Abstract
Perioperative graft failure following coronary artery bypass grafting (CABG) may result in acute myocardial ischaemia. Whether acute percutaneous coronary intervention, emergency reoperation or conservative intensive care treatment should be used is currently unknown. Between 2003 and 2009, 39 of the 5598 patients who underwent isolated CABG surgery underwent early postoperative coronary angiography for suspected myocardial ischaemia. Following angiography, two groups were identified: patients who underwent immediately reintervention (group 1); and those treated conservatively (group 2). Primary study endpoints were mortality and postoperative myocardial infarct size. Postoperative coronary angiography revealed early perioperative bypass graft failure in 32 of 39 patients. Acute percutaneous coronary intervention was performed in 15 patients, redo-CABG in 4 patients and conservative treatment in 13 patients. The number of failing bypass grafts were significantly higher in group 1 compared with group 2 (P = 0.0251). A trend toward lower post-procedural peak cardiac troponin T and creatinine phosphokinase serum levels in group 1 was observed (163.0 vs. 206.0 and 4.35 vs. 5.53, respectively) (P = 0.0662 and 0.1648). Early reintervention may limit the extent of myocardial cellular damage compared with conservative medical strategy in patients with myocardial ischaemia due to early graft failure.
Keywords: Coronary artery bypass graft surgery, Coronary artery imaging, Myocardial infarction, Surgery complications
INTRODUCTION
Perioperative myocardial infarction (MI) is a serious complication following coronary artery bypass graft (CABG) surgery with an incidence between 5 and 10% [1]. Postoperative MI significantly raises [2]. Since patients are unable to express classical clinical symptoms of myocardial ischaemia, the diagnosis of this complication is a clinical challenge. Surgeons rely on electrocardiogram (EKG) modifications (new ST segment alterations or new Q wave), refractory malignant arrhythmias, elevation of cardiac biomarkers, persistent low cardiac output syndrome (LCOS) and new echocardiography wall motion abnormalities to identify possible myocardial injury [1, 3–5].
Myocardial damage following CABG surgery is due to two different causes classified as graft or non-graft related [6, 7]. The incidence of early graft dysfunction is ∼3% [5]. Non-graft-related ischaemia is related to inappropriate myocardial protection, excessive surgical manipulations, and air or plaque embolization [8]. Graft-related injury is associated with: early graft thrombosis, anastomotic stenosis, bypass kinks, overstretching or tension, significant spasm or incomplete revascularization [4, 7]. Hence, discrimination between graft-related ischaemic events from other reasons [6] must be made rapidly. Early reintervention has been proposed to allow myocardial rescue to preserve ventricular function after CABG surgery [9] since MI is associated with congestive heart failure and significant adverse outcomes [3, 8].
The primary objective of this study was to describe specific angiographic findings in patients with early graft dysfunction to determine the most appropriate management.
METHODS
This study was performed as a retrospective analysis. Between January 2003 and December 2009, data were collected from 5598 consecutive patients who receive isolated CABG surgery. From this group, 39 underwent early coronary angiography for signs of myocardial ischaemia and suspicion of early graft dysfunction at the Montreal Heart Institute (MHI). Early graft failure was defined as suspected graft dysfunction occurring within the first 72 h after the CABG procedure. Following angiographic diagnosis, patients were divided in two groups according to the treatment strategy chosen. The first group consisted of patients with early aggressive treatment of graft dysfunction [return to OR for CABG revision or percutaneous coronary intervention (PCI)], while the second group consisted of patients with observation in the ICU. This study was approved by the Review Board and the Ethics Committee of MHI.
CABG procedures were performed at the MHI by a group of 10 surgeons. Revascularization strategy was according to surgeon's preferences. The left internal mammary artery was anastomosed to the left anterior descending coronary artery and the right internal mammary artery or a saphenous vein graft were used to complete revascularization. Patency of grafts was assessed following the last proximal anastomosis with intraoperative Doppler. Transoesophageal echography (TEE) was routinely performed in the OR by experienced cardiac anaesthesiologists to evaluate myocardial and valvular function. TEE evaluations of regional wall motion abnormalities (RWMAs) were performed after the anaesthetic induction and after weaning from cardiopulmonary bypass. Measurements were done according to the standard guidelines using a 16-segment model [10].
