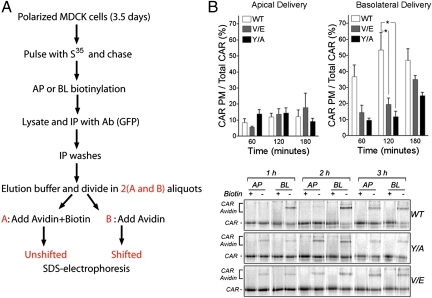
How exactly does YNQV contribute to CAR's basolateral localization? To answer this question, we investigated comparatively the biosynthetic delivery of WT and mutant CAR using a recently developed surface-biotinylation-avidin-shift (SBAS) assay that measures the surface-delivered fractions as a fraction of the total radioactively labeled pool of CAR (Materials and Methods) (24). We found that the delivery of both CAR-Y318A and CAR-V321E to the basolateral surface at 120 min was significantly reduced (Y318A = 13.71% and V321E = 17.45% compared with WT = 56.56%, P < 0.05) without a corresponding increase in apical delivery (Y318A = 14.39%, and V321E = 13.68% vs. WT = 13.34%) (Fig. 2; statistical analysis in Table S2). Thus, inactivation of the basolateral signal leads to intracellular retention of newly synthesized CAR. These experiments demonstrate that the basolateral signal of CAR acts as a canonical YXXΦ motif, critically dependent on both Y318 and V321, for the polarized biosynthetic delivery of CAR to the basolateral PM.
Fig. 2.
Polarized biosynthetic delivery of CAR is driven by its basolateral sorting signal YNQV. (A) Workflow for SBAS quantitative assay. This method allows measurement of the fraction of CAR-GFP delivered to apical or basolateral membranes (CAR avidin-shifted) as a fraction of the total protein synthesized (CAR avidin+biotin unshifted) radiolabeled with a pulse of S35cysteine/methionine. Amount delivered to each surface is calculated as: [intensity of the CAR-GFP band (75 kDa) in minus-Biotin lane] − [intensity of the CAR-GFP band (75 kDa) in plus-Biotin lanes]/(the intensity of the CAR-GFP band in minus-Biotin lane) × 100. This approach is superior to measuring the CAR-GFP displaced to MWs > 75 kDa by streptavidin because these complexes are more difficult to quantify. (B) Mutations V321E and Y318A in CAR's basolateral signal cause intracellular retention of the protein after 120 min relative to WT CAR (white bars; *P < 0.05). (Lower) One representative experiment. Bars represent the mean ± SEM (n ≥ 4). See Table S2 for statistical analysis.
YNQV Interacts with the Medium Subunits of AP-1A and AP-1B.
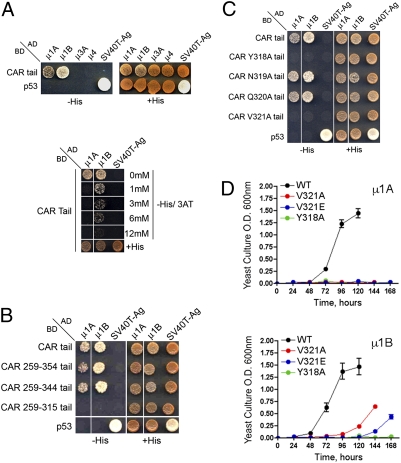
Our recent demonstration that clathrin is a key regulator of basolateral trafficking (25) suggests that a clathrin adaptor may be involved in biosynthetic delivery of CAR. To search for such an adaptor, we screened by Y2H analysis the interactions of CAR's cytoplasmic tail with the medium subunits of AP-1A, AP-1B, AP-3, and AP-4. These experiments demonstrated interactions of CAR's cytoplasmic tail with μ1A and μ1B, but not with μ3 and μ4 (Fig. 3A). Competition experiments with 3-Amino-1,2,4-triazole (3-AT), a competitive inhibitor of the HIS3 gene product, showed that the interaction with μ1B persisted in the presence of up to 6 mM 3-AT, whereas the interaction with μ1A was abolished by 1 mM 3-AT (Fig. 3A, Lower).
Fig. 3.
