Abstract
Tissue factor pathway inhibitor (TFPI) blocks thrombin generation via the extrinsic blood coagulation pathway. Because the severe bleeding in patients with hemophilia occurs from deficiency of intrinsic blood coagulation pathway factor VIII or IX, pharmacological agents that inactivate TFPI and, therefore, restore thrombin generation via the extrinsic pathway, are being developed for treatment of hemophilia. Murine models of combined TFPI and factor VIII deficiency were used to examine the impact of TFPI deficiency on bleeding and clotting in hemophilia. In breeding studies, Factor VIII null (F8−/−) did not rescue the embryonic death of TFPI null (Tfpi−/−) mice. Tfpi+/− did not alter the bleeding phenotype of F8−/− mice. However, total inhibition of intravascular TFPI through injection of anti-TFPI antibody mitigated tail vein bleeding. Interestingly, tail blood loss progressively decreased at doses greater than needed to totally inhibit plasma TFPI, suggesting that inhibition of a sequestered pool of TFPI released at the injury site mitigates bleeding. Because TFPI is sequestered within platelets and released following their activation, the function of platelet TFPI was examined in F8−/− mice lacking hematopoietic cell TFPI that was generated by fetal liver transplantation. Blood loss after tail transection significantly decreased in Tfpi+/−;F8−/− mice with hematopoietic Tfpi−/− cells compared with Tfpi+/−;F8−/− mice with Tfpi+/+ hematopoietic cells. Additionally, following femoral vein injury, Tfpi+/−;F8−/− mice with Tfpi−/− hematopoietic cells had increased fibrin deposition compared with identical-genotype mice with Tfpi+/+ hematopoietic cells. These findings implicate platelet TFPI as a primary physiological regulator of bleeding in hemophilia.
Keywords: hemostasis, Kunitz, coagulopathy
Hemophilia A and B are severe bleeding disorders caused by deficiency of blood coagulation factors VIII and IX (FVIII and FIX), respectively. Patients with hemophilia often suffer from spontaneous bleeding within the musculoskeletal system, such as hemarthrosis, that can result in disability at a young age if not treated with i.v. infusions of the missing coagulation factor. Early studies of blood coagulation in vitro described two blood coagulation pathways, an “intrinsic pathway” that is initiated by the contact of blood with a charged surface, and an “extrinsic pathway” that is initiated when factor VIIa (FVIIa), a serine protease in blood, is exposed to tissue factor (TF), a protein present in perivascular tissues (1, 2). Because FVIII and FIX are proteins of the intrinsic pathway, a classical question in blood coagulation has been to determine why patients with hemophilia have severe spontaneous bleeding despite having a fully functional extrinsic blood coagulation pathway (3). This question can be answered by understanding how the extrinsic clotting pathway is inhibited. The TF–FVIIa complex initiates coagulation by activating factors IX and X. However, the factor Xa (FXa) generated during initiation of coagulation promotes inhibition of TF–FVIIa by tissue factor pathway inhibitor (TFPI), making propagation of the coagulation cascade dependent on the activity of the FIXa–FVIIIa complex. Lack of TFPI inhibition of TF–FVIIa might consequently compensate for FIX or FVIII deficiency and mitigate bleeding in patients with hemophilia (4–7).
TFPI is a multivalent Kunitz-type protease inhibitor with domains that simultaneously bind and inhibit the active sites of FVIIa and FXa immediately after FX is activated by TF–FVIIa (8). It has been estimated that about 85% of intravascular TFPI is located on the surface of endothelial cells (9), with the remainder within platelets (10, 11) and in circulating plasma (12). Small amounts of TFPI have been identified on monocytes (13) and vascular smooth muscle cells (14) as well as other cell types (15). Although most intravascular TFPI is thought to be exposed to circulating blood, TFPI is also present within endothelial cells, with acute release following exposure to thrombin (16, 17), and within platelets, with release following dual agonist activation with thrombin plus collagen (10, 11). Interestingly, TFPI concentration progressively increases two- to threefold with time in blood samples obtained from a skin wound. This is likely the result of TFPI released from activated platelets accumulating within the growing blood clot (10).
Shortly after the discovery of TFPI, in vitro biochemical studies using purified proteins demonstrated that FXa generation in TF-initiated reactions was significantly reduced by TFPI when performed in the absence of FVIII and FIX but to a much lesser degree in their presence, suggesting a central role for TFPI inhibitory activity in the pathogenesis of bleeding in hemophilia (4, 7). Additional studies of human plasma clotting assays initiated with dilute TF demonstrated that anti-TFPI antibodies shorten the clotting time of hemophilia plasma more than normal plasma, suggesting that pharmacological inhibitors of TFPI may represent a novel treatment for hemophilia (5, 6). Consistent with these in vitro studies, in vivo studies performed in FVIII-deficient rabbits (18), dogs (19), and monkeys (20) have demonstrated that inhibition of TFPI activity reduces injury-induced blood loss in animal models of hemophilia.