Indexed cardiac output was available for all patients once temperature reached 36°C. All patients had a standard 12-lead EKG, TnT and CKMB serum level measurements in the ICU. These parameters were evaluated every 8 h and daily for 72 h. Intravenous nitroglycerin (1.2 mg/h) was administered in continuous perfusion for the first 24 h for all patients. American Society of Anaesthesiologists (325 mg) was administrated orally 6 h after surgery when was controlled.
Once the diagnosis of graft dysfunction was made, the treatment strategy was chosen after consultation with an interventional cardiologist and attending cardiac surgeon. Conservative treatment consisted of aggressive inotropic and intra-aortic balloon pumping support, intravenous heparin, beta-blockers and pain management. When PCI was chosen, the patient underwent immediate revascularization with percutaneous dilatation of the native coronary circulation. When redo CABG was chosen, the patient was immediately transferred to the OR and the failed graft was repaired or replaced.
Postoperative myocardial was evaluated according to the two treatment strategies. For each group, the highest TnT and CK-MB serum levels following CABG surgery were measured. In-hospital and 30-day mortality was also recorded for both treatment strategies.
Statistical analysis
Categorical variables are described as percentages, and the Fisher exact test was used to compare groups. Continuous variables are expressed as the mean value ± SD if normally distributed or the median value (interquartile range) if the distribution was skewed. The differences were tested by t-test or Wilcoxon rank-sum test when appropriate.
RESULTS
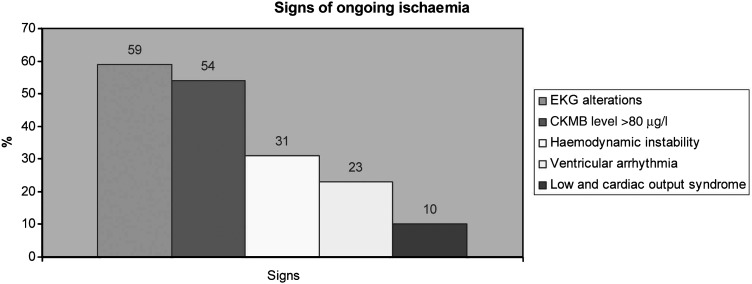
Thirty-nine patients (12 females: 27 males) had postoperative suspicion of early graft failure based on high CKMB (>80 µg/l) level (54%, n = 21), EKG alterations (59%, n = 23), haemodynamic instability (31%, n = 12), ventricular arrhythmia (23%, n = 9) and LCOS (10%, n = 4) (Fig. 1).
Figure 1:
Signs of ongoing ischaemia. EKG: electrocardiogram; CKMB: creatinine phosphokinase.
Of the 39 patients, 32 (82%) had early postoperative graft failure and 7 (18%) had graft unrelated conditions. Overall, early graft dysfunction was diagnosed in 0.6% (n = 32) of patients operated for CABG surgery in this study period (5598 cases).
Preoperative baseline characteristics and operative details are summarized for both groups in Tables 1 and 2, respectively. There were no significant differences between groups.
Table 1:
Preoperative characteristics
| Group 1 (n = 19) | Group 2 (n = 13) | P-value | |
|---|---|---|---|
| Age (years) | 65.9 ± 12.4 | 61.4 ± 10.2 | 0.2019 |
| Male | 11 (61.1) | 6 (60.0) | 0.2899 |
| BMI (kg/m2) | 27.1 ± 4.9 | 24.99 ± 7.2 | 0.2646 |
| Diabetes | 1 (5.5) | 3 (23.1) | 0.4420 |
| Hypertension | 14 (77.78) | 8 (61.5) | 0.7889 |
| Hyperlipidaemia | 14 (77.78) | 5 (38.5) | 0.3124 |
| Smoking | 7 (38.89) | 6 (46.2) | 0.6309 |
| COPD | 3 (16.67) | 2 (15.4) | 1.0000 |
| PVD | 8 (44.44) | 6 (46.2) | 0.7248 |
| Renal insufficiency | 0 | 1 (7.7) | 0.5882 |
| Previous MI | 13 (72.22) | 4 (30.1) | 0.2160 |
| Previous CABG | 3 (16.67) | 0 | 0.0441 |
| Previous PCI | 5 (27.78) | 1 (7.7) | 0.0702 |
| CCS >3 | 8 (44.44) | 6 (46.2) | 0.1027 |
| 3Vx | 3 (16.67) | 1 (7.7) | 0.354 |
| LMCA | 9 (50.0) | 5 (38.5) | 0.986 |
| Normal LVEF | 15 (83.3) | 8 (61.5) | 0.892 |
| ASA | 3.33 ± 0.69 | 3.67 ± 0.5 | 0.6592 |
| EuroSCORE | 10.16 ± 6.79 | 7.8 ± 7.6 | 0.125 |
Data are presented as mean ± SD or number (%).