CAR interacts with μ1A and μ1B but not with μ3A or μ4 through Y318 and V321. (A) Y2H assays demonstrated direct interactions of CAR's cytoplasmic tail with μ1A and μ1B. Competition with different concentrations of 3-AT shows that the interaction with μ1B was stronger than that with μ1A. (B) Deletion analysis indicates that the interaction of CAR's cytoplasmic tail with μ1A and μ1B involves amino acids 315–344. (C) Y2H assays performed on plates showed that mutations Y318A and V321A but not N319A or Q320A abolish CAR's interaction with μ1A and μ1B. (D) Y2H assays in liquid culture revealed variable interaction strengths between CAR and μ1B depending on the specific V321 substitution. Positive controls of interactions included double transformations with p53 and SV40 T-antigen, and negative controls were obtained by cotransformation of p53 with activation domain constructs and of SV40 T-antigen with BD constructs.
Analysis of progressive deletions of CAR's C-terminal tail identified a segment between amino acids 315 and 344, containing 318YNQV321, as the region responsible for the interactions with μ1A and μ1B in Y2H plate assays (Fig. 3B). Alanine mutagenesis of Y318 and V321 but not of N319 and Q320, abolished these interactions (Fig. 3C), demonstrating that the interations involved CAR's basolateral signal. Further analyses of these interactions by Y2H liquid-culture assays, which allow a more precise quantification of their relative strength, demonstrated that alanine substitution of Y318 completely abrogates the interaction of YXXΦ motif with both μ1A or μ1B, even after 7 d of culture (Fig. 3D). In contrast, conservative and nonconservative substitutions of V321 (V321A and V321E, respectively) had stronger inhibitory effects on the interaction with μ1A (Y318A = V321E = V321A < WT) than μ1B (Y318A < V321E < V321A < WT) (Fig. 3D). These results demonstrate that CAR's basolateral signal 318YNQV321 behaves as a canonical YXXΦ motif not only in directing CAR's basolateral localization and polarized trafficking, but also in mediating interactions with μ1A and μ1B.
Hydrophobic Pockets in μ1A and μ1B Interact with YNQV.
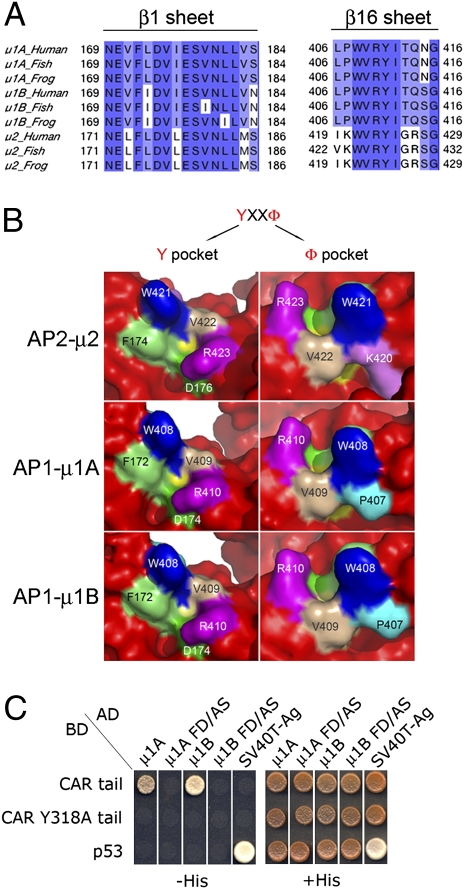
Are the interactions between AP-1A and AP-1B and CAR's basolateral sorting signal mediated by a hydrophobic pocket in μ1A or μ1B similar to the pocket that mediates the binding of AP-2's medium subunit μ2 to YXXΦ endocytic motifs (11)? Comparative analysis of μ1A, μ1B, and μ2 sequences demonstrates that the residues defining the pocket in μ2 are conserved in μ1A and μ1B and might mediate interactions with YxxΦ motifs (Fig. 4 A and B) (26). Mutagenesis of conserved phenylalanine and aspartic acid residues in this pocket, F172A and D174S, completely abolished the interaction of μ1A and μ1B with CAR (Fig. 4C). These results demonstrate that AP-1A and AP-1B interact with a YXXΦ-based basolateral sorting signal through hydrophobic pockets in their medium subunits, similar to those present in μ2. The pockets in μ1A and μ1B display differences in their ability to interact with YNQV, which may translate into differences in their ability to promote basolateral trafficking of CAR.
Fig. 4.
CAR's basolateral signal interacts with a conserved tyrosine recognition pocket in μ1A, and μ1B. (A) Sequence alignment analyses demonstrates that most of the amino acids conforming the pocket in AP-2 μ2 that binds YxxΦ motifs are conserved in both μ1A and μ1B. (B) Modeling of μ1A and μ1B based on the known structure of μ2 indicated conservation of the tertiary structure of the pocket, with the exception of K420 in human μ2 which is replaced by P407 in μ1A and μ1B. (C) Mutations F172S and D174S in μ1A and μ1B, involving phenylalanine and aspartic acid residues critical for interaction with Y residues of YxxΦ motifs, block interaction with CAR's cytoplasmic tail. p53 and SV40 T antigen were used as positive and negative controls, as in Fig. 2.