To further investigate the physiological mechanisms through which TFPI modulates bleeding in hemophilia, we undertook studies of genetically altered mice with combined FVIII deficiency and TFPI deficiency. The presence of FVIII deficiency did not rescue the embryonic lethal phenotype of TFPI null (Tfpi−/−) mice, and decreased TFPI plasma levels in Tfpi+/− mice did not alter the bleeding phenotype of Factor VIII null (F8−/−) mice. However, infusion of anti-TFPI polyclonal antibody at doses that totally inhibited plasma TFPI activity reduced blood loss in tail bleeding assays. Interestingly, tail blood loss continued to decrease at doses beyond that needed to totally inhibit plasma TFPI activity, suggesting inhibition of a sequestered pool of TFPI. When F8−/− mice were transplanted with fetal liver cells from Tfpi−/− embryos, they had significantly less blood loss in tail bleeding assays and significantly larger clot volume following vascular injury than mice transplanted with fetal liver cells from Tfpi+/+ embryos. These data suggest that TFPI present within hematopoietic cells, most likely the platelets accumulating at the site of vascular injury, is a physiological modulator of bleeding in mice with hemophilia A.
Results
F8−/− Does Not Rescue the Embryonic Lethal Phenotype of Tfpi−/− Mice.
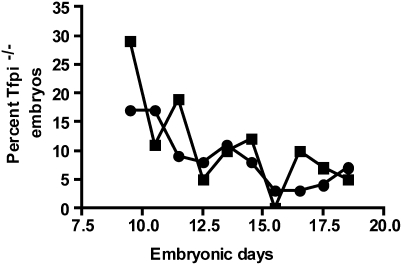
Tfpi+/−;F8−/− male and female mice were mated and the pups were genotyped at 3 wk of age. Of 195 pups, 83 were Tfpi+/+, 112 were Tfpi+/−, and 0 were Tfpi−/−, demonstrating that the loss of FVIII activity does not rescue the embryonic lethality of Tfpi−/− mice to weaning age. Timed matings were performed to determine whether FVIII deficiency prolongs the embryonic survival of Tfpi−/− embryos. There were no significant differences in the embryonic survival of Tfpi−/−;F8−/− embryos compared with that reported for Tfpi−/− embryos on a mixed C57BL/6 and 129SvPas background by Huang and coworkers (21) (Fig. 1). Thus, in contrast to extrinsic factor pathway deficiencies (22, 23), deficiency of the intrinsic pathway coagulation factor FVIII does not rescue the embryonic lethal phenotype of Tfpi−/− mice.
Fig. 1.
F8−/− does not rescue the embryonic lethality of Tfpi−/− mice. A total of 445 pups was genotyped from breeding of Tfpi+/−;F8−/− male and female mice, with a range of 21 to 67 pups on each embryonic day. The expected survival of Tfpi−/− mice from this mating is 25%, if no embryonic death occurs. There is no difference in embryonic survival between Tfpi−/−;F8−/− mice (■) and Tfpi−/−;F8+/+ mice (●), originally published by Huang and coworkers (21).
Tfpi+/− Does Not Alter the Bleeding Phenotype of Adult F8−/− Mice.
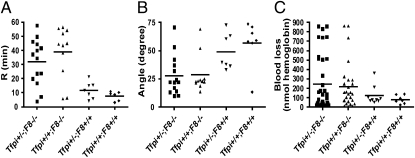
Thrombelastography (TEG) assays using whole-blood and tail vein bleeding assays were used to assess the effect of Tfpi+/− on bleeding in F8−/− mice. In TEG assays, the F8−/− mice had significantly longer R values (time for initiation of clot formation) and significantly decreased α angle (a measure of the kinetics of fibrin formation) than F8+/+ mice. However, the TEG R value and α angle in Tfpi+/−;F8−/− mice were not significantly different from those in Tfpi+/+;F8−/− mice (Fig. 2 A and B). Power calculations indicate that for the sample sizes used, the TEG assays had at least 80% power to detect a twofold difference between the two groups. Blood loss assayed as hemoglobin lost over 10 min following a 1-mm tail transection had very high variability between groups and demonstrated no significant difference in the amount of blood lost between Tfpi+/+;F8−/− and Tfpi+/−;F8−/− mice (Fig. 2C). Similarly, there was no difference in blood lost between F8+/+ and F8−/− mice or between Tfpi+/+ mice and Tfpi+/− mice (Fig. 2C). Power calculations indicate that for the sample sizes used, the tail bleeding assays had at least 80% power to detect a 2.5-fold difference between F8−/− mice with and without Tfpi+/− and to detect a fourfold difference between F8+/+ and F8−/− mice.
Fig. 2.
Tfpi+/− does not alter the bleeding phenotype of F8−/− mice. (A) The TEG R value (time for clot initiation) was significantly prolonged in F8−/− mice (▲) compared with F8+/+ mice (◆) (P = 0.0026) or Tfpi+/− mice (▼) (P = 0.018), but the presence of Tfpi+/− in F8−/− mice (■) did not decrease this prolongation (P = 0.59). (B) The TEG α angle (a measure of the kinetics of fibrin formation) was significantly decreased in Tfpi+/−;F8−/− mice (■) compared with Tfpi+/− mice (▼) (P = 0.017), but Tfpi+/−;F8−/− mice (■) were not different from F8−/− mice (▲) (P = 1). (C) In the 1-mm clip tail bleeding model, F8−/− mice (▲) showed a trend to bleed more than F8+/+ mice (◆) but it was not statistically significant (P = 0.23), whereas Tfpi+/−;F8−/− mice (■) had essentially identical blood loss as F8−/− mice (▲) (P = 1). Similarly, there was no difference between Tfpi+/− (▼) and Tfpi+/+ (◆) mice.