ASA: American Society of Anaesthesiologists; BMI: body mass index (kg/m2); CABG: coronary artery bypass graft; CCS: Canadian Cardiovascular Society; COPD: chronic congestive pulmonary disease; LMCA: left main coronary artery; LVEF: left ventricular ejection fraction; MI: myocardial infarction; PCI: percutaneous coronary intervention; PVD: peripheral vascular disease; 3Vx: three vessels coronary artery disease.
Table 2:
Intraoperative data
| Group 1 (n = 19) | Group 2 (n = 13) | P | |
|---|---|---|---|
| CBP time (min) | 71.33 ± 48.83 | 85.6 ± 36.89 | 0.2157 |
| Cross clamp time (min) | 49.56 ± 44.93 | 50 ± 27.33 | 0.7052 |
| No. of graft | 2.67 ± 0.77 | 2.9 ± 0.57 | 0.7845 |
| No. of anastomosis | 2.72 ± 0.75 | 3 ± 0.67 | 0.6362 |
Data are presented as mean ± SD.
CPB: cardiopulmonary bypass.
Angiographic findings
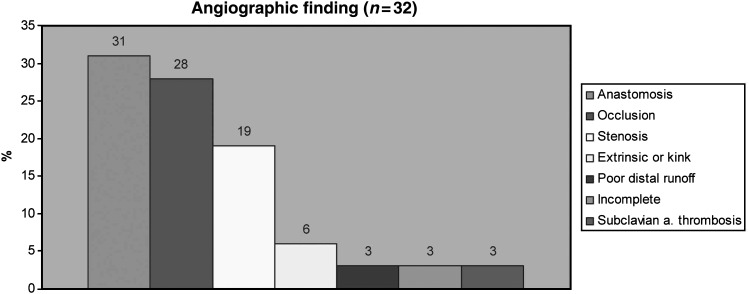
The angiographic findings are presented in Fig. 2. In patient with graft-related failure, findings were: graft thrombosis or severe stenosis (>50%) in 47% of patients (n = 15), anastomotic stenosis (>50%) in 31% (n = 10), extrinsic compression or graft kink in 9% (n = 3), insufficient distal runoff in 6% (n = 2), incomplete revascularization in 3% (n = 1) and subclavian artery thrombosis in 3% (n = 1). When comparing the two treatment options (conservative vs. repeat revascularization), group 1 had more graft failures per patient than group 2 (1.5 ± 0.7 compared to 1 ± 0.42 graft, P = 0.03). The type of conduits failing and myocardial territory with the failing conduit did not differ between the groups (Table 3).
Figure 2:
Angiographic finding. a: artery.
Table 3:
Angiographic finding (failing graft)
| Group 1 (n = 19) | Group 2 (n = 13) | P-value | |
|---|---|---|---|
| LIMA-LAD | 44% | 36% | 0.598 |
| RIMA-OM | 4% | 0% | 0.432 |
| SVG-D | 8% | 0% | 0.324 |
| SVG-OM | 20% | 29% | 0.876 |
| SVG-RCA | 24% | 36% | 0.903 |
D: diagonal artery; LAD: left descending artery; LIMA: left internal mammary artery; OM: obtuse marginal; RCA: right coronary artery; RIMA: right internal mammary artery; SVG: saphenous vein graft.
Outcomes
Myocardial injury following myocardial initial revascularization, as indicated by peak postoperative CKMB and TnT serum levels, was not statistically different between the two groups. However, there was a trend towards more extensive myocardial injury in the group with conservative treatment: CKMB: 175.25 ± 122.32 vs. 206.35 ± 131.1 µg/l, and TnT: 4.37 ± 5.79 vs. 5.53 ± 8.2 µg/l (P = 0.0662 and 0.1648, respectively). In-hospital or 30-day mortality was 15.8% in group 1 and 15.4% in group 2 (P = 0.74). This was not significant between the two groups (Table 4).