AP-1A Controls Basolateral Localization and Biosynthetic Delivery of CAR.
The demonstration that CAR's basolateral signal interacts with μ1A and μ1B suggests a role for AP-1A in the sorting of CAR and provides a molecular basis for our demonstration that AP-1B controls postendocytic sorting of this receptor (6). To characterize the role of AP-1A, we studied the effects of knocking-down AP-1A (A-KD), AP-1B (B-KD) as described elsewhere (24), or both adaptors together (AB-KD) on the polarized localization of CAR. Figs. S2 and S3 characterize cell lines used for these studies, including a unique MDCK cell line stably knocked-down for AP-1A.
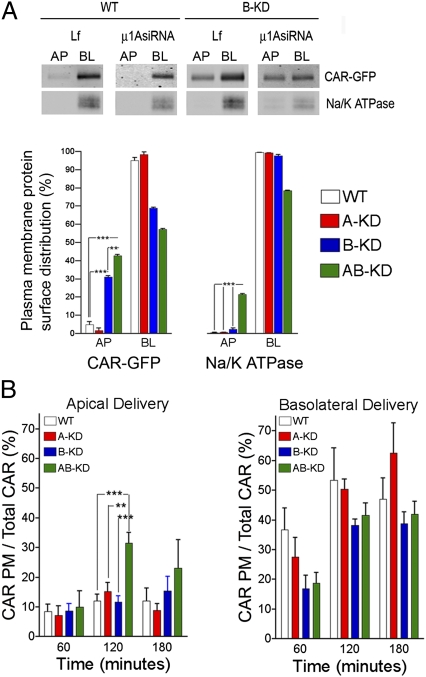
As previously shown (6), B-KD caused a substantial loss of CAR's basolateral polarity (68.9%, compared with 95.7% basolateral in WT MDCK cells, P < 0.001) (Fig. 5A and Table S3). In contrast, CAR was normally polarized (98.4% basolateral) in A-KD cells (Fig. 5A). Strikingly, AB-KD cells showed a larger loss of CAR's basolateral polarity than B-KD cells (57.1% basolateral, P < 0.01). Interestingly, the decrease in CAR's basolateral localization observed in B-KD and AB-KD MDCK cells was accompanied by a corresponding increase in CAR's apical localization (WT 4%, A-KD 2%, B-KD 32%, AB-KD 42%). Parallel experiments demonstrated that AB-KD also reduced the steady-state polarity of Na/K ATPase, although to a smaller extent (WT 99.4% basolateral, AB-KD 78.5%). However, Na/K ATPase polarity was not significantly affected by either B-KD 97.6%, or A-KD (99.4%) alone. These experiments establish that AP-1A plays a role in the basolateral steady-state distribution of CAR.
Fig. 5.
AP-1A and AP-1B co-operate in the basolateral sorting of CAR. (A) Steady-state localization. Domain selective biotinylation demonstrates significant loss of basolateral distribution of CAR-GFP in B-KD (68.93 ± 0.65%), even higher in AB-KD (57.15 ± 0.55%) MDCK cells, relative to WT (95.92 ± 1.58%) and A-KD (98.37 ± 1.48%) in MDCK cells. Endogenous Na/K ATPase was significantly depolarized only in AB-KD compared with WT, A-KD, or B-KD MDCK cells. (B) Biosynthetic delivery. After 2 h of biosynthetic delivery, AB-KD cells display 31.60 ± 3.47% of radioactively labeled CAR in the apical membrane, significantly higher than WT (13.34 ± 2.13%), A-KD (16.89 ± 3.17%), and B-KD (11.63 ± 2.11%) MDCK cells. Symbols represent the Mean ± SEM. All points were at least assayed in triplicate. See Tables S3 and S4 for statistical analysis. **P < 0.01, ***P < 0.001.
Is AP-1A involved in biosynthetic sorting of CAR? To answer this question, we studied the biosynthetic delivery of CAR in single and double knock-down MDCK cells, using the SBAS assay described above (Fig. 2) (24). We found that AB-KD increased by ∼2.5-fold the apical missorting of CAR after 2 h of chase (WT = 13.34%, AB-KD 31.60%) (see statistical anaylsis in Table S4); in contrast, neither A-KD nor B-KD significantly increased apical missorting of CAR (A-KD = 16.63%, B-KD = 11.63%) (Fig. 5B). In contrast with our results with CAR mutants (Fig. 2), we did not observe any significant intracellular retention of CAR upon knock-down of both adaptors involved in its basolateral sorting. These results show that AP-1A controls biosynthetic delivery of CAR in partnership with AP-1B.