Anti-TFPI Antibody Infusion Reduces Blood Loss in F8−/− Mice in Tail Bleeding Assays.
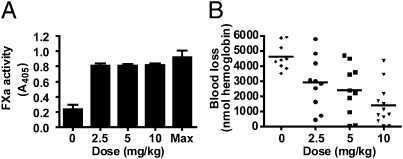
Intravenous infusion of a polyclonal anti-mouse TFPI antibody was used to investigate how inhibition of intravascular TFPI activity altered tail bleeding in F8−/− mice. The antibody was dosed in progressively increasing amounts from 0 to 10 mg/kg. Activity assays demonstrated that plasma TFPI was totally inhibited following infusion of 2.5 mg/kg antibody, with no change in the residual plasma TFPI activity as the antibody dose increased to 10 mg/kg (Fig. 3A). Blood loss assayed as hemoglobin lost over 20 min following a 4-mm tail transection progressively decreased from 4,615 ± 287 nmol hemoglobin in the untreated group to 2,921 ± 537, 2,406 ± 530, and 1,419 ± 386 nmol hemoglobin (mean ± SEM) in the groups treated with 2.5, 5, and 10 mg/kg antibody, respectively (Fig. 3B). A posttest for linear trend was statistically significant (test for linear trend, r2 = 0.40, P = 0.000024), demonstrating that direct inhibition of intravascular TFPI effectively reduces tail bleeding in mice with hemophilia. The antibody fully inhibited plasma TFPI at the lowest dose (2.5 mg/kg). This suggests that other sources of intravascular TFPI accessible to antibody binding, such as that on the endothelium surface, are also inhibited. Therefore, we expected this dose to maximally prevent tail blood loss. However, a progressive decrease in tail blood loss with increasing antibody dosage was observed (Fig. 3B), suggesting that the anti-TFPI antibody may reduce bleeding through inhibition of TFPI stored within platelets (10, 11).
Fig. 3.
Intravenous injection of a polyclonal anti-TFPI antibody mitigates bleeding in F8−/− mice. (A) Plasma FXa activity in samples from F8−/− mice treated with different i.v. doses of polyclonal anti-mouse TFPI antibody or a vehicle control shows essentially total inhibition of TFPI activity at all doses of antibody tested. Data are presented as mean ± SD. Max represents data for plasma samples obtained from vehicle control-treated mice that were spiked with 100 μg/mL of the anti-mouse TFPI antibody and is the maximum total inhibition of plasma TFPI activity. (B) Treatment of F8−/− mice with increasing doses of a polyclonal anti-mouse TFPI antibody produces progressively smaller amounts of blood loss following 4-mm tail transection (test for linear trend, r2 = 0.40, P = 0.000024).
Lack of Hematopoietic Cell TFPI Reduces Blood Loss from F8−/− Mice in Tail Vein Bleeding Assays.
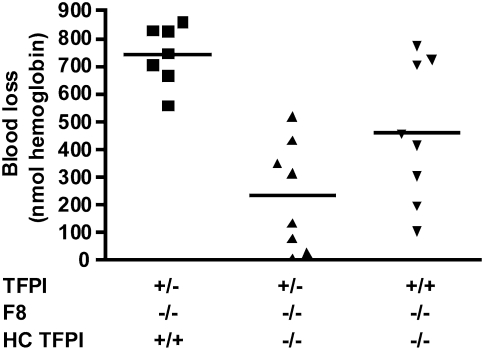
Megakaryocytes, which produce TFPI, are the major hematopoietic source of TFPI (10, 11). We have previously demonstrated that lethally irradiated Tfpi+/− mice can be rescued by transplantation with Tfpi−/− fetal liver cells, the rescued mice lack platelet TFPI activity, and the TFPI plasma concentration in these mice is unaffected (24). To investigate how platelet TFPI alters bleeding in mice with hemophilia, blood loss assayed as hemoglobin lost over 10 min following a 1-mm tail transection was measured in Tfpi+/−;F8−/− mice transplanted with either Tfpi+/+ or Tfpi−/− fetal liver cells. Tfpi+/−;F8−/− mice were chosen for the initial transplantation experiments because they have one-half the plasma TFPI concentration and one-half the amount of endothelial TFPI as wild-type mice, and their use limits the confounding effects that other sources of TFPI may have on bleeding while maximizing the effects of platelet TFPI. The Tfpi+/−;F8−/− mice transplanted with Tfpi+/+ cells lost 744 nmol hemoglobin whereas those transplanted with Tfpi−/− cells lost only 233 nmol hemoglobin (P = 0.00015), demonstrating that loss of hematopoietic cell TFPI significantly reduces bleeding in mice with hemophilia (Fig. 4). The hemostatic effect of transplanted Tfpi−/− fetal liver cells suggests a physiologically relevant contribution of platelet TFPI to bleeding in hemophilia. Therefore, additional studies were performed using Tfpi+/+;F8−/− mice transplanted with Tfpi−/− fetal liver cells to investigate the effect of the loss of hematopoietic TFPI in mice with normal amounts of plasma and endothelial TFPI. These mice had an intermediate amount of bleeding compared with the other two groups studied (458 nmol hemoglobin) that was statistically less than the Tfpi+/−;F8−/− mice transplanted with Tfpi+/+ fetal liver cells (P = 0.033), further demonstrating the effect of hematopoietic cell TFPI on bleeding in hemophilia (Fig. 4). There was a trend toward less bleeding in Tfpi+/−;F8−/− mice transplanted with Tfpi−/− fetal liver cells compared with Tfpi+/+;F8−/− mice transplanted with Tfpi−/− fetal liver cells (P = 0.067) (Fig. 4). Also of note, in the tail transection bleeding assays, it appears that irradiation of mice for transplantation causes F8−/− mice to consistently bleed at the higher end of the observed range than F8−/− mice that have not been irradiated (compare hemoglobin lost from 1-mm tail transection in Figs. 2 and 4). Finally, because there was no difference in blood loss between Tfpi+/+ and Tfpi+/− when on either an F8+/+ or F8−/− background (Fig. 2C), it can reasonably be expected that bleeding time studies of Tfpi+/+;F8−/− mice transplanted with Tfpi+/+ fetal liver cells will be similar to the results obtained with Tfpi+/−;F8−/− mice transplanted with Tfpi+/+ fetal liver cells. Therefore, the use of animals for these experiments could not be justified.