Table 4:
Postoperative characteristics
| Group 1 (n = 19) | Group 2 (n = 13) | P-value | |
|---|---|---|---|
| No. of graft failure | 1.5 ± 0.42 | 1 ± 0.7 | 0.0251 |
| Delay before angiography (h) | 16.08 ± 21.71 | 10.96 ± 22.65 | 0.3598 |
| Intubation time (h) | 45.67 ± 57.84 | 44.78 ± 75.14 | 0.6185 |
| Peak CKMB (µg/l) | 175.25 ± 122.32 | 206.35 ± 131.1 | 0.0662 |
| Peak troponin T (µg/l) | 4.37 ± 5.79 | 5.53 ± 8.2 | 0.1648 |
| In-hospital mortality (%) | 3 (15.8%) | 2 (15.4%) | 0.7373 |
Data are presented as mean ± SD.
Intraoperative TEE prior to suspected myocardial ischaemia
In the 32 patients with graft failure, 8 patients (20.5%) (true positive) showed new intraoperative RWMAs as detected by TEE, 21 patients (53.8%) had normal examinations (false negative), no report was available in 8 patients (20.5%) and 2 examinations (5.1%) were excluded due to poor imaging quality. The sensitivity of TEE to detect graft failure was 20%, specificity 25%, positive predictive and negative values were 62.5% and 4.8%, respectively.
DISCUSSION
The aim of this study was to determine the appropriate treatment for patients with early graft dysfunction following CABG surgery. The major findings of the present study are: (i) patients with early re-intervention for early graft failure after CABG surgery had a higher number of graft/patient failure compared to patient managed conservatively, (ii) even with more graft failure per patient, there was a trend toward smaller size of MI in the early aggressive re-intervention group compared to the conservative group, and (iii) coronary angiography was a good tool to discriminate the aetiology of postoperative infarction (graft related or non-graft related).
Two important reports addressing revascularization of patient after angiographic confirmation of early graft failure have been published [4, 5]. Thielmann et al. demonstrated that conservative management of patients with early graft failure with large myocardial area at risk failed to prevent the development of severe myocardial pump failure. Early PCI can limit the progression towards LCOS and that early aggressive re-revascularization can limit the extent of myocardial cellular damage. Rasmussen et al. demonstrated that graft failure or incomplete revascularization was the cause of myocardial ischaemia early after CABG and that re-operation could be performed with low additional risks.
Overall, the incidence of early graft dysfunction is 3% following cardiac surgery [5]. The rate of graft occlusion before discharge varies from 3 to 12% for vein grafts, 3 to 4% for radial arteries and 1 to 2.5% for internal mammary arteries [11, 12].
Prevention of postoperative graft failure starts with harvesting vessels for CABG. A special attention must be taken not to injure the endothelium. At the end of the procedure, intraoperative assessment of the technical adequacy of distal graft anastomoses is of paramount importance. This holds true particularly for off-pump CABG, where technical errors requiring revision for distal anastomoses have been reported in up to 9.9% of cases [13]. Hence, non-permeable graft must be repaired or replaced rapidly when detected in the OR. The most common method of assessing graft patency intraoperatively is transit-time flowmetry, but this is of limited value since coronary flow is affected in many ways by coronary resistance and morphology of the anastomosis. This method is less accurate than angiography and may fail to detect non-occlusive, but haemodynamically significant stenosis [14]. Despite those limitations, the Doppler analysis of arterial and venous grafts is the simplest and the most widely available method to assess graft patency in the operating room. Intraoperative TEE has proven useful in detecting acute myocardial ischaemia and to predict postoperative MI [15]. However, in this study, the sensitivity of new intraoperative RMWAs to predict graft failure was rather low (20%) with a specificity of 25%. Possible explanations include that limited two regional wall motion analysis, one before CBP and one after CBP, and different loading conditions may influence regional wall motion analysis as well as inter observers differences, and the possibility of missing new RWMAs when performed in real-time. Moreover, ischaemic episodes may occur in the post-operative period after TEE probe removal. In this retrospective study, RWMAs with TEE were of limited value to predict early postoperative CABG failure. However, 20% of potential graft failures could have been corrected at the time of the initial surgical intervention.
The diagnosis of graft failure should be rapidly confirmed by coronary angiography when the clinical status of the patient allows it. Although this procedure has several important risks such as thromboembolic events, bleeding and contrast dye-induced nephropathy, recent data demonstrate that this procedure can be performed safely [4]. In our opinion, redo CABG without coronary angiography should be reserved for unstable patients who cannot wait for a definite angiographic diagnosis.
