Abstract
Whole, fertile plants of Silene stenophylla Ledeb. (Caryophyllaceae) have been uniquely regenerated from maternal, immature fruit tissue of Late Pleistocene age using in vitro tissue culture and clonal micropropagation. The fruits were excavated in northeastern Siberia from fossil squirrel burrows buried at a depth of 38 m in undisturbed and never thawed Late Pleistocene permafrost sediments with a temperature of −7 °C. Accelerator mass spectrometry (AMS) radiocarbon dating showed fruits to be 31,800 ± 300 y old. The total γ-radiation dose accumulated by the fruits during this time was calculated as 0.07 kGy; this is the maximal reported dose after which tissues remain viable and seeds still germinate. Regenerated plants were brought to flowering and fruiting and they set viable seeds. At present, plants of S. stenophylla are the most ancient, viable, multicellular, living organisms. Morphophysiological studies comparing regenerated and extant plants obtained from modern seeds of the same species in the same region revealed that they were distinct phenotypes of S. stenophylla. The first generation cultivated from seeds obtained from regenerated plants progressed through all developmental stages and had the same morphological features as parent plants. The investigation showed high cryoresistance of plant placental tissue in permafrost. This natural cryopreservation of plant tissue over many thousands of years demonstrates a role for permafrost as a depository for an ancient gene pool, i.e., preexisting life, which hypothetically has long since vanished from the earth's surface, a potential source of ancient germplasm, and a laboratory for the study of rates of microevolution.
Keywords: plants of Late Quaternary, Beringia ice complex, paleoenvironment, ancient genetic resources, natural cryobank
The long-term conservation of viable biological material is an important scientific challenge. Low and extreme low temperature preservation is often used (1–3) and the most widespread natural subzero depository, permafrost (∼20% of the earth's surface), is now under extensive investigation. This considerable frozen mass, up to several hundred meters deep, harbors a vast variety of viable ecological and morphological microbial groups: anaerobic and aerobic, spore-forming and non–spore-forming bacteria, green algae and cyanobacteria, yeast, actino- and micromycetes, and protozoa (4, 5). All have survived under permafrost conditions since the time of its formation.
The age of retrieved and cultivated biota corresponds to the longevity of the permanently frozen state of the embedding strata and dates back tens to hundreds of thousands of years and even more. Permafrost thawing due to anthropogenic or natural impacts exposes relict life to modern ecosystems where the ancient biota may resume physiological activity and may become part of present-day biological systems and processes. Microorganisms and their metabolic end products, the pigments chlorophyll and phaeophytin, biologically active free intra- and extracellular enzymes, biogenic gases and bacterial, archaeal, and fungal DNA occur in permafrost (6–8). Permanently frozen deposits also contain ancient evidence of higher plants, e.g., DNA preserved from 10 to 400 kya, for which plant DNA sequences are known (9) as well as viable spores of moss species. However, to date, no viable flowering plant remains have been discovered from these ancient permafrost sediments.
Outside the permafrost zone the longevity of seeds in soil has been studied during the last 45 y in many places, including archaeological sites (10, 11). At the moment, the oldest viable seeds with the ability to germinate were radiocarbon dated to the first and eighth centuries of the Common Era. These are, respectively, Phoenix dactylifera found near the Dead Sea (12) and Nelumbo nucifera found in northeastern China (13).
The burrows from which our study material derived were buried in permanently frozen loess-ice deposits on the right bank of lower Kolyma River, northeastern Siberia (14). These deposits (Fig. 1A) are representative of a widely distributed, so-called Late Pleistocene ice complex found throughout the eastern Arctic, which has continuously accumulated during the last 60 ky. This complex comprises icy silts with a network of large syngenetic polygonal ice wedges and is richly fossil bearing (pollen, insects, plants, macrofossil, and mammal fossils). The studied burrows (Fig. 1B) were found in three exposures: Zelyony Mys, Stanchikovsky Yar, and Duvanny Yar. The last represents a key cross-section of Late Quaternary environmental history in the eastern Arctic studied by many scientists. All burrows were found at depths of 20–40 m from the present day surface and located in layers containing bones of large mammals such as mammoth, wooly rhinoceros, bison, horse, deer, and other representatives of fauna from the age of Mammoths as well as plant remnants. The deposits were formed under tundra–steppe conditions. The Late Pleistocene age marine isotope stage 3 (MIS 3), a period between 60 and 27 ky ago during the last glacial cycle of these layers, was previously confirmed by radiocarbon, oxygen-isotope, and palynological analyses of plant and bone remnants (15, 16). The sediments have high ice content (35–80%). The present day mean annual ground temperature on the studied watershed is −7 °C, and the depth of seasonal thawing ranges, depending on the landscape features, from 40 to 70 cm.
