Abstract
Cardiomyocyte contraction and relaxation are controlled by Ca2+ handling, which can be regulated to meet demand. Indeed, major reduction in sarcoplasmic reticulum (SR) function in mice with Serca2 knockout (KO) is compensated by enhanced plasmalemmal Ca2+ fluxes. Here we investigate whether altered Ca2+ fluxes are facilitated by reorganization of cardiomyocyte ultrastructure. Hearts were fixed for electron microscopy and enzymatically dissociated for confocal microscopy and electrophysiology. SR relative surface area and volume densities were reduced by 63% and 76%, indicating marked loss and collapse of the free SR in KO. Although overall cardiomyocyte dimensions were unaltered, total surface area was increased. This resulted from increased T-tubule density, as revealed by confocal images. Fourier analysis indicated a maintained organization of transverse T-tubules but an increased presence of longitudinal T-tubules. This demonstrates a remarkable plasticity of the tubular system in the adult myocardium. Immunocytochemical data showed that the newly grown longitudinal T-tubules contained Na+/Ca2+-exchanger proximal to ryanodine receptors in the SR but did not contain Ca2+-channels. Ca2+ measurements demonstrated a switch from SR-driven to Ca2+ influx-driven Ca2+ transients in KO. Still, SR Ca2+ release constituted 20% of the Ca2+ transient in KO. Mathematical modeling suggested that Ca2+ influx via Na+/Ca2+-exchange in longitudinal T-tubules triggers release from apposing ryanodine receptors in KO, partially compensating for reduced SERCA by allowing for local Ca2+ release near the myofilaments. T-tubule proliferation occurs without loss of the original ordered transverse orientation and thus constitutes the basis for compensation of the declining SR function without structural disarrangement.
Keywords: excitation–contraction coupling, transgenic mice
In cardiomyocytes, Ca2+ is cycled across the plasmalemma and across the membrane of the sarcoplasmic reticulum (SR). These two systems interact closely. Ca2+ influx through L-type Ca2+ channels (CaV1.2) in the T-tubules triggers Ca2+ release from ryanodine receptors (RyR) in the SR membrane. The resulting rise in cytosolic Ca2+ concentration elicits contraction as Ca2+ binds to the myofilmants. Removal of Ca2+ from the cytosol is governed by the SR calcium ATPase 2 (SERCA2), which recycles Ca2+ into the SR, and to a lesser extent by the Na+/Ca2+-exchanger (NCX), which removes Ca2+ from the cell.
The two Ca2+ cycling systems compete for Ca2+, and this competition can be shifted to meet demand. This can occur on a beat-to-beat basis (1) but also in the longer term by altered expression of proteins. For example, contractile function in surviving tissue after myocardial infarction is initially enhanced by augmented transsarcolemmal Ca2+ cycling (2). We have also observed that marked enhancement of plasmalemmal Ca2+ fluxes efficiently compensate for near-total loss of SR function after conditional Serca2 knockout (KO) (3, 4). Here we report that such adaptation of Ca2+ handling capacity requires alterations in cardiomyocyte ultrastructure. When SERCA2 expression was reduced, we observed a reduction in the SR volume and collapse of the remaining SR. Importantly, however, a proliferation of longitudinal T-tubules (LTTs) containing NCX facilitates plasmalemmal Ca2+ flux as a means to compensate for the declining SR function.
Results
Serca2 Disruption Induces SR Collapse and Volume Reduction.