Surprisingly, 24–35% of patients undergoing coronary angiography after CABG for early graft dysfunction had normal patent grafts [4]. In this contemporary study, seven patients (18%) underwent coronary angiography for graft failure suspicion, and had normal grafts. These observations show that graft failure is the most common cause of ischaemia when suspicion is high. Angiographic confirmation of graft failure should be obtained whenever possible before return to the OR to avoid unnecessary reoperation. In this study, 0.6% of all patients having CABG underwent a coronary angiography for suspicion of early graft failure during the first 72 h. This is an underestimation of the postoperative incidence of MI, since patients without any diagnostic or therapeutic procedures were excluded from the analysis.
Postoperative graft failure is not a benign condition. It is of interest to note that in-hospital mortality is 15.8 and 15.4% in groups 1 and 2, respectively. This mortality is higher than the one predicted by the EuroSCORE (10.2 ± 6.8 and 7.8 ± 7.6 in groups 1 and 2, respectively).
Limitations
The results of this study should be interpreted with caution since it is a retrospective study of a single centre experience. Early graft dysfunction remains a rare event. However, the differences observed between the two groups may guide us in the management of our patients.
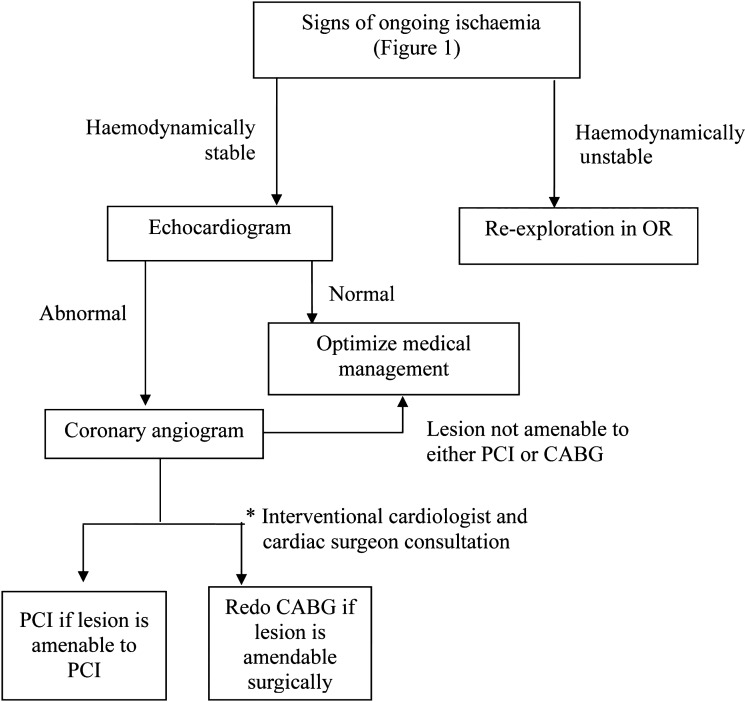
In conclusion, early graft failure after surgery is an infrequent but serious complication after CABG. Coronary angiography is a reliable tool to confirm the diagnosis and to determine the reason of ongoing ischaemia. This study suggests that once diagnosis of early graft dysfunction is confirmed, early re-intervention for repeat revascularization of the myocardium using PCI or redo surgery may be superior to conservative support management in selected cases. We propose a new algorithm of management for patient with signs of ongoing ischaemia early after CABG surgery (Fig. 3).
Figure 3:
Proposed algorithm for the management of early postoperative coronary artery bypass graft failure.
Conflict of interest: none declared.