Fig. 1.
Late Pleistocene loess-ice deposits of Duvanny Yar, Kolyma Lowland. (A) General view. (B) Burrow of ground squirrel buried in permafrost deposits.
The ice complexes formed immediately on sedimentation, with concurrent freezing from below and have remained undisturbed since their formation. No events of permafrost degradation were observed in this continuous Late Pleistocene sequence. The presence of vertical ice wedges demonstrates that it has been continuously frozen and never thawed. Accordingly, the fossil burrows and their content have never been defrosted since burial and simultaneous freezing (17).
Seventy fossil burrows were found and more than 30 of them have been investigated so far. The plant material from fossil burrows has been dated by the radiocarbon method as being 28–32 ky old (Table 1). During burrow construction by animals, the chambers with seed and fruit storage were built against the frozen boundary of the permafrost sediments. This feature of storage chamber construction allowed high quality preservation of their biological material.
Table 1.
Radiocarbon age of material taken from fossil burrows of Kolyma Lowland (Northern Yakutia)
| Cross-section | Burrow | Material | Radiocarbon age, y |
| Zelyony Mys | Р-923 | Plant remnants | 32,800 ± 400 (IEMEZH 1178)* |
| Zelyony Mys | Р-917 | Plant remnants | 30,500 ± 700 (IEMEZH 1179) |
| Stanchikovski Yar | Р-1010 | Plant remnants | 27,700 ± 300 (GIN 10874) |
| Stanchikovski Yar | Р-820 | Seeds and fruits | 28,200 ± 600 (IEMEZH 1190) |
| Duvanny Yar | Р-1075 | Seeds and fruits | 31,800 ± 310 (Beta 157195) |
*The laboratory number of the sample given by the institute that carried out the analysis is indicated in parentheses. IEMEZH, transliteration from the Russian abbreviation of the former name of the Institute of Ecology and Evolution, Russian Academy of Sciences; GIN, Geological Institute, Russian Academy of Sciences; Beta, Beta Analytic.
The storage chambers in the fossil burrows contain a great supply of plant seeds and fruits. The number of seeds and fruits reaches up to 600–800 thousand in some chambers.
Direct in situ measurements of γ-radiation were made in the borehole crossing the burrow buried horizon on Duvanny Yar. The observation showed that in ice complex the ground radiation level provided by natural radionuclides was 0.23 μGy/h or 2.01 mGy/y on average. These data correlate well with an estimate of ground radiation made using elemental analysis of the radioactive elements in ice complex samples from other boreholes in this area: ∼2 mGy/y (18) and they are similar to the ground radiation levels reported for natural radionuclides surrounding the above-mentioned seeds of N. nucifera in China. In this case, the total γ-radiation dose accumulated by 1,300-y-old fruits was calculated at −3 Gy. On the basis of these data, the total γ-radiation dose accumulated by 28- to 32-ky-old fruits was calculated as 0.07 kGy. This is now the maximal reported dose after which tissues remain viable and seeds can still germinate.
Observations of seed viability in relation to environment show that their longevity increases as the seed storage temperature and moisture decrease (19, 20). This same situation is seen for seed viability in the permafrost. First, at subzero temperatures the rates of biochemical reactions and biological processes become extremely slow and ensure preservation of the biological system. Second, in frozen ground, ice makes up 93–98% of total water volume (21); this frozen environment is effectively a “biologically dry” environment and favors conservation (22). There is only one previous report of plants (Lupinus arcticus) grown from seeds deeply buried within lemming burrows in permanently frozen silt in the central Yukon (23). However, after 40 y these seeds were dated using the accelerator mass spectrometry radiocarbon method (AMS) and it was concluded that they were not of Pleistocene age (>10 ky old), but modern seeds that apparently contaminated the ancient sample (24).
The aim of our studies was regeneration of plants from seeds and fruits buried in the Late Pleistocene permafrost, 28–32 ky old and excavated from fossil burrows (Fig. 1) of Urocitellus parryii (an Arctic species of ground squirrel).
