Abstract
We compare results of a hybrid monopolar vs. a hybrid bipolar thoracoscopic approach employing radiofrequency (RF) sources for the surgical treatment of lone atrial fibrillation (LAF). From January 2008 to June 2010, 19 patients (35.1%) underwent RF monopolar/monolateral RF ablation, whereas 35 (64.9%) had RF bipolar/bilateral thoracoscopic ablation. One-year time-related prevalence of postoperative AF was 13.3 (11.0–17.4) and 5.2% (4.2–6.7), in monopolar and bipolar groups, respectively (P < 0.001). It was 21.1 (17.6–24.9) vs. 8.2% (5.1–11.6) in long standing persistent (P < 0.001), 13.2 (10.6–17.8) vs. 3.8% (1.4–6.9) in persistent (P < 0.001) and 5.6 (2.8–8.3) vs. 3.2% (1.0–6.5) in paroxysmal AF (P = 0.64). At 12 months, estimated prevalence of anti-arrhythmic drugs was 26 (22.4–30.1) and 18.0% (15.5–21.7, P = 0.04), whereas prevalence of Warfarin use was 48.2 (44.2–52.2) and 29.0% (26.2–33.1, P < 0.001) in the monopolar and bipolar groups, respectively. Left atrial (LA) reverse remodelling occurred in 47.3% of monopolar patients (n = 9) and in 77.1% of bipolar patients (P = 0.03). The hybrid bilateral approach with a bipolar device for the treatment of LAF showed a good 1-year success rate independently of the AF type and seems to be the better choice for longstanding persistent and persistent LAF.
Keywords: Atrial fibrillation, Ablation, Minimally invasive, Catheter ablation, Surgical ablation
INTRODUCTION
The hybrid procedure has recently been introduced to overcome the shortcomings of percutaneous catheter ablation and a video-assisted thoracoscopic epicardial procedure and, together, to combine their advantages [1]. The technique originally described combines a bilateral thoracoscopic epicardial approach employing bipolar radiofrequency (RF) followed by a percutaneous trans-septal procedure [2]. In order to reduce the invasiveness of the procedure, we introduced, in selected patients, a hybrid single-sided approach employing a monopolar RF source through a right thoracoscopy.
We present our short-term experience with the hybrid procedure in lone atrial fibrillation (LAF) and compare results of the hybrid monopolar vs. the hybrid bipolar RF ablation.
MATERIALS AND METHODS
Patient population
The Ethics Committee approved the study and waived the need for patient consent according to the national law regulating observational retrospective studies (Dutch WMO law). However, all patients signed an informed consent to use data for scientific purposes.
Between January 2008 and June 2010, 54 consecutive patients with LAF underwent a single step hybrid minimally invasive surgical treatment of LAF combined with a percutaneous endocardial step employing a RF source. Nineteen patients (35.1%) underwent RF monopolar-right thoracoscopic ablation, whereas 35 (64.9%) underwent RF bipolar bilateral thoracoscopic procedure. All operations were performed by the same cardiac surgeon (M.L.M.).
LAF was defined following American College of Cardiology/American Heart Association/European Society of Cardiology (ACC/AHA/ESC) guidelines [3] and updated ESC Guidelines were followed to distinguish the type of AF and to score the AF-related symptoms [4].
Indication for minimally invasive surgery was based on the Heart Rhythm Society/European Heart Rhythm Association/European Cardiac Arrhythmia Society (HRS/EHRA/ECAS) Guidelines [5].
Patient characteristics are shown in Table 1. No significant difference was detected between groups.