Discussion
We chose CAR for this study because of the growing realization of its physiological importance as a regulator of the barrier function of epithelia and because its basolateral sorting signal consists of a typical YXXΦ motif, 318YNQV321 (5). Our studies show that Y318 and a hydrophobic residue at position 321 are required for steady-state basolateral localization of CAR (Fig. 1), for polarized biosynthetic delivery of CAR to the basolateral membrane (Fig. 2), and for interaction with the medium subunits of AP-1A and AP-1B (Figs. 3 and 4), which are involved in basolateral trafficking of this receptor (Fig. 5, further discussed below). Our experiments further show that variations in the hydrophobicity of the Φ321 residue causes variations in the strength of the basolateral signal (i.e., a differential ability to target basolaterally CAR versus the apical marker p75) (Fig. 1). This type of variation likely contributes to the variable polarity of PM proteins in different tissues, as recently reported for monocarboxylate transporters (23) (for additional discussion of this issue, see ref. 27). The results reported here are unique in demonstrating how a basolateral PM protein with a canonical YxxΦ motif is sorted along its biosynthetic and recycling routes through direct interactions with two clathrin adaptors. Although this concept has been established by biochemical and structural studies for interactions between endocytic signals and AP-2 required for endocytosis (9, 11), it had not been previously established for basolateral trafficking.
A second major result in this report is the identification of AP-1A as a clathrin adaptor that regulates the basolateral localization of CAR. A Y2H screen identified AP-1A and AP-1B as the only AP adaptors that interact via their medium subunits μ1A and μ1B with the basolateral signal of CAR (Fig. 3A). Further computer modeling and Y2H experiments (Fig. 4) demonstrated that these interactions occurred between the Y318 and V321 residues in CAR's signal and conserved phenylalanine and aspartic acid residues in μ1A and μ1B, as previously shown for the interactions between YXXΦ motifs and AP-2's μ2 subunit required for receptor internalization at the PM. Biochemical trafficking assays demonstrated that AP-1A KD causes a disruption of the biosynthetic delivery of CAR in the background of MDCK cells previously knocked-down of AP-1B (Fig. 5). We do not have a clear explanation of why knock-down of both AP-1A and AP-1B cause apical missorting with little intracellular retention (Fig. 5B) but ablation of CAR's basolateral signal causes intracellular retention of CAR with little apical missorting (Fig. 2B). All experiments in this article together demonstrate that AP-1A regulates, in cooperation with AP-1B, the biosynthetic delivery of CAR through interactions of these adaptors with CAR's basolateral sorting signal.
What is the cellular compartment where AP-1A performs its sorting function? Experiments in a separate study (24) indicate that in polarized MDCK cells: (i) AP-1A localizes preferentially to the trans Golgi network (TGN), whereas AP-1B localizes preferentially to common recycling endosomes (CRE); and (ii) AP-1A knock-down enhances trafficking of basolateral proteins into CRE. These studies, together with previous work on AP-1B (6, 8), support a scenario in which AP-1A controls biosynthetic trafficking from the TGN to the basolateral membrane, whereas AP-1B controls recycling of basolateral proteins and biosynthetic trafficking of PM proteins that enter CRE upon exit from the TGN. This scenario suggests a mechanism for the compensation of AP-1A knock-down by AP-1B and is consistent with the well-established function of AP-1A in promoting exit from the TGN of various membrane proteins, such as the mannose-6-phosphate receptor (28, 29), vesicular stomatitis virus (VSV) G protein (30), and the K channel Kir 2.1 (31).
Our finding of a unique role for the ubiquitous clathrin adaptor AP-1A in basolateral protein sorting is timely, as growing evidence indicates that AP-1B is not a universal basolateral sorting adaptor. For example, it has been shown that several epithelia do not express AP-1B—for example, liver (12, 32), retinal pigment epithelium (6), and kidney proximal tubule (33)—and that knockout of μ1B in mice is not lethal (34). That both AP-1A and AP-1B play key roles in basolateral trafficking highlight the importance of AP-1 as a master regulator of epithelial polarity, consistent with other findings implicating AP-1 in neuronal polarity (35) and as a key requirement in early development of multicellular organisms (36, 37). Recent observations indicating that AP-1B levels may be decreased in some forms of Crohn disease and that mice knockout for AP-1B develop colon inflamation (34), suggest that investigating changes in polarity of CAR may provide new avenues for translational research in intestinal disease.