Previous studies (25) revealed the return of some physiological activity to the cells and tissues of seeds and fruits of four plant species from burrows in the permafrost deposits during in vitro culture. Enlargement of the cotyledon was detected in a sedge seed (Carex sp., Cyperaceae) from burrow P-923 of Zelyony Mys locality (Table 1). Cell division activity was detected in several radicles of Arctous alpina (Ericaceae). The burrows P-1026 and P-1068 (Stanchikovsky Yar exposure) with Arctous seeds are located at the same level and near burrow P-1010, for which radiocarbon analysis has been done. Radicle activation was also detected in seeds of Rumex arcticus (Polygonaceae) from burrow P-1075. One Rumex seed germinated up to the cotyledon release stage but the primary shoot did not develop because of shoot apical meristem degradation. The cotyledon explants isolated from this seedling were cultured on agar media and showed initiation of callusing but did not continue further. Eruptions of radicle and subsequent callusing occurred in vitro in Silene stenophylla Ledeb. seeds from burrow P-923 of the Zelyony Mys exposure. However, further proliferation of callus tissue was aborted as was the case with R. arcticus. Primary root development also stopped because of damaged embryo and storage tissues. Nevertheless, these preliminary results showed a real possibility for obtaining material for whole plant regeneration.
In these earlier studies, we found that the seeds of S. stenophylla were the most promising for further investigation because they had some physiological vitality and viability. Seeds of this species are dark in color and relatively small (0.6 mm wide, 0.8 mm long). S. stenophylla is a perennial herbaceous plant from the family Caryophyllaceae. Seeds of wild species of this family demonstrated high resistance to both nondeep and deep freezing (26). Abundant and numerous seeds and fruits of S. stenophylla were found in most of the burrows. Many experiments were carried out with seeds and with both open (ripe) and closed (unripe) fruits of this species. Seeds taken from immature fruits often had a funiculus, a seedstalk, by which the seed was attached to the placenta. These funiculi showed tissue enlargement in vitro (27) and, importantly, these observations led us to focus on investigations of immature fruit tissues of ancient Silene. Here, we describe the regeneration of whole plants from placental tissue of such fruits.
Results
Several immature fruits were found at a depth of 38 m in a seed conglomerate from the Duvanny Yar burrow P-1075 (Table 1). Silene seeds and fruits were dominant in this burrow and were in a state of good morphological preservation. AMS radiocarbon dating showed them to be 31,800 ± 300 y old (Beta-157195).
Organogenesis of Shoots and Microclonal Propagation.
Our in vitro tissue culture method was adopted from one for the regeneration of ancient plants (28, 29). Organogenesis of adventitious shoots was induced in vitro directly from fragments of the placental tissue of three immature uninjured fruits. A modification of Murashige and Skoog (30) and Anderson (31) nutrient media was used for initiation of organogenesis and multiplication of shoots. Apart from ancient plants, those from extant seeds of the same species, and from the same region, were grown in vitro as a control. During micropropagation in vitro the ancient and extant plants were different, with the ancient plants producing up to 1.5–2 times more buds, whereas the extant plants produced roots more rapidly.
Vegetative Growth and Flowering.
Rooted plants were transplanted to plastic pots with appropriate soil and placed in a growth room with controlled light and temperature. Under these conditions, plants grew and developed flowers and fruits and set seeds (Fig. 2). As is typical for S. stenophylla as a perennial plant, flowering and seed set occurred during the second year of the potted plants’ cultivation.
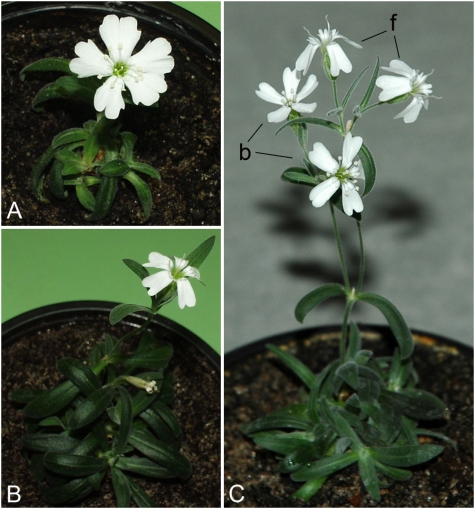
Fig. 2.
Fruiting plants of Silene stenophylla regenerated from tissue of fossil fruits. (Scale bar, 50 mm.)
Thirty-six ancient plants (12 from each fruit) and 29 extant plants were morphologically tested. All ancient plants were morphologically identical. During vegetative development, the ancient and extant plants were morphologically indistinguishable from one another. However, at the flowering stage they showed different corolla shape: petals of extant flowers were obviously wider and more dissected (Fig. 3). Moreover, all flowers of the extant plants were bisexual (b) (Fig. 3A), whereas the primary flowers (two to three in number) of each ancient plant were strictly female (f) (Fig. 3 B and C, f), and then bisexual flowers were formed on each ancient plant (Fig. 3C, b).
Fig. 3.
Flowering plants of Silene stenophylla. (A) Plant grown in vitro culture from seed of an extant plant. (B) Plant regenerated in vitro culture from tissue of fossil fruit with primary strictly female flower. (C) Plant regenerated from tissue of fossil fruit with both female (f) and bisexual (b) flowers.