Table 1:
Hybrid procedures: preoperative data (n = 54)
| Monopolar (n = 19) | Bipolar (n = 35) | P-value | |
|---|---|---|---|
| Age | 61.2 ± 8.6 | 57.1 ± 9.5 | 0.073 |
| M/F | 16/3 (84.2/15.8) | 24/11 (68.5/31.4) | 0.23 |
| BMI | 27.6 ± 4.6 | 26.8 ± 3.6 | 0.84 |
| Diabetes | 0 (0) | 1 (2.8) | 0.71 |
| Hypertension | 7 (36.8) | 15 (42.8) | 0.55 |
| TIA/CVA | 1 (5.3) | 0 (0) | 0.28 |
| Type of preoperative AF | |||
| Paroxysmal | 5 (26.3) | 16 (45.7) | 0.054 |
| Persistent | 4 (21.1) | 8 (22.8) | |
| Long-standing persistent | 10 (52.6) | 11 (31.5) | |
| Prevalence of AF (68% CI) | |||
| Paroxysmal | 20.0 [16.8–23.3] | 22.3 [19.2–257] | 0.74 |
| Persistent | 34.7 [31.2–38.9] | 32.6 [29.1–36.9] | |
| Long-standing persistent | 63.6 [59.9–67.1] | 61.3 [57.4–65.0] | |
| EHRA Score | 4 [3–4] | 4 [3–4] | >0.9 |
| Duration of preoperative AF (years) | 5 [3–8.5] | 5 [4.2–9.0] | 0.66 |
| Anti-arrhythmic drugs | |||
| Amiodaron | 2 (10.5) | 7 (20.0) | 0.08 |
| Dysopiramide | 1 (5.3) | 2 (5.7) | |
| Flecainide | 6 (31.5) | 15 (42.8) | |
| Propaphenon | 1 (5.3) | 2 (5.7) | |
| Sotalol | 5 (26.3) | 11 (31.4) | |
| Electrical cardioversion | 15 (78.9) | 17 (48.5) | 0.06 |
| Previous catheter ablation | |||
| For AF | 6 (31.5) | 12 (34.3) | 0.65 |
| For atrial flutter | 5 (26.3) | 9 (25.7) | |
| Preoperative pace maker | 2 (10.5) | 2 (5.3) | 0.88 |
| Anticoagulant status | |||
| Sodium Warfarin | 16 (84.2) | 30 (85.7) | 0.76 |
| Aspirin | 5 (26.3) | 10 (28.5) | |
| LAVI (ml/m2) | 47 ± 11 | 50 ± 12 | 0.08 |
| LAMax (ml/m2) | 49 ± 20 | 52 ± 25 | 0.63 |
| LAMin (ml/m2) | 30 ± 15 | 32 ± 18 | 0.90 |
| LAEF (%) | 38 ± 12 | 38 ± 11 | 0.90 |
| LA A-P (cm) | 5.0 ± 0.5 | 5.2 ± 0.5 | 0.77 |
| LA S-I (cm) | 6.4 ± 0.5 | 6.3 ± 0.7 | 0.60 |
Normal data were presented as mean ± 1 standard deviation (SD), non-parametric data as median and [interquartile range] and discrete data as percentages (%).
M/F: male/female; BMI: body mass index; AF: atrial fibrillation; CI: confidence interval; EHRA: European Heart Rhythm Association; LAVI: (biplane) left atrial volume index; LAMax: maximum left atrial volume; LAMin: minimum left atrial volume; LAEF: left atrial emptying fraction; LA A-P: left atrial antero-posterior diameter; LA S-I: left atrial antero-posterior diameter.
Follow-up and assessment of AF
All patients were followed-up according to the Heart Rhythm Society/European Heart Rhythm Association/European Cardiac Arrhythmia Society (HRS/EHRA/ECAS) expert consensus statement on catheter and surgical ablation of AF [5]. Outcomes were reported following the Society of Thoracic Surgeon (STS) guidelines [6].
Seven-day Holter monitoring (HM) was performed after 3 months, 6 months and 1 year. All patients reached 1-year follow-up. Monitoring was carried out with an external loop recorder (Del Mar Reynolds, Spacelabs Healthcare, Issaquah, WA, USA) and analysed with Lifescreen Software (Del Mar Reynolds, Spacelabs Healthcare, Issaquah, WA, USA). For analysis, three rhythms were considered postoperative AF:AF, atrial flutter or atrial tachycardia lasting more than 30 s. In addition, all electrocardiograms (ECG) performed at the discretion of referring physicians/cardiologists during the first 3 months after surgery and in the time between Holter examinations were included when patients had at least two records available for the analysis. Each ECG and Holter was treated as discrete data to calculate estimated prevalence [5]. A total of 628 postoperative Holter/ECG data were retrieved (bilateral thoracoscopy, n = 408; right thoracoscopy, n = 222).