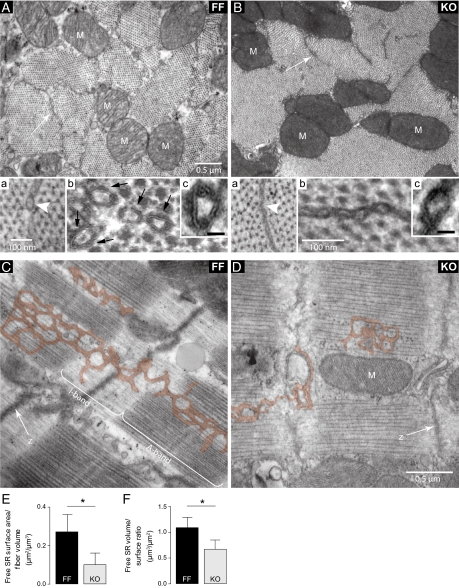
SR ultrastructure was markedly altered in KO (Fig. 1). Morphometric analysis of electron micrographs showed that SR surface area per cell volume declined by 63% at 7 wk after induction of KO [Serca2flox-flox mice (FF): 0.27 ± 0.09; KO: 0.10 ± 0.06 μm2/μm3; P < 0.05; Fig. 1]. A 38% reduction in volume-to-surface ratio indicated that remaining SR in KO had collapsed (FF: 1.09 ± 0.20; KO: 0.67 ± 0.18 μm3/100 μm2; P < 0.05; Fig. 1), resulting in an overall decrease to 24% of the SR/cell volume ratio. SR collapse was also detected at an earlier time point when surface area was not greatly diminished (Fig. S1).
Fig. 1.
SR collapse and volume reduction in KO cardiomyocytes. (A and B) Cross-sections of cardiomyocytes at the A-band level show that SR profiles were less prominent in KO (arrows). (A, a and B, a) High-magnification images indicate SR collapse in KO (arrowheads). (A, b and B, b) Contrast enhancement with tannic acid revealed discontinuous dense bands in FF (black arrows) composed of the superimposed outlines of the SERCA heads. The absence of these bands in KO confirmed the marked reduction of SERCA content. (A, c and B, c) Higher magnifications of tannic acid-enhanced images. (Scale bar, 20 nm.) (C and D) Longitudinal sections of cardiomyocytes show that free SR (pseudocolored) is much less prominent in KO. M, mitochondria; z, z-lines. (E and F) Mean data from morphometric analysis: SR surface area per cell volume (E) and free SR volume-to-surface ratio (F). n = 32–57 cells; *P < 0.05.
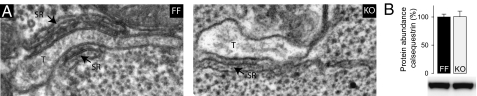
We have previously shown a 97% reduction of SERCA2 in KO (4). In the present study the near-total absence of SERCA was confirmed by electron microscopy with enhanced contrast. Aggregated SERCA heads were visible as dense bands on the cytoplasmic surface of the free SR in FF but not in KO (Fig. 1 A, b and c and B, b and c). In contrast, there was no alteration in the structure of junctional SR domains associated with T-tubules, which bear surface RyR and contain calsequestrin (Fig. 2). This confirmed the existence of a primary free SR defect not affecting the SR domains dedicated to Ca2+ release. The relative volume of mitochondria per fiber volume was unchanged (FF: 31% ± 7%; KO: 31% ± 5% of cell volume excluding nuclei).
Fig. 2.
Unaltered dyads in KO cardiomyocytes. (A) Representative electron micrographs show no apparent structural changes at the level of the dyad in KO cardiomyocytes. T, T-tubule. (B) Expression levels of calsequestrin were unaltered in KO hearts; n = 6.
Increased Abundance of Longitudinal T-Tubules in Serca2 KO.
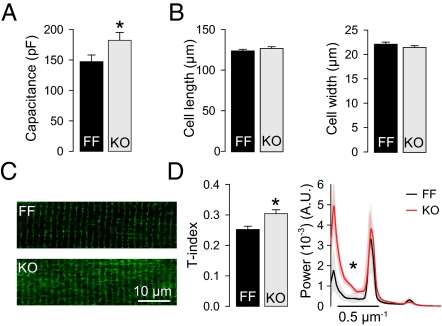
Although SR size was reduced, cell capacitance measurements revealed a 24% increase in relative surface area in KO compared with FF (Fig. 3A). This could not be explained by an increase in cell size because cell dimensions showed no sign of cell hypertrophy (Fig. 3B). Greater surface area was instead explained by a 21% increase in T-tubule density (Fig. 3 C and D). This was confirmed by fast Fourier transformations (FFTs) of the confocal images: KO cells exhibited greater power of the first peak (zero-order component), which represents the mean fluorescence signal. The remaining peaks of the FFTs, at ≈0.5-μm−1 intervals, were similar in both groups, indicating maintained organization of transverse elements at the level of the z-lines in KO (Fig. 3D). However, greater between-peak power in KO indicated an increased fraction of tubules located at the levels of the A-band (LTTs). Taken together, increased cell surface area in KO myocytes was explained by growth of new LTTs between z-lines.