Fig. 4.
Absence of hematopoietic cell TFPI (HC TFPI) mitigates bleeding in F8−/− mice. Tfpi+/−;F8−/− mice transplanted with Tfpi−/− hematopoietic cells (▲) lost significantly less blood than Tfpi+/−;F8−/− mice transplanted with Tfpi+/+ hematopoietic cells (■) (P = 0.00015) following 1-mm tail transection. Tfpi+/+;F8−/− mice transplanted with Tfpi−/− hematopoietic cells (▼) also bled significantly less than Tfpi+/−;F8−/− mice transplanted with Tfpi+/+ hematopoietic cells (■) (P = 0.033). Finally, there was a trend for Tfpi+/−;F8−/− mice transplanted with Tfpi−/− hematopoietic cells (▲) to bleed less than Tfpi+/+;F8−/− mice transplanted with Tfpi−/− hematopoietic cells (▼) (P = 0.067). With 80% power and a common SD of 200, this experiment had the ability to detect a difference in means of 300 units or more.
Lack of Hematopoietic Cell TFPI Activity Produces Increased Clot Volume Following Vascular Injury in F8−/− Mice.
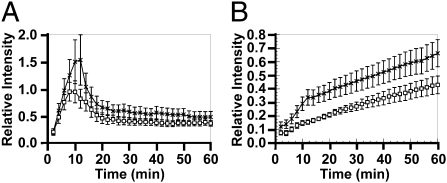
An electrolytic femoral vein injury model (25) was used to investigate how hematopoietic cell TFPI deficiency alters clot formation following vascular injury. In these experiments, Tfpi+/−;F8−/− mice transplanted with Tfpi−/− cells had increased platelet and fibrin deposition compared with mice transplanted with Tfpi+/+ cells. Platelet clot volume peaked at ∼10 min, where it was seen to be ∼50% greater in mice with Tfpi−/− platelets versus mice with Tfpi+/+ platelets (Fig. 5A), although this trend did not reach statistical significance. Fibrin deposition increased continuously and reached statistical significance (P < 0.05) at 10–60 min with increases of 50–100% (Fig. 5B). This increase occurred in the first 10 min, coinciding with the net accumulation of platelets within the thrombus and continued with a parallel increase from 10 to 60 min.
Fig. 5.
Absence of hematopoietic cell TFPI causes increased thrombus volume in F8−/− mice in a femoral vein electrolytic injury model. (A) Accumulation of platelets and (B) fibrin at the site of injury in mice in Tfpi+/−;F8−/− mice transplanted with Tfpi+/+ hematopoietic cells (□; n = 9) or with Tfpi−/− hematopoietic cells (X; n = 15). Data points were determined every 2 min, with error bars showing the SEM. The fibrin accumulation in mice lacking hematopoietic cell TFPI was significantly greater than in mice transplanted with Tfpi+/+ hematopoietic cells at 10–60 min (P < 0.05).
Discussion
A detailed investigation of how TFPI anticoagulant activity contributes to the severity of bleeding in hemophilia was performed through study of mice with combined TFPI and FVIII deficiencies. Key findings include: (i) Total FVIII deficiency does not alter the embryonic lethal phenotype of Tfpi−/− embryos; (ii) Tfpi+/− does not significantly impact the bleeding phenotype of F8−/− mice, but blood loss in tail bleeding assays is reduced when intravascular TFPI activity is blocked by infusion of anti-TFPI antibody; (iii) blood loss in tail bleeding assays is significantly reduced in Tfpi+/−;F8−/− mice with Tfpi−/− hematopoietic cells compared with those with Tfpi+/+ hematopoietic cells. The lack of hematopoietic cell TFPI is furthermore shown to reduce blood loss in tail bleeding assays of Tfpi+/+;F8−/− mice with Tfpi−/− hematopoietic cells compared with Tfpi+/−;F8−/− mice with Tfpi+/+ hematopoietic cells; and (iv) complete elimination of hematopoietic cell TFPI in Tfpi+/−;F8−/− mice produces a phenotype that shows increased fibrin production in clots formed following femoral vein injury. Collectively, these results demonstrate that whereas the physiological functions of TFPI and FVIII do not overlap in a manner that impacts embryonic survival, selective elimination of TFPI from hematopoietic cells, including platelets, reduces bleeding and increases clot volume following vascular injury in mice with hemophilia A.