Seed Production.
The plants were tested for their sexual fertility. It should be noted that S. stenophylla is allogamous and requires cross-fertilization for sexual reproduction to occur. Flowers of the ancient plants were pollinated artificially using pollen from other ancient plants; pollination of extant plants was performed similarly. The time from artificial pollination of flowers to ripening of first seeds took 8–9 wk. Laboratory germination of seeds taken from regenerated ancient plants was 100% and that of seeds from control plants was 86–90%. The first generation cultivated from seeds obtained from regenerated plants progressed through all developmental stages and showed the same morphological features as the parent plants. The taxonomic identification of both ancient and extant regenerated plants was verified at the Department of Higher Plants, Moscow State University, Moscow, Russia. The ancient plants were considered to be a distinct phenotype from the extant plants.
Discussion
The present study uniquely demonstrates that viable placental tissue, from immature fruits of the flowering plant S. stenophylla is preserved in Late Pleistocene sediments. These sediments date between 30,000 and 32,000 y in age and were deposited in an undisturbed permafrost environment. Under tissue culture and micropropagation, the placental tissue was able to differentiate and grow to become fertile adult plants.
The principal points of discussion in support of this discovery are: The provenance and age of the fruits, the basis for preservation, the resilience of placental tissue, phenotypic plasticity, and the opportunities provided by permafrost environments for preserving ancient and modern germplasm of higher plants.
Provenance.
The fruits, from which placental tissue was obtained, came from similar and immediately adjacent ice conglomerates that were used to establish the age of the deposits. We dated the age of the seeds from the closed fruits and regenerated the plants from the placental tissue to which these very seeds were attached in the process of their formation. Thus, we ensured “direct radiocarbon dating.”
In view of the Porsild et al. (23) paper on seeds of Lupinus from the Yukon and its refutation by Zazula et al. (24), showing that contamination of old seed by recent seed had occurred in lemming burrows within Pleistocene deposits, we were careful to assess the possibility that this may have happened with our material. We have high confidence that no contamination took place after the onset of freezing. The fossil burrows are well below the seasonal thawing (active) layer and more than 35 m below the permafrost table. Further, we know of no animals that burrow deeper than the active layer. The permafrost table is the upper boundary of deep sediments within which physical and biogeochemical exchanges occur and through which external factors and processes do not cross. The embedding over- and underlying sediments are firmly cemented together and are often totally filled in by ice. These sediments represent a closed system where there are no water-bearing horizons or water infiltration. Thus, within the permafrost sediments the penetration of seeds to the deeper frozen layers could not have occurred from outside; they exist in situ. Further, penetration along pores can also be virtually ruled out because the diameter of the average pore is less than 0.1 mm, compared with seed diameters of 0.6–0.8 mm and diameters of 2–4 mm for fruits.
Basis for Preservation.
The food storage chambers of squirrels are built adjacent to ice wedges and frozen sediment. The material within burrows and the embedding strata provide important evidence of rapid freezing and subsequent preservation without defrosting. Gubin et al. (17) have demonstrated that studied individual burrows have only a single entrance at ground level and the fossil burrows were sealed by loess and became rapidly and permanently frozen. As cited in the Introduction, it is well known that representatives of nearly all single-cell and lower plant biota can survive over long geological periods at subzero temperatures and biologically dry milieu of the permafrost. The long-term subzero temperature regime of the permafrost preserves living tissue through stabilization via dehydration, which leads to a considerable decrease of biochemical and metabolic activities. Thus, it is reasonable to expect that higher plant tissues, especially those associated with reproductive processes, can be expected to survive better within the permafrost sediments than in any other known habitat. It is also possible that low doses of radiation also might have an effect on the viability of biological objects (13, 32).
Tissue Resilience.
Among the various approaches used to seek the viability of seed and fruit material in this study, only the placental tissues from immature fruits had sufficient vitality to be cultured and grown to whole, healthy, fertile adult plants. An explanation for the success with placental tissue may lie with the high metabolic activity of these tissues as reported by Pontovich (33). The placenta is involved in the regulation and transport of nutrient substances synthesized in its own tissues as well as in other tissues of a plant. The high concentration of organic substances, including sucrose, responsible for the cryoresistance, is the likely reason that allowed high-quality preservation of fruits and their contents in permafrost. The morphogenic factor, composed of active phenolic compounds, is localized in the placenta (33). Phenolic compounds, accumulating in plant tissues under low temperatures, are well known for their protective function and may be produced as a stress response. The high morphogenic potential of placental tissue of the fossil fruits observed here may, therefore, result from special metabolic properties not only of the placental tissue itself but of the whole organism, which developed under the cold, dry climatic conditions of Late Pleistocene. Well-preserved placentae were observed only in fruits with intact and undamaged pericarp. The good preservation of placenta is probably further due to the hermetic conditions in the immature fruit. Finally, immature tissues and organs are known to be more regenerative in vitro than mature tissues and organs (29).