Materials and Methods
Cell Culture and Cell Lines.
MDCK cells were maintained in DMEM containing 5% (vol/vol) FBS. WT and B-KD MDCK cell lines expressing hCAR-GFP and WT MDCK cell lines expressing CAR-Y318A-GFP, CAR-V321A-GFP and CAR-V321E-GFP were generated using selection in hygromycin (200 μg/mL). For biochemical experiments, MDCK cell lines were plated at confluency (3 × 105 cells/cm2) on 12-mm Transwell chambers (Corning; Cat # 3412). Cells were allowed to polarize for 3.5 d and medium was replaced every day. To knock down μ1A transiently, we followed a previously published protocol (8) using a μ1A-specific siRNA (GTGCTCATCTGCCGGAATT) (24). Briefly, WT or μ1B-KD MDCK cells in suspension culture (4 × 106) were treated with 5 μL of 40 mM siRNA and subjected to three rounds of electroporation with Amaxa Nucleofector kit V, spaced every 3 d, and plated after the last round on Transwell chambers at a density of 1 × 106 cells per 24-mm filter. RT-PCR and Western blot were used to corroborate the absence of μ1A (Fig. S2).
Protein Modeling and Alignment.
Alignment of μ1A, μ1B, and μ2 cDNAs from human, fish, and frog was done with CLUSTAL software. Structural models of human AP1M1 (μ1A), AP1M2 (μ1B), and AP2M1 (μ2) proteins were obtained using the automated homology-modeling server SWISS-MODEL (38). A crystal structure of rat AP2M1 generated recently (39) was applied as the template to guide the structural alignment of human AP1M1, AP1M2, and AP2M1 proteins. The SWISS-MODEL server positively scored final results.
CAR Biosynthetic Delivery Assay.
Biosynthetic delivery of CAR-GFP to apical and basolateral PM domains was measured by a recently described SBAS quantitative assay (24) (Fig. 2). Briefly, [35S]-pulse–labeled MDCK cells were chased in chase medium for various times, chilled with ice-cold Ca, Mg-HBSS, and subjected to domain-selective surface biotinylation. Cells were rinsed twice with ice-cold Ca, Mg-HBSS and once with 25 mM triethanolamine-HCl, pH 7.8, 0.25 M sucrose, 0.5 mM Cl2Ca, 1 mM MgCl2 (BB) followed by two successive 20-min incubations at 4 °C with 3 mg⋅mL−1 sulfo-NHS-LC-Biotin in BB, added to the apical (0.6 mL) or to the basolateral (0.3 mL) sides. Cells were rinsed twice with BB and incubated at 4 °C for 20 min with 40 mM ethanolamine-HCl in BB followed by a quick rinse with Ca,Mg-free HBSS. Cells were lysed in 50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 25 mM KCl, 2 mM EDTA, 1% Na-deoxycholate, 0.1% SDS, 1% Triton X-100, supplemented with 1 mM PMSF and 15 μg⋅mL−1 Leupeptin/Pepstatin/Antipain and 37 μg⋅mL−1 benzamidine-HCl for 30 min at 4 °C. Lysates, cleared by centrifugation (18,000 × g for 10 min), were subjected to immunoprecipitation with rabbit anti-GFP antibodies and protein A-Agarose. The immunoprecipitated protein was eluted by boiling samples with 40 mM Tris-HCl pH 9.0, 1.5% SDS and 20 mM DTT (170 μL per sample) for 10 min and divided into two identical 80-μL aliquots: (i) one aliquot was mixed with 55 μL of a freshly made solution of 500 μg⋅mL−1 avidin, 35% (vol/vol) glycerol, and 15 mg⋅mL−1 bromophenol blue in water (avidin sample); (ii) the other aliquot was mixed with 55 μL of the same solution supplemented with 3 mM biotin (biotin-avidin sample). Both samples were incubated at 60 °C for 10 min and analyzed by SDS/PAGE. Avidin binds tightly to biotinylated proteins in avidin-sample but not in biotin-avidin sample, generating a shift in electrophoretic mobility that discriminates surface (biotinylated) from intracellular (nonbiotinylated) proteins. The difference between total and intracellular samples represents the amount of protein transported to the cell surface. This assay quantifies the amount of cargo proteins delivered to the apical and basolateral cell surfaces as a percentage of the total amount of cargo protein (total = surface + intracellular) (24). The difference between total labeling and the sum of apical and basolateral labeling represents the intracellular fraction of the protein.