Mice lacking functional TFPI succumb to an apparent consumptive coagulopathy during development and do not survive embryogenesis (21). This embryonic lethal phenotype can be rescued by breeding Tfpi+/− mice with mice that have low amounts of TF (22) or that lack FVIIa (23), demonstrating that embryonic survival requires a proper physiological balance between pro- and anticoagulant reactions regulating the extrinsic blood coagulation pathway. The failure of FVIII deficiency to rescue Tfpi−/− embryos suggests that unfettered activation of FX by TF–FVIIa results in consumptive coagulopathy that does not require further amplification through the intrinsic coagulation pathway to be devastating for embryonic development.
Previous studies have demonstrated that Tfpi+/− contributes to a severe thrombosis in mice when in the presence of an underlying procoagulant state, such as the factor V Leiden mutation (26) or decreased thrombomodulin function (27). Conversely, studies performed here show that breeding of Tfpi+/− to F8−/− mice does not alter the hemophilia bleeding phenotype, as assessed using whole-blood TEG and tail bleeding assays. Thus, a 50% reduction in TFPI activity is not sufficient to alter hemophilia bleeding in this mouse model. However, treatment of F8−/− mice with an anti-TFPI polyclonal antibody to reduce TFPI activity resulted in decreased blood loss in tail bleeding assays, demonstrating that generalized intravascular inhibition of TFPI activity limits bleeding in this murine model of hemophilia, as has previously been demonstrated in rabbit (18), canine (19), and monkey (20) models of hemophilia A. Interestingly, the lowest concentration of antibody used in these studies, 2.5 mg/kg, which corresponds to about 7.5-fold more antibody than TFPI in mouse plasma on a molar basis, essentially totally blocked plasma TFPI activity and also presumably other forms of intravascular TFPI accessible to antibody binding, such as that expressed on the endothelium surface, yet tail bleeding progressively decreased with increasing concentration of antibody. These data suggested that additional antibody may be required to effectively inhibit sequestered pools of TFPI that are released at the site of vascular injury, such as platelet TFPI, which is localized within platelets and released following dual activation with collagen plus thrombin (10, 11).
Our laboratory has demonstrated that mice lacking hematopoietic TFPI have increased thrombus growth following electrolytic vascular injury (24). Based on these findings, we suggested that a physiological activity of platelet TFPI is the inhibition of blood-borne TF procoagulant activity accumulating within a growing thrombus (24). This hypothesis is supported by the work of Hrachovinová and coworkers, who demonstrated that TF-bearing microparticles, generated by infusion of P-selectin–Ig chimeras, corrected the plasma clotting time and tail vein bleeding time of F8−/− mice (28). In addition, Massberg and coworkers demonstrated that following vascular injury, TFPI is captured by externalized neutrophil nucleosomes and then efficiently degraded by neutrophil serine proteases, producing localized intravascular thrombosis (29). The results from these studies further suggest that the absence of platelet TFPI may improve hemostasis in hemophilia by increasing the functional blood-borne TF activity at the site of vascular injury. To further investigate the function of platelet TFPI in regulating bleeding in F8−/− mice, Tfpi+/−;F8−/− mice transplanted with either Tfpi+/+ or Tfpi−/− hematopoietic cells were produced. Tail bleeding assays were then used to assess the function of hematopoietic cell TFPI in hemostasis. Tfpi+/−;F8−/− mice transplanted with Tfpi+/+ cells bled more than Tfpi+/+/F8−/− mice transplanted with Tfpi−/− cells, that, in turn, bled more than Tfpi+/−;F8−/− mice transplanted with Tfpi−/− cells. Because platelets are the primary hematopoietic cell accumulating at the site of vascular injury, it is most likely a deficiency in platelet TFPI, as opposed to other hematopoietic cells, that produces the decreased bleeding observed in these experiments. In addition, a sequestered pool of TFPI has been identified in endothelial cells that may be released at the site of vascular injury and contribute to the observed phenotype (16). However, because the nontransplanted Tfpi+/−;F8−/− mice and Tfpi+/+/F8−/− mice had identical tail bleeding whereas Tfpi+/−;F8−/− mice transplanted with Tfpi−/− cells had a strong trend (P = 0.067) toward less bleeding than the Tfpi+/+/F8−/− mice transplanted with Tfpi−/− cells, it appears that the effect of endothelial TFPI is best observed in the absence of hematopoietic cell TFPI activity.
A femoral vein vascular injury model using Tfpi+/−;F8−/− mice transplanted with either Tfpi+/+ or Tfpi−/− hematopoietic cells was used as a second model system investigating the function of hematopoietic cell TFPI in F8−/− mice that allows for visualization and quantification of fibrin and platelets within the growing thrombus (25). Consistent with a physiological role for platelet TFPI in the inhibition of blood-borne TF, the mice transplanted with Tfpi−/− cells accumulated more fibrin within the clot over the first 10 min following injury and developed larger thrombi in response to the vascular injury.