Fig. 3.
Increased abundance of LTTs in KO cardiomyocytes. (A) Cell capacitance was increased in KO (n = 33–38). (B) Cell size was unaltered in KO (n = 102–104). (C) Representative images of di-8-ANNEPS–stained cells. (D) Mean data show increased T-tubule density (T-index) and increased between-peak power in FFT plots, indicating newly grown LTTs; n = 10–19; A.U., arbitrary units; *P < 0.05.
Newly Grown T-Tubules in KO Contain NCX Proximal to RyR.
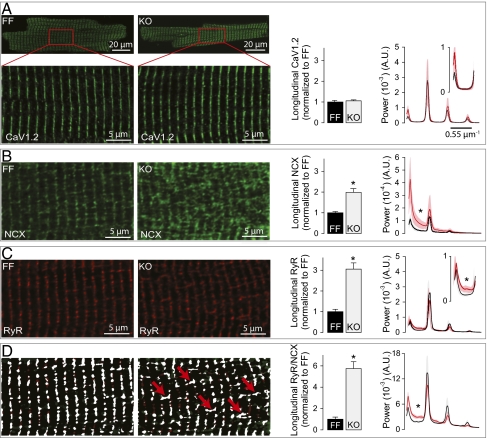
We have previously observed increased expression of CaV1.2 and NCX in KO (3, 4), which promotes enhanced plasmalemmal Ca2+ cycling. Presently, we examined whether such changes were facilitated by T-tubule expansion. Immunocytochemical analysis showed that CaV1.2 was localized exclusively in transversely oriented T-tubule elements in both FF and KO and not in the newly added LTTs (Fig. 4A). However, we found increased expression of NCX in LTTs (Fig. 4B). Surprisingly, RyR foci, usually located exclusively at the level of the z-lines, also appear at the A bands, in proximity to the NCX molecules of LTTs (Fig. 4 C and D). These changes were confirmed quantitatively by measuring the relative amount of longitudinal staining (histograms in Fig. 4; Methods) and by FFT analysis (curves in Fig. 4). The power of the peaks of the FFT plots reflects the amount of staining at the level of the z-lines, whereas the between-peak power reflects the amount of staining at the levels of the A-band. The protein distributions determined experimentally were incorporated in a mathematical model (see below, SI Methods and Fig. S2). The increased amount of LTTs was confirmed in electron micrographs (Fig. S3). We also found more junctions between LTTs and SR (dyads) in the A-band in KO compared with FF (Fig. S3).
Fig. 4.
Newly grown LTTs in KO cardiomyocytes contain NCX apposing RyR in the SR. (A–C) Representative confocal images of cardiomyocytes stained for CaV1.2 (A), NCX (B), and RyR (C). (D) Images from B and C were thresholded using the Otsu method, and pixels above threshold in both images are shown in white pixels. Red arrows indicate longitudinal segments where NCX and RyR are proximal. Histograms: Relative abundance of LTTs in KO normalized to FF (n = 8–16). Mean FFTs of confocal images (Right, n = 11–17) show unaltered labeling of CaV1.2, NCX, and RyR in the transverse segments and increased labeling of NCX and RyR but not CaV1.2 in the LTTs. A.U., arbitrary units; *P < 0.05.
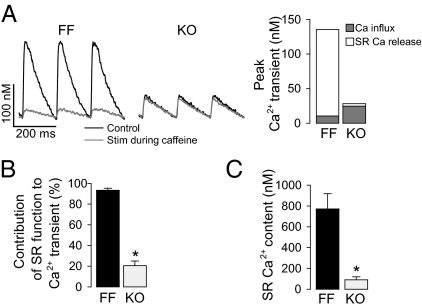
Contribution of SR Function to Ca2+ Transient Is Dramatically Reduced.