REFERENCES
- 1.Chaitman BR, Alderman EL, Sheffield LT, Tong T, Fisher L, Mock MB, et al. Use of survival analysis to determine the clinical significance of new Q waves after coronary bypass surgery. Circulation. 1983;67:302–9. doi: 10.1161/01.cir.67.2.302. [DOI] [PubMed] [Google Scholar]
- 2.Jain U, Wallis DE, Moran JF. Significance of electrocardiographic ST elevation during coronary artery bypass surgery. Anesth Analg. 1994;78:638–43. doi: 10.1213/00000539-199404000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Force T, Hibberd P, Weeks G, Kemper AJ, Bloomfield P, Tow D, et al. Perioperative myocardial infarction after coronary artery bypass surgery. Clinical significance and approach to risk stratification. Circulation. 1990;82:903–12. doi: 10.1161/01.cir.82.3.903. [DOI] [PubMed] [Google Scholar]
- 4.Rasmussen C, Thiis JJ, Clemmensen P, Efsen F, Arendrup HC, Saunamäki K, et al. Significance and management of early graft failure after coronary artery bypass grafting: feasibility and results of acute angiography and re-re-vascularization. Eur J Cardiothorac Surg. 1997;12:847–52. doi: 10.1016/s1010-7940(97)00268-6. [DOI] [PubMed] [Google Scholar]
- 5.Thielmann M, Massoudy P, Jaeger BR, Neuhäuser M, Marggraf G, Sack S, et al. Emergency re-revascularization with percutaneous coronary intervention, reoperation, or conservative treatment in patients with acute perioperative graft failure following coronary artery bypass surgery. Eur J Cardiothorac Surg. 2006;30:117–25. doi: 10.1016/j.ejcts.2006.03.062. [DOI] [PubMed] [Google Scholar]
- 6.Thielmann M, Massoudy P, Schmermund A, Neuhäuser M, Marggraf G, Kamier M, et al. Diagnostic discrimination between graft-related and non-graft-related perioperative myocardial infarction with cardiac troponin I after coronary artery bypass surgery. Eur Heart J. 2005;26:2440–7. doi: 10.1093/eurheartj/ehi437. [DOI] [PubMed] [Google Scholar]
- 7.Holmvang L, Jurlander B, Rasmussen C, Thiis JJ, Grande P, Clemmensen P. Use of biochemical markers of infarction for diagnosing perioperative myocardial infarction and early graft occlusion after coronary artery bypass surgery. Chest. 2002;121:103–11. doi: 10.1378/chest.121.1.103. [DOI] [PubMed] [Google Scholar]
- 8.Raabe DS, Jr, Morise A, Sbarbaro JA, Gundel WD. Diagnostic criteria for acute myocardial infarction in patients undergoing coronary artery bypass surgery. Circulation. 1980;62:869–78. doi: 10.1161/01.cir.62.4.869. [DOI] [PubMed] [Google Scholar]
- 9.Breuer M, Schütz A, Gansera B, Eichinger W, Weingartner J, Kemkes B. Intraoperative local fibrinolysis as emergency therapy after early coronary artery bypass thrombosis. Eur J Cardiothorac Surg. 1999;15:266–70. doi: 10.1016/s1010-7940(99)00010-x. [DOI] [PubMed] [Google Scholar]
- 10.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, et al. American Heart Association Writing Group on Myocardial Segmentation and Registration for Cardiac Imaging. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–42. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 11.Fitzgibbon GM, Kafka HP, Leach AJ, Keon WJ, Hooper GD, Burton JR. Coronary bypass graft fate and patient outcome: angiographic follow-up of 5,065 grafts related to survival and reoperation in 1,388 patients during 25 years. J Am Coll Cardiol. 1996;28:616–26. doi: 10.1016/0735-1097(96)00206-9. [DOI] [PubMed] [Google Scholar]
- 12.Goldman S, Copeland J, Mortiz T, Henderson W, Zadina K, Ovitt T, et al. Starting aspirin therapy after operation. Effects on early graft patency. Department of Veterans Affairs Cooperative Study Group. Circulation. 1991;84:520–6. doi: 10.1161/01.cir.84.2.520. [DOI] [PubMed] [Google Scholar]
- 13.D'Ancona G, Karamanoukian HL, Soltoski P, Salerno TA, Bergsland J. Changing referral pattern in off-pump coronary artery bypass surgery: a strategy for improving surgical results. Heart Surg Forum. 1999;2:246–9. [PubMed] [Google Scholar]
- 14.Hol PK, Fosse E, Mork BE, Lundblad R, Rein KA, Lingaas PS, et al. Graft control by transit time flow measurement and intraoperative angiography in coronary artery bypass surgery. Heart Surg Forum. 2001;4:254–7. discussion 257–8. [PubMed] [Google Scholar]
- 15.Leung JM, O'Kelly B, Browner WS, Tubau J, Hollenberg M, Mangano DT. Prognostic importance of postbypass regional wall-motion abnormalities in patients undergoing coronary artery bypass graft surgery. SPI Research Group. Anesthesiology. 1989;71:16–25. doi: 10.1097/00000542-198907000-00004. [DOI] [PubMed] [Google Scholar]