Statistical Analysis.
Data were analyzed using one-way ANOVA followed by the Bonferroni multiple comparisons posttest (GraphPad Prism).
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants EY08538 and GM34107 (to E.R.-B.); a European Molecular Biology Organization fellowship (to J.M.C.-G.); the Dyson Foundation and by the Research to Prevent Blindness Foundation; and by the Intramural Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R.M. and J.S.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1117949109/-/DCSupplemental.
References
- 1.Verdino P, Witherden DA, Havran WL, Wilson IA. The molecular interaction of CAR and JAML recruits the central cell signal transducer PI3K. Science. 2010;329:1210–1214. doi: 10.1126/science.1187996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Witherden DA, et al. The junctional adhesion molecule JAML is a costimulatory receptor for epithelial gammadelta T cell activation. Science. 2010;329:1205–1210. doi: 10.1126/science.1192698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freimuth P, Philipson L, Carson SD. The coxsackievirus and adenovirus receptor. Curr Top Microbiol Immunol. 2008;323:67–87. doi: 10.1007/978-3-540-75546-3_4. [DOI] [PubMed] [Google Scholar]
- 4.Bergelson JM, et al. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 5.Cohen CJ, Gaetz J, Ohman T, Bergelson JM. Multiple regions within the coxsackievirus and adenovirus receptor cytoplasmic domain are required for basolateral sorting. J Biol Chem. 2001;276:25392–25398. doi: 10.1074/jbc.M009531200. [DOI] [PubMed] [Google Scholar]
- 6.Diaz F, et al. Clathrin adaptor AP1B controls adenovirus infectivity of epithelial cells. Proc Natl Acad Sci USA. 2009;106:11143–11148. doi: 10.1073/pnas.0811227106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gan Y, McGraw TE, Rodriguez-Boulan E. The epithelial-specific adaptor AP1B mediates post-endocytic recycling to the basolateral membrane. Nat Cell Biol. 2002;4:605–609. doi: 10.1038/ncb827. [DOI] [PubMed] [Google Scholar]
- 8.Gravotta D, et al. AP1B sorts basolateral proteins in recycling and biosynthetic routes of MDCK cells. Proc Natl Acad Sci USA. 2007;104:1564–1569. doi: 10.1073/pnas.0610700104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Traub LM. Tickets to ride: Selecting cargo for clathrin-regulated internalization. Nat Rev Mol Cell Biol. 2009;10:583–596. doi: 10.1038/nrm2751. [DOI] [PubMed] [Google Scholar]
- 10.Ohno H, et al. Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science. 1995;269:1872–1875. doi: 10.1126/science.7569928. [DOI] [PubMed] [Google Scholar]
- 11.Owen DJ, Evans PR. A structural explanation for the recognition of tyrosine-based endocytotic signals. Science. 1998;282:1327–1332. doi: 10.1126/science.282.5392.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohno H, et al. Mu1B, a novel adaptor medium chain expressed in polarized epithelial cells. FEBS Lett. 1999;449:215–220. doi: 10.1016/s0014-5793(99)00432-9. [DOI] [PubMed] [Google Scholar]
- 13.Fölsch H, Ohno H, Bonifacino JS, Mellman I. A novel clathrin adaptor complex mediates basolateral targeting in polarized epithelial cells. Cell. 1999;99:189–198. doi: 10.1016/s0092-8674(00)81650-5. [DOI] [PubMed] [Google Scholar]
- 14.Simmen T, Höning S, Icking A, Tikkanen R, Hunziker W. AP-4 binds basolateral signals and participates in basolateral sorting in epithelial MDCK cells. Nat Cell Biol. 2002;4:154–159. doi: 10.1038/ncb745. [DOI] [PubMed] [Google Scholar]
- 15.Nishimura N, Plutner H, Hahn K, Balch WE. The delta subunit of AP-3 is required for efficient transport of VSV-G from the trans-Golgi network to the cell surface. Proc Natl Acad Sci USA. 2002;99:6755–6760. doi: 10.1073/pnas.092150699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez A, Rodriguez-Boulan E. Clathrin and AP1B: Key roles in basolateral trafficking through trans-endosomal routes. FEBS Lett. 2009;583:3784–3795. doi: 10.1016/j.febslet.2009.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brewer CB, Roth MG. A single amino acid change in the cytoplasmic domain alters the polarized delivery of influenza virus hemagglutinin. J Cell Biol. 1991;114:413–421. doi: 10.1083/jcb.114.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin S, Naim HY, Roth MG. Tyrosine-dependent basolateral sorting signals are distinct from tyrosine-dependent internalization signals. J Biol Chem. 1997;272:26300–26305. doi: 10.1074/jbc.272.42.26300. [DOI] [PubMed] [Google Scholar]