Anticoagulation and anti-arrhythmic therapy
Anti-arrhythmic drugs (AAD) were given postoperatively to all patients but, although we recommend discontinuing AAD 3 months after ablation if the patients appear to be AF free, their continued use was at the discretion of referring cardiologists.
Electrical cardioversion was not attempted for patients who remained in AF after the surgical procedure and was reserved for patients who were still in AF after 3 and 6 months.
Warfarin was administered on postoperative day 2 with INR target of 2.5 and stopped after 3 months if the Holter recording showed a sinus rhythm or patients had a low thromboembolic risk (CHADS2 score <2).
Echocardiography
Echocardiography was performed preoperatively and at follow-up appointments using a commercially available echocardiographic system(Philips iE33; Philips Medical Systems, Eindhoven, The Netherlands) and analysed ‘off-line’ by an experienced echocardiographer (F.L.) using the Xcelera software (Philips Medical Systems).
LA maximum antero-posterior (A-P) and LA superior–inferior (S-I) diameters, LA maximum volume (LA max) and LA minimum volume (LA min) were measured [7]. LA emptying fraction (LAEF) was calculated as ([La max − LA min]/La max) × 100. LA maximum volume was also measured by the biplane area–length method [8] and indexed to body surface area (LAVI) LA remodelling (LARR) was defined as a reduction in LAVI ≥ 15% at late follow-up [9].
Surgery
Indication for minimally invasive surgery was based on current guidelines [5]. The right thoracoscopic approach was preferred for patients with greater than or equal moderate chronic obstructive pulmonary disease following European Respiratory Society (ERS) diagnostic criteria [10].
Surgery was carried out as previously described [1]. The lesion set in the two groups is shown in Table 2. More details can be found online (http://www.sandrogelsomino.eu/supplementalfiles/interactcardiovasctoracsurg/2011/ICVTS2011-Surgery.pdf).
Table 2:
Hybrid procedures: lesion set (n = 54)
| Monopolar (n = 19) | Bipolar (n = 35) | |
|---|---|---|
| Left atrium | ||
| Right PVs isolation | 19 (100) | 35 (100) |
| Left PVs isolation | 19 (100) | 35 (100) |
| Inferior line | 19 (100) | 31 (88.5) |
| Roof line | 19 (100) | 32 (91.4) |
| Isthmus lesion | 3 (15.7) | 7 (20.0) |
| Endocardial gaps closure | 17 (88.4) | 5 (14.2) |
| Excision of LA appendage | – | 8 (22.8) |
| Closure of LA appendage with a clip | – | 7 (20.0) |
| Right atrium | ||
| Superior vena cava to inferior vena cava lesion | – | 10 (28.5) |
| Superior vena cava circumferential isolation | – | 8 (22.8) |
| Inferior vena cava circumferential isolation | – | 3 (8.5) |
| Cavo-tricuspid isthmus line | 2 (10.5) | 3 (8.5) |
| Ablation of autonomic Ganglia | 19 (100) | 35 (100) |
Discrete data were presented as numbers (percentage).
PVs: pulmonary veins; LA: left atrium.
Statistical analysis
Normal values were expressed as mean ± 1 standard deviation (SD), non-normal values as median and interquartile range (IQR) and categorical variables as percentages. t-Test, Wilcoxon and McNemar's tests were employed where appropriate.
We analysed all intermittent data available in terms of time-related prevalence of atrial fibrillation with a multiphase hazard decomposition method. Time-related prevalences are presented as percentages with asymmetric 68% confidence limits (CLs), comparable to ±1 standard error. The CLs for AF prevalence were obtained with the bootstrap percentile method.