Phenotypic Plasticity.
Phenotypic plasticity, as a response to temperature conditions and photoperiod, has been reported for other members of Caryophyllaceae (34, 35). The genotype of the ancient plants may be different from that of the modern plants and may have developed under the severe climatic conditions of Late Pleistocene. The revealing of sexual dimorphism of ancient plants may also indicate that ontogeny of paleo- and extant plants were formed under different ecological conditions (36).
Significance.
Naturally occurring permanently frozen sediments offer an important opportunity for the discovery of wild plant species, preservation of biological material, studying the conditions for cryopreservation, and developing germplasm collections. We consider it essential to continue permafrost studies in search of an ancient genetic pool, that of preexisting life, which hypothetically has long since vanished from the earth's surface.
Conclusion
Late Pleistocene plant tissue of S. stenophylla, naturally preserved in permafrost, can be regenerated using tissue culture and micropropagation to form healthy sexually reproducing plants. The source of the ancient tissue is the placentae of immature S. stenophylla fruits found in buried squirrel caches. At present, plants of S. stenophylla are the unique representative of ancient higher plants to be cultivated successfully. Their high cryoresistance over many thousands of years demonstrates the value of permafrost sediments as a cryodepository, a potential source of ancient germplasm, and a natural laboratory for studies of crypopreservation of ancient genetic resources and rates of microevolution. In this sense, development of the Svalbard Global Seed Vault (Spits Bergen, Norway) is of great interest and importance. Duplicates of seeds of cultivated plants from different germplasm collections are to be stored in this institution (www.nordgen.org/sgsv/).
The squirrel burrows with seeds have been identified within Late Pleistocene ice complex not only in eastern Siberia, but also in Alaska and Yukon (37, 38). This indicates that the whole Beringia has a great potential as storage of ancient life preserved in permafrost. Further investigations of morphological, functional, biochemical, and cytogenetic features of regenerated plants, as well as their seminal progeny, and comparison with extant species will allow us to obtain new data on the mechanisms of cryoresistance of plant cells, their adaptation potential, and additional information about environmental conditions of Late Pleistocene.
Materials and Methods
Biological Samples.
Many seeds and fruits from collection sites in permafrost have well preserved coats (testa and pericarp), which allow their taxonomic identification (39). Our collection of extant seeds and fruits of Kolyma lowland plants, along with seed collections of the Botanical Institute, Russian Academy of Sciences, St. Petersburg, Russia, were used for identification of ancient seeds. To date, 38 species are identified. The identity of S. stenophylla seeds was also confirmed by scanning electron microscopy using TESLA BS-300 (40).
The accumulation of seeds and fruits in fossil burrows appeared as icy conglomerates. Seeds and fruits excavated from fossil burrows were immediately transferred to sterile bags, kept frozen in the field, and transported frozen to the laboratory at a temperature of −10 °C. Samples selected for the study were defrosted and examined by light microscopy to select undamaged firm seeds and fruits for investigation.
Media for Culture in Vitro.
The following media were used: Medium 1: Murashige and Skoog basal nutrient medium (MS) rich in nutrients was supplemented with vitamins (41), ascorbic acid 2 mg l−1, glycine 2 mg l−1, myo-inositol 100 mg l−1, casein hydrolysate 500 mg l−1, 6-benzylaminopurine (BAP) 2 mg l−1, kinetin 1 mg l−1, dichlorophenoxyacetic acid (2,4-D) 2 mg l−1, gibberellic acid (GA) 1 mg l−1, and 3% sucrose. Medium 2: same as medium 1, to which 10% coconut milk was added. Medium 3: a nutrient-poor medium, consisting of the basal MS medium with sucrose concentration reduced to 1% and supplemented only with kinetin (0.2 mg l−1) and indole-3-acetic acid (IAA) (0.1 mg l−1). Modified Anderson basal nutrient medium (ABM) (31), with monobasic sodium phosphate replaced by monobasic potassium phosphate and supplemented with ascorbic acid 2 mg l−1, glycine 2 mg l−1, myo-inositol 100 mg l−1, adenine 30 mg l−1, kinetin 0.5 mg l−1, BAP 1 mg l−1, GA 2 mg l−1, IAA 0.1 mg l−1, 1% sucrose, and vitamins (41).
Methods.
Preparation of tissue samples for culture.