- 19.Le Bivic A, et al. An internal deletion in the cytoplasmic tail reverses the apical localization of human NGF receptor in transfected MDCK cells. J Cell Biol. 1991;115:607–618. doi: 10.1083/jcb.115.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matter K, Hunziker W, Mellman I. Basolateral sorting of LDL receptor in MDCK cells: The cytoplasmic domain contains two tyrosine-dependent targeting determinants. Cell. 1992;71:741–753. doi: 10.1016/0092-8674(92)90551-m. [DOI] [PubMed] [Google Scholar]
- 21.Weisz OA, Rodriguez-Boulan E. Apical trafficking in epithelial cells: Signals, clusters and motors. J Cell Sci. 2009;122:4253–4266. doi: 10.1242/jcs.032615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le Gall AH, Powell SK, Yeaman CA, Rodriguez-Boulan E. The neural cell adhesion molecule expresses a tyrosine-independent basolateral sorting signal. J Biol Chem. 1997;272:4559–4567. doi: 10.1074/jbc.272.7.4559. [DOI] [PubMed] [Google Scholar]
- 23.Castorino JJ, et al. Basolateral sorting signals regulating tissue-specific polarity of heteromeric monocarboxylate transporters in epithelia. Traffic. 2011;12:483–498. doi: 10.1111/j.1600-0854.2010.01155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gravotta D, et al. Novel role of the clathrin adaptor AP-1A in basolateral polarity. Dev Cell. 2012 doi: 10.1016/j.devcel.2012.02.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deborde S, et al. Clathrin is a key regulator of basolateral polarity. Nature. 2008;452:719–723. doi: 10.1038/nature06828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonifacino JS, Dell'Angelica EC. Molecular bases for the recognition of tyrosine-based sorting signals. J Cell Biol. 1999;145:923–926. doi: 10.1083/jcb.145.5.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Philp N, Shoshani L, Cereijido M, Rodriguez-Boulan E. Epithelial domains. In: Nabi R, editor. Cellular Domains. Hoboken, NJ: John Wiley and Sons; 2011. [Google Scholar]
- 28.Doray B, Ghosh P, Griffith J, Geuze HJ, Kornfeld S. Cooperation of GGAs and AP-1 in packaging MPRs at the trans-Golgi network. Science. 2002;297:1700–1703. doi: 10.1126/science.1075327. [DOI] [PubMed] [Google Scholar]
- 29.Waguri S, et al. Visualization of TGN to endosome trafficking through fluorescently labeled MPR and AP-1 in living cells. Mol Biol Cell. 2003;14:142–155. doi: 10.1091/mbc.E02-06-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chi S, Cao H, Chen J, McNiven MA. Eps15 mediates vesicle trafficking from the trans-Golgi network via an interaction with the clathrin adaptor AP-1. Mol Biol Cell. 2008;19:3564–3575. doi: 10.1091/mbc.E07-10-0997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma D, et al. Golgi export of the Kir2.1 channel is driven by a trafficking signal located within its tertiary structure. Cell. 2011;145:1102–1115. doi: 10.1016/j.cell.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koivisto UM, Hubbard AL, Mellman I. A novel cellular phenotype for familial hypercholesterolemia due to a defect in polarized targeting of LDL receptor. Cell. 2001;105:575–585. doi: 10.1016/s0092-8674(01)00371-3. [DOI] [PubMed] [Google Scholar]
- 33.Schreiner R, et al. The absence of a clathrin adapter confers unique polarity essential to proximal tubule function. Kidney Int. 2010;78:382–388. doi: 10.1038/ki.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takahashi D, et al. The epithelia-specific membrane trafficking factor AP-1B controls gut immune homeostasis in mice. Gastroenterology. 2011;141:621–632. doi: 10.1053/j.gastro.2011.04.056. [DOI] [PubMed] [Google Scholar]
- 35.Dwyer ND, Adler CE, Crump JG, L'Etoile ND, Bargmann CI. Polarized dendritic transport and the AP-1 mu1 clathrin adaptor UNC-101 localize odorant receptors to olfactory cilia. Neuron. 2001;31:277–287. doi: 10.1016/s0896-6273(01)00361-0. [DOI] [PubMed] [Google Scholar]