As recently reviewed (30), TFPI is produced in several alternatively spliced isoforms that differ in their C-terminal region. Adult mouse tissues produce TFPIβ in all major vascular beds (31) whereas TFPI in mouse platelets, as well as in human platelets, is produced as the highly evolutionarily conserved α isoform (24). The selective production of TFPIα by platelets, compared with the production of TFPIβ by other mouse tissues, suggests that platelet TFPI may have a unique function in the regulation of hemostasis and/or thrombosis. Pharmaceutical agents for treatment of hemophilia that inhibit TFPI are in development (19, 20). The data presented here suggest that development of pharmaceutical agents that specifically target platelet TFPI, but not endothelial TFPI, may allow hemostasis at the site of vascular injury in patients with hemophilia while minimizing the potential risk for thrombosis that could result from total intravascular inhibition of TFPI activity.
Materials and Methods
Transgenic Mice.
The Medical College of Wisconsin Institutional Animal Care and Use Committee approved all animal experiments performed at the Blood Center of Wisconsin and Medical College of Wisconsin. The Ethical Review Council at Novo Nordisk and the Danish Animal Experiments Inspectorate, Ministry of Justice, approved the animal studies conducted at Novo Nordisk. Mice heterozygous for a TFPI mutation (TFPItm1Gjb), such that the first Kunitz domain is not produced (Tfpi+/−), and backcrossed onto the C57BL/6 background for over 10 generations were a gift from George Broze, Jr. (Washington University, St. Louis, MO) (21). Mice homozygous for a disrupted exon 16 of FVIII (B6;129S4-F8tm1Kaz/J) were purchased from The Jackson Laboratory and Taconic (32). F8−/− mice were on a mixed background of C57BL/6J and 129SvPas. Female F8−/− mice were bred with male Tfpi+/− mice to produce doubly heterozygous offspring. These mice were then mated to produce Tfpi+/−; F8−/− offspring.
Timed Matings.
Tfpi+/−;F8−/− female and male mice were mated. Embryos were harvested at various gestational ages and genotyped for the TFPItm1Gjb mutation. The gestational age of litters was determined by embryo morphology (33).
Fetal Liver Transplantation.
Fetal livers were harvested at embryonic day 14.5 and Tfpi+/+ or Tfpi−/− cell suspensions were transplanted into lethally irradiated recipient male Tfpi+/+;F8−/− or Tfpi+/−;F8−/− mice as previously described (24) to generate mice containing Tfpi+/+ or Tfpi−/− hematopoietic cells.
Thrombelastography Assays.
Whole blood was collected by either vena cava or aorta puncture and directly made into 10% volume 0.13 M sodium citrate. Assays were performed with 340 μL of blood and 20 μL of 200 mM calcium chloride without further activation of coagulation, using a TEG instrument (thrombelastograph coagulation analyzer; Haemoscope) for analysis. The following parameters were recorded: clot time (R) defined as time to a 2-mm amplitude of the TEG trace, and maximal velocity of clot formation (α angle) defined as the peak level of the first derivative of the TEG trace.
Tail Bleeding Assays.
Tail bleeding assays were performed in two different laboratories using slightly different techniques based on the approved protocols at the respective institutions. In studies examining the effect of anti-TFPI antibody, F8−/− mice anesthetized using 80 mg/kg sodium pentobarbital i.p. were placed on their abdomen with the tail immersed in 37 °C saline. Different amounts of polyclonal rabbit anti-mouse TFPI antibody (NNC 0172-0000-1000; Novo Nordisk) or saline control were injected into a lateral tail vein 5 min before the distal 4 mm of the tail was transected. After 20 min, the tail was removed from the saline and blood loss was determined following centrifugation and lysis of red blood cells, measuring the amount of hemoglobin spectrophotometrically at 550 nm. In this assay, the central artery is transected and bleeding is arterial and venous. Alternatively, tail bleeding assays with mice having combined genetic deficiency of FVIII and TFPI were performed using essentially the same technique except that anesthesia was induced with 120 mg/kg ketamine and 15 mg/kg xylazine i.p. followed by 1-mm transection of the tail with removal from the saline after 10 min. In this assay the central artery is not transected and the bleeding is primarily venous.
Plasma TFPI Activity.
Blood was sampled 30 min postadministration of polyclonal rabbit anti-mouse TFPI antibody or saline control. Functional plasma TFPI was estimated using the Actichrome TFPI Activity Assay, which is a chromogenic assay for measuring the ability of TFPI to inhibit the catalytic activity of FXa in human plasma (American Diagnostica). The assay was performed in compliance with the manufacturer's instructions, but due to species differences the human TFPI calibrator could not be applied and the optimal sample dilution in terms of response window was found to be 50-fold in contrast to the 20-fold recommended for human samples. The response window was defined as the difference in FXa activity between samples from control animals and the same samples spiked in vitro with 100 μg/mL of the polyclonal rabbit anti-mouse TFPI antibody, thus representing full saturation of functional plasma TFPI.
Venous Injury Model.
In vivo thrombus formation was evaluated in Tfpi+/−;F8−/− fetal liver recipient mice at 8–12 wk posttransplantation using an electrolytic femoral vein injury model (25). In brief, anesthesia was induced using i.p. sodium pentobarbital (50 mg/kg). Mice were preinjected with platelets obtained from donor mice of the same genotype/transplant group that were prelabeled with Vybrant DiD (Invitrogen); monoclonal anti-fibrin antibody, which does not bind fibrinogen (hybridoma cells were a gift from Marschall Runge, University of North Carolina, Chapel Hill, NC), was labeled with Alexa Fluor-532 (Invitrogen) and coinjected. Electrolytic injuries were created in femoral veins using a steel microsurgical needle applied to the outer surface of each vessel, with 1.5 V of positive direct current delivered for 30 s. Vessels were illuminated uniformly with beam-expanded green (532 nm) and red (650 nm) laser light. Fluorescent images were captured over a 60-min interval with a low-light video camera attached to an operating microscope at 100× magnification; video images were taken every 2 min for analysis of relative fluorophore intensity (ImageJ software; National Institutes of Health) within the thrombus zone and normalized for interanimal comparisons.