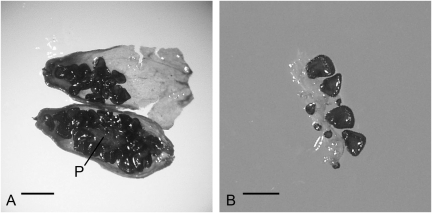
Immature fruits were carefully cleaned, washed with tap water, and successively surface sterilized with 0.1% solution of corrosive sublimate (mercury bichloride HgCl2) with addition of Tween-80 (0.4 mL in 1,000 mL of solution) and with 70% ethanol. The tissue was washed with sterile distilled water after each disinfectant. Right after disinfection, fruits were dissected under sterile conditions. A dissected, immature fruit of S. stenophylla, having placental tissue and a fragment of the placenta retained with attached seeds at different developmental stages, is shown in Fig. 4. Fragments of the placenta were cultivated in vitro on media 1–3. Reagents were purchased from Sigma-Aldrich.
Fig. 4.
Immature fruit of Silene stenophylla from burrow buried in permafrost more than 30,000 y ago. (A) Dissected fruit showing seeds and placenta (P). (B) Fragment of placenta with seeds at different developmental stages. (Scale bars, 1 mm.)
Explant culture.
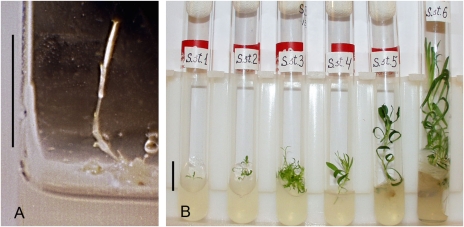
Explants, isolated from placentae of three different immature fruits, were cultured on the three different media (1–3) described above. Organogenesis was observed on both rich- and poor-nutrient media. Successful initiation of shoots on different media occurred, presumably due to high morphogenic and physiological potential of the placental tissue. The first shoot initiated from placental tissue is shown in Fig. 5A. The first formed shoots were etiolated as a result of being cultivated under dark conditions. When the cultures were transferred to sufficient light, shoots synthesized chlorophyll and made active growth. After 5–20 d, numerous adventitious shoots were being formed. On the poor medium, primary shoot initiation was retarded up to 7–9 d, compared with explants cultivated on both rich media (medium 1 and medium 2). Morphogenesis took place only in placental tissues from unripe fruits.
Fig. 5.
Clonal micropropagation of Silene stenophylla regenerated from placenta tissue of immature 30,000-y-old fruits buried in permafrost deposits. (А) Initial shoot initiated from placental tissue in vitro. (В) Stages of clonal micropropagation from primary shoots to rooted plants. (Scale bars, 20 mm.)
Microclonal propagation.
Shoots were multiplied through microclonal propagation in vitro on modified ABM (Fig. 5B). For micropropagation, the medium was switched from MS to ABM because of high shoot extension on MS media containing high levels of nitrogen. Plants cultivated from different fruits were maintained as separate lineages. Apart from ancient plants, those from extant seeds of the same species, and from the same region, were grown in vitro as a control lineage. All cultivation stages of ancient material were repeated in the control lineage.
Green shoots were rooted on half-strength modified ABM without the organic compounds mentioned above and further grown under low light (2,000 lx, 16/8 h light/dark period) at 22–26 °C. Rooted plants were transferred to plastic pots with high-moor peat (50%) and sand (or vermiculite, 50%) and placed in a growth room. Plants were watered as needed with distilled water and fertilized using ABM diluted 1:4. To provide adaptation for aseptic conditions, regenerated plants were cultivated in high humidity and decreased night temperature (8–12 °C) for 70–90 d. Adapted vegetative plants were then transplanted to a potting medium with mineral nutrients, peat, sand, and modern cryozem soil taken from an area with contemporary S. stenophylla. To initiate the generative stage of plant development, lighting conditions close to those Arctic values were maintained (5,000 lx, 18/6 h and then 20/4 light/dark period) at a constant temperature of 18 °C.
Acknowledgments
This paper is dedicated to Dr. David Gilichinsky, the longtime Head of Geocryology Lab. A pioneer in studying microorganisms in Siberian and Antarctic permafrost, his achievement attracted scientists from all over the world to research on permafrost life systems. We thank Dr. N. V. Obrucheva for discussions and encouragement, Dr. A. G. Devyatov for verification and identification of the plants, and Professors P. J. Webber, M. M. Webber, and M. A. Holland for help with scientific editing.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
2Deceased February 18, 2012.