- 36.Lee J, Jongeward GD, Sternberg PW. unc-101, a gene required for many aspects of Caenorhabditis elegans development and behavior, encodes a clathrin-associated protein. Genes Dev. 1994;8:60–73. doi: 10.1101/gad.8.1.60. [DOI] [PubMed] [Google Scholar]
- 37.Zizioli D, et al. Early embryonic death of mice deficient in gamma-adaptin. J Biol Chem. 1999;274:5385–5390. doi: 10.1074/jbc.274.9.5385. [DOI] [PubMed] [Google Scholar]
- 38.Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL workspace: A web-based environment for protein structure homology modelling. Bioinformatics. 2006;22:195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- 39.Jackson LP, et al. A large-scale conformational change couples membrane recruitment to cargo binding in the AP2 clathrin adaptor complex. Cell. 2010;141:1220–1229. doi: 10.1016/j.cell.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fields IC, et al. v-SNARE cellubrevin is required for basolateral sorting of AP-1B-dependent cargo in polarized epithelial cells. J Cell Biol. 2007;177:477–488. doi: 10.1083/jcb.200610047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomas DC, Roth MG. The basolateral targeting signal in the cytoplasmic domain of glycoprotein G from vesicular stomatitis virus resembles a variety of intracellular targeting motifs related by primary sequence but having diverse targeting activities. J Biol Chem. 1994;269:15732–15739. [PubMed] [Google Scholar]
- 42.Monlauzeur L, Rajasekaran A, Chao M, Rodriguez-Boulan E, Le Bivic A. A cytoplasmic tyrosine is essential for the basolateral localization of mutants of the human nerve growth factor receptor in Madin-Darby canine kidney cells. J Biol Chem. 1995;270:12219–12225. doi: 10.1074/jbc.270.20.12219. [DOI] [PubMed] [Google Scholar]
- 43.Prill V, Lehmann L, von Figura K, Peters C. The cytoplasmic tail of lysosomal acid phosphatase contains overlapping but distinct signals for basolateral sorting and rapid internalization in polarized MDCK cells. EMBO J. 1993;12:2181–2193. doi: 10.1002/j.1460-2075.1993.tb05866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rajasekaran AK, et al. TGN38 recycles basolaterally in polarized Madin-Darby canine kidney cells. Mol Biol Cell. 1994;5:1093–1103. doi: 10.1091/mbc.5.10.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohno H, Fournier MC, Poy G, Bonifacino JS. Structural determinants of interaction of tyrosine-based sorting signals with the adaptor medium chains. J Biol Chem. 1996;271:29009–29015. doi: 10.1074/jbc.271.46.29009. [DOI] [PubMed] [Google Scholar]
- 46.Adair-Kirk TL, Dorsey FC, Cox JV. Multiple cytoplasmic signals direct the intracellular trafficking of chicken kidney AE1 anion exchangers in MDCK cells. J Cell Sci. 2003;116:655–663. doi: 10.1242/jcs.00260. [DOI] [PubMed] [Google Scholar]
- 47.Geffen I, et al. Related signals for endocytosis and basolateral sorting of the asialoglycoprotein receptor. J Biol Chem. 1993;268:20772–20777. [PubMed] [Google Scholar]
- 48.Lodge R, Lalonde JP, Lemay G, Cohen EA. The membrane-proximal intracytoplasmic tyrosine residue of HIV-1 envelope glycoprotein is critical for basolateral targeting of viral budding in MDCK cells. EMBO J. 1997;16:695–705. doi: 10.1093/emboj/16.4.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ohno H, et al. Interaction of endocytic signals from the HIV-1 envelope glycoprotein complex with members of the adaptor medium chain family. Virology. 1997;238:305–315. doi: 10.1006/viro.1997.8839. [DOI] [PubMed] [Google Scholar]
- 50.Ohka S, Ohno H, Tohyama K, Nomoto A. Basolateral sorting of human poliovirus receptor alpha involves an interaction with the mu1B subunit of the clathrin adaptor complex in polarized epithelial cells. Biochem Biophys Res Commun. 2001;287:941–948. doi: 10.1006/bbrc.2001.5660. [DOI] [PubMed] [Google Scholar]
- 51.Höning S, Griffith J, Geuze HJ, Hunziker W. The tyrosine-based lysosomal targeting signal in lamp-1 mediates sorting into Golgi-derived clathrin-coated vesicles. EMBO J. 1996;15:5230–5239. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.