Statistics.
The main analysis tool used in the TEG and tail bleeding times in Figs. 2 and 5 is Welch's t test, which does not assume equal variances, with the familywise type 1 error rate controlled at 5% using Holm's method. Adjusted P values are reported. In addition, the values in Fig. 2 were log-transformed before the analysis to improve normality. A two-tailed t test using GraphPad Prism software was used to assign significance to the venous injury model evaluated at 10, 20, 30, and 60 min. Blood loss in the studies investigating the effect of the anti-TFPI antibody was analyzed using ANOVA and linear regression analysis. A probability value of <0.05 was used to assign statistical significance.
Acknowledgments
We thank Annette Jøns and Lene Kureer for expert technical assistance, and Aniko Szabo for statistical assistance. This work was funded by National Heart, Lung, and Blood Institute Grants HL068835 (to A.E.M.), HL091469 (to S.A.M.), and EB007582 (to B.C.C.), by a research grant from Novo Nordisk (to A.E.M.), and by Novo Nordisk (P.B.J., M.B.H., and L.C.P. are employees of Novo Nordisk).
Footnotes
Conflict of interest statement: A.E.M. receives research grant funding from Novo Nordisk. P.B.J., M.B.H., and L.C.P. are employees of Novo Nordisk.
This article is a PNAS Direct Submission.
References
- 1.Davie EW, Ratnoff OD. Waterfall sequence for intrinsic blood clotting. Science. 1964;145:1310–1312. doi: 10.1126/science.145.3638.1310. [DOI] [PubMed] [Google Scholar]
- 2.MacFarlane RG. An enzyme cascade in the blood clotting mechanism, and its function as a biochemical amplifier. Nature. 1964;202:498–499. doi: 10.1038/202498a0. [DOI] [PubMed] [Google Scholar]
- 3.Broze GJ., Jr The role of tissue factor pathway inhibitor in a revised coagulation cascade. Semin Hematol. 1992;29(3):159–169. [PubMed] [Google Scholar]
- 4.Repke D, et al. Hemophilia as a defect of the tissue factor pathway of blood coagulation: Effect of factors VIII and IX on factor X activation in a continuous-flow reactor. Proc Natl Acad Sci USA. 1990;87:7623–7627. doi: 10.1073/pnas.87.19.7623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nordfang O, Valentin S, Beck TC, Hedner U. Inhibition of extrinsic pathway inhibitor shortens the coagulation time of normal plasma and of hemophilia plasma. Thromb Haemost. 1991;66:464–467. [PubMed] [Google Scholar]
- 6.Welsch DJ, Novotny WF, Wun TC. Effect of lipoprotein-associated coagulation inhibitor (LACI) on thromboplastin-induced coagulation of normal and hemophiliac plasmas. Thromb Res. 1991;64:213–222. doi: 10.1016/0049-3848(91)90120-l. [DOI] [PubMed] [Google Scholar]
- 7.van 't Veer C, Hackeng TM, Delahaye C, Sixma JJ, Bouma BN. Activated factor X and thrombin formation triggered by tissue factor on endothelial cell matrix in a flow model: Effect of the tissue factor pathway inhibitor. Blood. 1994;84:1132–1142. [PubMed] [Google Scholar]
- 8.Baugh RJ, Broze GJ, Jr, Krishnaswamy S. Regulation of extrinsic pathway factor Xa formation by tissue factor pathway inhibitor. J Biol Chem. 1998;273:4378–4386. doi: 10.1074/jbc.273.8.4378. [DOI] [PubMed] [Google Scholar]
- 9.Bajaj MS, Kuppuswamy MN, Saito H, Spitzer SG, Bajaj SP. Cultured normal human hepatocytes do not synthesize lipoprotein-associated coagulation inhibitor: Evidence that endothelium is the principal site of its synthesis. Proc Natl Acad Sci USA. 1990;87:8869–8873. doi: 10.1073/pnas.87.22.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Novotny WF, Girard TJ, Miletich JP, Broze GJ., Jr Platelets secrete a coagulation inhibitor functionally and antigenically similar to the lipoprotein associated coagulation inhibitor. Blood. 1988;72:2020–2025. [PubMed] [Google Scholar]
- 11.Maroney SA, et al. Active tissue factor pathway inhibitor is expressed on the surface of coated platelets. Blood. 2007;109:1931–1937. doi: 10.1182/blood-2006-07-037283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Novotny WF, Girard TJ, Miletich JP, Broze GJ., Jr Purification and characterization of the lipoprotein-associated coagulation inhibitor from human plasma. J Biol Chem. 1989;264:18832–18837. [PubMed] [Google Scholar]
- 13.Ott I, et al. Regulation of monocyte procoagulant activity in acute myocardial infarction: Role of tissue factor and tissue factor pathway inhibitor-1. Blood. 2001;97:3721–3726. doi: 10.1182/blood.v97.12.3721. [DOI] [PubMed] [Google Scholar]