References
- 1.Veprintsev BN, Rott NN. Conserving genetic resources of animal species. Nature. 1979;280:633–634. doi: 10.1038/280633a0. [DOI] [PubMed] [Google Scholar]
- 2.Withers LA. Cryopreservation and storage of germplasm. In: Dixon RA, editor. Plant Cell Culture. A Practical Approach. Oxford: IRL; 1985. pp. 169–191. [Google Scholar]
- 3.Stanwood PC. Cryopreservation of seed germplasm for genetic conservation. In: Kartha KK, editor. Cryopreservation of Plant Cells and Organs. Boca Raton, FL: CRC; 1985. pp. 199–226. [Google Scholar]
- 4.Gilichinsky DA, Rivkina EM. Permafrost microbiology. In: Reitner J, Thiel V, editors. Encyclopedia of Geobiology. New York: Springer; 2011. pp. 726–732. [Google Scholar]
- 5.Steven B, Léveillé R, Pollard WH, Whyte LG. Microbial ecology and biodiversity in permafrost. Extremophiles. 2006;10:259–267. doi: 10.1007/s00792-006-0506-3. [DOI] [PubMed] [Google Scholar]
- 6.Vorobyova E, et al. The deep cold biosphere: Facts and hypothesis. FEMS Microbiol Rev. 1997;20:277–290. [Google Scholar]
- 7.Lydolph MC, et al. Beringian paleoecology inferred from permafrost-preserved fungal DNA. Appl Environ Microbiol. 2005;71:1012–1017. doi: 10.1128/AEM.71.2.1012-1017.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vishnivetskaya TA, et al. Bacterial community in ancient Siberian permafrost as characterized by culture and culture-independent methods. Astrobiology. 2006;6:400–414. doi: 10.1089/ast.2006.6.400. [DOI] [PubMed] [Google Scholar]
- 9.Willerslev E, et al. Diverse plant and animal genetic records from Holocene and Pleistocene sediments. Science. 2003;300:791–795. doi: 10.1126/science.1084114. [DOI] [PubMed] [Google Scholar]
- 10.Odum S. Germination of ancient seeds: Floristical observations and experiments with archaeologicaly dated soil samples. Dansk Botanisk Arkiv. 1965;24:1–70. [Google Scholar]
- 11.Priestly DA. Seed Ageing: Implications for Seed Storage and Persistence in the Soil. Ithaca, NY: Comstock Associates; 1986. [Google Scholar]
- 12.Sallon S, et al. Germination, genetics, and growth of an ancient date seed. Science. 2008;320:1464. doi: 10.1126/science.1153600. [DOI] [PubMed] [Google Scholar]
- 13.Shen-Miller J, et al. Long-living lotus: Germination and soil γ-irradiation of centuries-old fruits, and cultivation, growth, and phenotypic abnormalities of offspring. Am J Bot. 2002;89:236–247. doi: 10.3732/ajb.89.2.236. [DOI] [PubMed] [Google Scholar]
- 14.Gubin SV, Khasanov BF. Fossil burrows of mammals in the loess-ice deposits of the Kolyma-Indigirka lowland. Dokl Biol Sci. 1996;346:26–27. [Google Scholar]
- 15.Sher AV. Pleistocene mammals and stratigraphy of the Far Northeast USSR and North America. Int Geol Rev. 1974;16:1–284. [Google Scholar]
- 16.Kaplina TN, Lozhkin AV. Age and history of accumulation of the ‘‘Ice Complex’’ of the Maritime Lowlands of Yakutia. In: Velichko AA, Wright HE, Barnosky CW, editors. Late Quaternary Environments of the Soviet Union. Minneapolis: University of Minnesota Press; 1984. pp. 147–151. [Google Scholar]
- 17.Gubin SV, et al. [The possible contribution of late pleistocene biota to biodiversity in present permafrost zone] Zh Obshch Biol. 2003;64:160–165. [PubMed] [Google Scholar]
- 18.McKay CP. The Deep Biosphere: Lessons for Planetary Exploration. In: Fredrekson JK, Fletcher M, editors. Subsurface Microbiology and Biogeochemistry. New York: Wiley-Liss; 2001. pp. 315–327. [Google Scholar]
- 19.Ellis RH, Roberts EH. Improved equations for the prediction the seed longevity. Ann Bot (Lond) 1980;45:13–30. [Google Scholar]
- 20.Murdoch AJ, Ellis RH. In: Dormancy, Viability and Longevity/CAB International Seeds: The Ecology of Regeneration in the Plant Communities. 2nd Ed. Fenner N, editor. Wallingford, UK: CAB International; 2000. pp. 183–214. Chapter 8. [Google Scholar]
- 21.Andersen DM. Ice nucleation and substrate-ice interface. Nature. 1967;216:563–566. [Google Scholar]