It was unclear why RyRs would be localized apposed to NCX in LTTs when, with such marked reductions in SERCA levels and SR volume, the SR would not be expected to provide significant Ca2+ release. Therefore, we investigated SR function by measuring Ca2+ transients in the absence or presence of caffeine at near-physiological stimulation frequency (Fig. 5A). Under control conditions, Ca2+ transients were markedly reduced in magnitude in KO. However, when SR stores were depleted in the presence of 10 mM caffeine, Ca2+ transients were larger in KO than in FF (P < 0.05), owing to increased Ca2+ influx. We have previously shown that greater Ca2+ influx in KO results from increased Ca2+ entry via both CaV1.2 and NCX (4, 5). SR contributed to ≈90% of the Ca2+ transient amplitude in FF and to ≈20% in KO (Fig. 5B). Thus, despite a switch from a predominantly SR driven Ca2+ transient in FF to a predominantly Ca2+ influx-driven Ca2+ transient in KO, there was still a relatively important SR contribution in KO. SR content in KO cardiomyocytes was ≈10% of FF values (Fig. 5C).
Fig. 5.
Contribution of SR function to Ca2+ transients. (A) Steady-state Ca2+ transients in cardiomyocytes field stimulated (6 Hz) before and during presence of 10 mM caffeine. Histogram: Mean values of the Ca2+ transient magnitudes (n = 17–19). (B) Contribution of SR function to Ca2+ transient magnitude derived from experiments in A. (C) SR Ca2+ content measured as peak caffeine-elicited Ca2+ transient (n = 18–19).
Longitudinal T-Tubules Partially Compensate for Loss of SR Function.
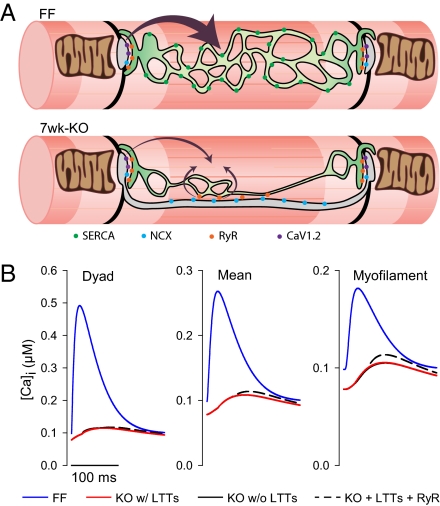
To investigate whether the presence of LTTs could contribute to maintaining the Ca2+ transient when SR contribution was blunted, we established a simple mathematical model (SI Methods). In this model, Ca2+ entry by NCX was enhanced by action potential prolongation and higher cytosolic Na+ concentration in KO as observed experimentally (4, 5) (see Tables S1 and S2 for model parameters). Modeling data showed that Ca2+ entry through NCX in the LTTs could trigger release from proximal RyRs (Fig. 6A), boosting systolic Ca2+ levels near the myofilaments (Fig. 6B). Thus, these results suggest that the presence of NCX in LTTs and apposing RyRs in KO may partially compensate for the reduction in SERCA.
Fig. 6.
LTTs in KO may partially compensate for the reduction in SERCA. (A) Schematic representation of the remodeling of SR and T-tubules in KO. In FF, the SR (green) has a characteristic fenestrated morphology, and the T-tubules (blue) mainly occur at the z-lines (black). In KO, SR is reduced and T-tubule occurrence in the longitudinal direction is increased. Although Ca2+ release occurs primarily at the z-lines in FF, release of Ca2+ from RyR apposing LTTs in KO can boost the relatively small Ca2+ transient near the myofilaments.*P < 0.05. (B) Modeled Ca2+ transients at the dyad (Left), overall (Center), and at the myofilaments (midway between dyads, Right) for FF (blue), KO with (full black line), and without (red) LTTs and with LTTs and triggering of RyR midway between the dyads (dashed black curve).