- 14.Caplice NM, et al. Expression of tissue factor pathway inhibitor in vascular smooth muscle cells and its regulation by growth factors. Circ Res. 1998;83:1264–1270. doi: 10.1161/01.res.83.12.1264. [DOI] [PubMed] [Google Scholar]
- 15.Bajaj MS, Birktoft JJ, Steer SA, Bajaj SP. Structure and biology of tissue factor pathway inhibitor. Thromb Haemost. 2001;86:959–972. [PubMed] [Google Scholar]
- 16.Lupu C, Lupu F, Dennehy U, Kakkar VV, Scully MF. Thrombin induces the redistribution and acute release of tissue factor pathway inhibitor from specific granules within human endothelial cells in culture. Arterioscler Thromb Vasc Biol. 1995;15:2055–2062. doi: 10.1161/01.atv.15.11.2055. [DOI] [PubMed] [Google Scholar]
- 17.Lupu C, Kruithof EK, Kakkar VV, Lupu F. Acute release of tissue factor pathway inhibitor after in vivo thrombin generation in baboons. Thromb Haemost. 1999;82:1652–1658. [PubMed] [Google Scholar]
- 18.Erhardtsen E, et al. Blocking of tissue factor pathway inhibitor (TFPI) shortens the bleeding time in rabbits with antibody induced haemophilia A. Blood Coagul Fibrinolysis. 1995;6:388–394. doi: 10.1097/00001721-199507000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Prasad S, et al. Efficacy and safety of a new-class hemostatic drug candidate, AV513, in dogs with hemophilia A. Blood. 2008;111:672–679. doi: 10.1182/blood-2007-07-098913. [DOI] [PubMed] [Google Scholar]
- 20.Waters EK, et al. Aptamer ARC19499 mediates a procoagulant hemostatic effect by inhibiting tissue factor pathway inhibitor. Blood. 2011;117:5514–5522. doi: 10.1182/blood-2010-10-311936. [DOI] [PubMed] [Google Scholar]
- 21.Huang ZF, Higuchi D, Lasky N, Broze GJ., Jr Tissue factor pathway inhibitor gene disruption produces intrauterine lethality in mice. Blood. 1997;90:944–951. [PubMed] [Google Scholar]
- 22.Pedersen B, Holscher T, Sato Y, Pawlinski R, Mackman N. A balance between tissue factor and tissue factor pathway inhibitor is required for embryonic development and hemostasis in adult mice. Blood. 2005;105:2777–2782. doi: 10.1182/blood-2004-09-3724. [DOI] [PubMed] [Google Scholar]
- 23.Chan JC, et al. Factor VII deficiency rescues the intrauterine lethality in mice associated with a tissue factor pathway inhibitor deficit. J Clin Invest. 1999;103:475–482. doi: 10.1172/JCI5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maroney SA, Cooley BC, Ferrel JP, Bonesho CE, Mast AE. Murine hematopoietic cell tissue factor pathway inhibitor limits thrombus growth. Arterioscler Thromb Vasc Biol. 2011;31:821–826. doi: 10.1161/ATVBAHA.110.220293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooley BC. In vivo fluorescence imaging of large-vessel thrombosis in mice. Arterioscler Thromb Vasc Biol. 2011;31:1351–1356. doi: 10.1161/ATVBAHA.111.225334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eitzman DT, et al. Lethal perinatal thrombosis in mice resulting from the interaction of tissue factor pathway inhibitor deficiency and factor V Leiden. Circulation. 2002;105:2139–2142. doi: 10.1161/01.cir.0000017361.39256.82. [DOI] [PubMed] [Google Scholar]
- 27.Maroney SA, Cooley BC, Sood R, Weiler H, Mast AE. Combined tissue factor pathway inhibitor and thrombomodulin deficiency produces an augmented hypercoagulable state with tissue-specific fibrin deposition. J Thromb Haemost. 2008;6(1):111–117. doi: 10.1111/j.1538-7836.2007.02817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hrachovinová I, et al. Interaction of P-selectin and PSGL-1 generates microparticles that correct hemostasis in a mouse model of hemophilia A. Nat Med. 2003;9:1020–1025. doi: 10.1038/nm899. [DOI] [PubMed] [Google Scholar]
- 29.Massberg S, et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med. 2010;16:887–896. doi: 10.1038/nm.2184. [DOI] [PubMed] [Google Scholar]
- 30.Maroney SA, Ellery PE, Mast AE. Alternatively spliced isoforms of tissue factor pathway inhibitor. Thromb Res. 2010;125(Suppl 1):S52–S56. doi: 10.1016/j.thromres.2010.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maroney SA, et al. Temporal expression of alternatively spliced forms of tissue factor pathway inhibitor in mice. J Thromb Haemost. 2009;7:1106–1113. doi: 10.1111/j.1538-7836.2009.03454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bi L, et al. Targeted disruption of the mouse factor VIII gene produces a model of haemophilia A. Nat Genet. 1995;10(1):119–121. doi: 10.1038/ng0595-119. [DOI] [PubMed] [Google Scholar]
- 33.Theiler K. The House Mouse: Atlas of Embryonic Development. New York: Springer; 1989. [Google Scholar]