- 22.Gilichinsky DA, Wagener S, Vishnivetskaya TA. Permafrost microbiology. Permafrost and Periglacial Processes. 1995;6:281–291. [Google Scholar]
- 23.Porsild AE, Harington CR, Mulligan GA. Lupinus arcticus Wats. grown from seeds of Pleistocene age. Science. 1967;158:113–114. doi: 10.1126/science.158.3797.113. [DOI] [PubMed] [Google Scholar]
- 24.Zazula GD, Harington CR, Telka AM, Brock F. Radiocarbon dates reveal that Lupinus arcticus plants were grown from modern not Pleistocene seeds. New Phytol. 2009;182:788–792. doi: 10.1111/j.1469-8137.2009.02818.x. [DOI] [PubMed] [Google Scholar]
- 25.Yashina SG, Gubin SV, Shabaeva EV, Egorova EF, Maksimovich SV. Viability of higher plant seeds of late pleistocene age from permafrost deposits as determined by in vitro culturing. Dokl Biol Sci. 2002;383:151–154. doi: 10.1023/a:1015350209946. [DOI] [PubMed] [Google Scholar]
- 26.Tikhonova VL, Yashina SG. Long-term storage of endangered wild plant seeds. In: Turpaev TM, editor. Physiology and General Biology Reviews. Amsterdam: Harwood Academic Publishers; 1997. pp. 1–33. [Google Scholar]
- 27.Yashina SG, Gakhova EN, Gubin SV. Permafrost as a natural cryobank of late Pleistocene and modern plant germplasm. In: Haeberli W, Brandova D, editors. Permafrost. Extended Abstracts Reporting Current Research and New Information. Switzerland: Glaciology and Geomorphodynamics Group, Geography Department, University of Zurich; 2003. pp. 187–188. [Google Scholar]
- 28.Butenko RG. Culture of Isolated Tissues and the Physiology of Plant Morphogenesis. Moscow: Nauka; 1964. in Russian. [Google Scholar]
- 29.Tisserat B. Embryogenesis, organogenesis and plant regeneration. In: Dixon RA, editor. Plant Cell Culture. A Practical Approach. Oxford: IRL; 1985. pp. 79–126. [Google Scholar]
- 30.Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. [Google Scholar]
- 31.Anderson WC. Rooting of tissue cultured rhododendrons. Proc Inter Plant Prop Soc. 1978;28:135–139. [Google Scholar]
- 32.Kuzin AM. Role of Natural Radioactivity and Secondary Biogenic Radiation in a Phenomenon of Life. Moscow: Nauka; 2002. in Russian. [Google Scholar]
- 33.Pontovich VE. Culture of tissues of reproductive organs. In: Butenko R, editor. Culture of Plant Organs, Tissues and Cells. Moscow: Nauka; 1970. pp. 7–20. in Russian. [Google Scholar]
- 34.Macdonald SE, Chinnappa CC, Reid DM. Studies on Stellaria longipes Goldie complex: Phenotypic plasticity. I. Response of stem elongation to temperature and photoperiod. Can J Bot. 1984;62:414–419. [Google Scholar]
- 35.Chinnappa CC, Donald GM, Sasidharan R, Emery RJN. The biology of Stellaria longipes (Caryophyllaceae) Can J Bot. 2005;83:1367–1383. [Google Scholar]
- 36.Khryanin VN. Role of phytohormones in sex differentiation in plants. Russ J Plant Physiol. 2002;49:545–551. [Google Scholar]
- 37.Gaglioti BV, Barnes BM, Zazula GD, Beaudoin AB, Wooller MJ. Late Pleistocene paleoecology of arctic ground squirrel (Urocitellus parryii) caches and nests from Interior Alaska's mammoth steppe ecosystem, USA. Quat Res. 2011;76:373–382. [Google Scholar]
- 38.Zazula GD, Froese DG, Elias SA, Kuzmina S, Mathewes RW. Arctic ground squirrels of the mammoth-steppe: Paleoecology of Late Pleistocene middens (24 000-29 450 14C yr BP), Yukon Territory, Canada. Quat Sci Rev. 2007;26:979–1003. [Google Scholar]
- 39.Gubin SV, Maximovich SV, Zanina OG, Stakhov VL. Morphogenetics of plant remains from paleosols and rodent burrows buried in permafrost of the Late Pleistocene (32 - 28,000 BP) In: Gyulai G, editor. Plant Archaeogenetics. New York: Nova Science; 2011. pp. 11–21. [Google Scholar]
- 40.Başli GA, et al. Light and scanning electron microscopic analysis of Silene stenophylla seeds excavated from Pleistocene-Age (Kolyma) Anadolu Univ J Sci Technol. 2009;10:161–167. [Google Scholar]
- 41.White PR. The Cultivation of Animal and Plant Cells. New York: Ronald Press; 1963. [Google Scholar]