Discussion
In this study, we observed that cardiomyocytes from mice with conditional Serca2 KO exhibited striking SR volume reduction and collapse, coupled with increased T-tubule abundance. These morphological changes enhance plasmalemmal Ca2+ flux and also the efficiency of SR Ca2+ release, because newly grown T-tubules contain NCX in proximity to RyR. Indeed, an increased number of junctions between LTTs and SR resembling dyads were observed in electron micrographs in KO (Fig. S3). Thus, adult cardiomyocytes possess a striking ability to remodel T-tubules to compensate for loss of SR function.
The marked reduction in SR volume observed in KO would be expected to counteract the decrease in free SR [Ca2+], by decreasing the effective volume into which the remaining [Ca2+] is diluted, thus helping to maintain RyR sensitivity and facilitating Ca2+ release. Such alterations may explain how SR Ca2+ release can occur in these myocytes despite a ≈90% reduction in SR Ca2+ content. The causes of this SR structural remodeling are unclear. However, SR collapse may directly result from reduced turgor due to loss of SR Ca2+ and associated water. SR structure can also be altered by endoplasmic reticulum/SR stress signaling (6), and these pathways are activated at early time points where we presently observed SR collapse (Fig. S1). These structural alterations seem to be opposite those reported after calsequestrin deletion, where marked SR volume expansion helps maintain SR Ca2+ content (7). Taken together, these findings show impressive SR lability.
Increased capacitance of KO myocytes indicates increased surface area, which was traced to the presence of new LTTs. T-tubules of both skeletal and cardiac muscles occasionally extend into longitudinally oriented elements that connect adjacent transverse networks (8). The increased frequency of LTTs in KO mimics the disposition during early differentiation, when tubules are essentially longitudinal (9). Although KO animals exhibit heart failure symptoms (3, 4), there are significant differences between the increased presence of LTTs observed here and T-tubule remodeling normally reported during heart failure development (10–15). First, whereas the transverse component of the network and its association with dyads are disarranged in classic heart failure models (16), KO cardiomyocytes exhibit a maintained transverse and unaltered dyadic architecture. Second, the changes in our system result in an overall increase rather than decrease in T-tubule density, which we believe allows for compensatory increases in plasmalemmal Ca2+ fluxes. However, it will be important to examine whether LTTs appear as a result of regulated growth rather than disarray of existing T-tubules in heart failure. Interestingly, we observed that LTTs formed junctions with SR that resemble dyads (Fig. S3), but no CaV1.2 staining was observed in these regions (Fig. 4). Thus, we suggest that dyads might form even in the absence of CaV1.2.
In KO, NCX molecules were localized in newly grown LTTs. As already shown (4) Ca2+ entry via NCX is facilitated in KO by cytosolic Na+ accumulation and action potential prolongation. Thus, NCX expressed in LTTs could act as triggers of proximal RyRs, as predicted in our mathematical model (Fig. 6). In support of this prediction, Ca2+ transients elicited by NCX alone (presence of 20 μM nifedipine) are significantly reduced in magnitude when SR stores are depleted using caffeine (Fig. S4). The modeling results also suggest that Ca2+ release at these sites more efficiently triggers contraction because these RyRs are closer to the myofilaments (SI Results and Discussion). NCX in the LLTs therefore likely mediates both Ca2+ entry and removal in the neighborhood of the myofilaments.
In conclusion, we observe dramatic SR volume loss and collapse after KO. Proliferation of LTTs containing NCX facilitates plasmalemmal Ca2+ flux, which can trigger release of Ca2+ through RyR proximal to myofilaments to compensate for the declining SR function.
Methods
Protein distribution and ultrastructure of T-tubules and SR were determined by electron and confocal microscopy in KO 7-wk after Serca2 disruption. FF mice served as controls. Standard patch-clamp techniques were used. Refer to SI Methods for details.
Supplementary Material
Acknowledgments
This study was supported by The Research Council of Norway, The South-Eastern Norway Regional Health Authority, Anders Jahre's Fund for the Promotion of Science, Oslo University Hospital Ullevål, University of Oslo, National Institutes of Health Grant RO1 HL 48093 (to C.F.-A.), and European Union Project FP7-HEALTH-2010.2.4.2-4 (“MEDIA-Metabolic Road to Diastolic Heart Failure”).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1120172109/-/DCSupplemental.
References
- 1.Dibb KM, Eisner DA, Trafford AW. Regulation of systolic [Ca2+]i and cellular Ca2+ flux balance in rat ventricular myocytes by SR Ca2+, L-type Ca2+ current and diastolic [Ca2+]i. J Physiol. 2007;585:579–592. doi: 10.1113/jphysiol.2007.141473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mørk HK, et al. Increased cardiomyocyte function and Ca2+ transients in mice during early congestive heart failure. J Mol Cell Cardiol. 2007;43:177–186. doi: 10.1016/j.yjmcc.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Andersson KB, et al. Moderate heart dysfunction in mice with inducible cardiomyocyte-specific excision of the Serca2 gene. J Mol Cell Cardiol. 2009;47:180–187. doi: 10.1016/j.yjmcc.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 4.Louch WE, et al. Sodium accumulation promotes diastolic dysfunction in end-stage heart failure following Serca2 knockout. J Physiol. 2010;588:465–478. doi: 10.1113/jphysiol.2009.183517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li L, et al. Calcium dynamics in the ventricular myocytes of SERCA2 knockout mice: A modeling study. Biophys J. 2011;100:322–331. doi: 10.1016/j.bpj.2010.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu XH, et al. Cardiomyocyte-specific disruption of Serca2 in adult mice causes sarco(endo)plasmic reticulum stress and apoptosis. Cell Calcium. 2011;49:201–207. doi: 10.1016/j.ceca.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Knollmann BC, et al. Casq2 deletion causes sarcoplasmic reticulum volume increase, premature Ca2+ release, and catecholaminergic polymorphic ventricular tachycardia. J Clin Invest. 2006;116:2510–2520. doi: 10.1172/JCI29128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soeller C, Cannell MB. Examination of the transverse tubular system in living cardiac rat myocytes by 2-photon microscopy and digital image-processing techniques. Circ Res. 1999;84:266–275. doi: 10.1161/01.res.84.3.266. [DOI] [PubMed] [Google Scholar]
- 9.Franzini-Armstrong C. Veratti and beyond: Structural contributions to the study of muscle activation. Rend Fis Acc Lincei. 2002;13:289–323. [Google Scholar]
- 10.Song LS, et al. Orphaned ryanodine receptors in the failing heart. Proc Natl Acad Sci USA. 2006;103:4305–4310. doi: 10.1073/pnas.0509324103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wasserstrom JA, et al. T-tubule remodeling causes Ca2+ cycling defects during the progression to heart failure in the intact spontaneously hypertensive rat heart. Biophys J. 2011;100:180a. [Google Scholar]
- 12.Wei S, et al. T-tubule remodeling during transition from hypertrophy to heart failure. Circ Res. 2010;107:520–531. doi: 10.1161/CIRCRESAHA.109.212324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swift F, et al. Altered Na+/Ca2+-exchanger activity due to downregulation of Na+/K+-ATPase alpha2-isoform in heart failure. Cardiovasc Res. 2008;78:71–78. doi: 10.1093/cvr/cvn013. [DOI] [PubMed] [Google Scholar]
- 14.Louch WE, et al. T-tubule disorganization and reduced synchrony of Ca2+ release in murine cardiomyocytes following myocardial infarction. J Physiol. 2006;574:519–533. doi: 10.1113/jphysiol.2006.107227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heinzel FR, et al. Remodeling of T-tubules and reduced synchrony of Ca2+ release in myocytes from chronically ischemic myocardium. Circ Res. 2008;102:338–346. doi: 10.1161/CIRCRESAHA.107.160085. [DOI] [PubMed] [Google Scholar]
- 16.Louch WE, Sejersted OM, Swift F. There goes the neighborhood: Pathological alterations in T-tubule morphology and consequences for cardiomyocyte Ca2+ handling. J Biomed Biotechnol. 2010;2010:503906. doi: 10.1155/2010/503906. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.