Analyses of the prevalence of AF do not account for anti-arrhythmic medications. Prevalence of anti-arrhythmic medication use was estimated by mixed modelling based on medication use at each follow-up assessment. With the same method, we estimated the prevalence of Warfarin use at the time of each follow-up. Finally, the use of electrical cardioversion was analysed as a repeated event and is presented as cumulative incidence (events per patient) [6] and the Gray's test was employed to compare two different cumulative incidence curves.
Statistical analyses were performed using SPSS release 12.0 (SPSS, Chicago, IL, USA) and Curve Expert Professional release1.0.1 (D.G. Hyams, Chattanooga, TN, USA). P-values <0.05 were considered significant.
RESULTS
Early results
In the monopolar group, none of the patients showed entrance and/or exit block after the epicardial ablation. Seventeen patients had at least one PV not isolated, which needed an endocardial touch-up. After completing PV isolation endocardially, all these patients had a conduction delay superior to 200 ms at the level of the posterior wall of the LA, but none had block.
In the bipolar group, two patients with paroxysmal AF had no extra lesions besides PV antral ablation because AF was no longer inducible. In three patients with inducible AF after PV ablation, we performed an additional roof line and no more arrhythmias were seen. A roof and an inferior line were carried out in the other 30 patients of the bilateral group. During endocardial mapping, 30 patients (86%) showed entrance and exit block, whereas in 3 patients (8.5%) there was a clear gap at the junction of the right superior PV with the roof of the left atrium, 1 patient (2.8%) showed a gap in the lateral portion of the roof line and 1 patient (2.8%) had a gap in the middle of the inferior line. These gaps were closed endocardially.
It was possible to complete all the procedures as planned without any conversion to cardiopulmonary bypass. Median operative time was 216 min (IQR 132–391) in the monolateral procedure and 268 min (IQR186–477) in the bilateral procedure (P < 0.001). There were neither early deaths nor complications during the postoperative course. Median intensive care unit and median in-hospital length of stay were comparable in the two groups (6.9 h [IQR4.0–14.0] vs. 6.2 h [5.5–13.4], P = 0.61; 3.6 days [2.7–4.3] vs. 3.4 days [2.6–4.1], P = 0.8).
Follow-up and AF recurrence
No patient died during the follow-up. There was a significant improvement in median EHRA (European Hear Rhythm Association) score in both groups (both, 1 [IQR 1–2], P < 0.001 vs. baseline). Finally, no thromboembolic event occurred during the follow-up.
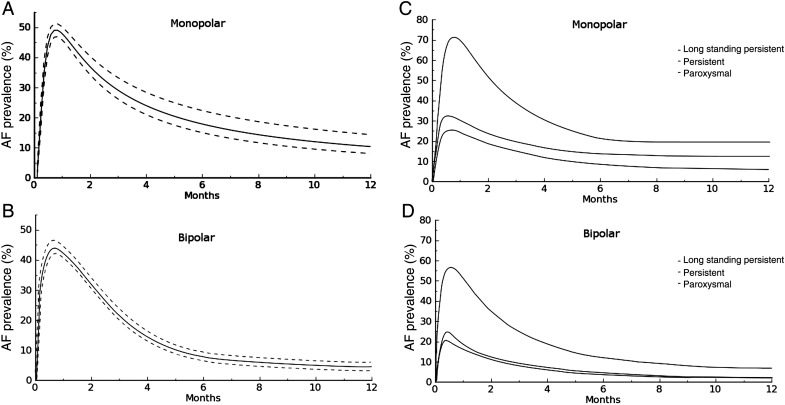
Time-related prevalence of postoperative AF (Fig. 1a and b) in patients who underwent surgery using the monolateral thoracoscopic approach or bilateral thoracoscopic ablation were 44.4 (41.3–47.4) and 35.5% (33.2–38.2) at 2 weeks (P = 0.06), 30.4 (27.3–34.9) and 27.4% (24.5–31.4) at 3 months (P = 0.08), 14.2 (11.6–18.1) and 6.6% (5.8–8.3) at 6 months (P = 0.001), 13.3 (11.0–17.4) and 5.2% (4.2–6.7) at 12 months (P < 0.001), respectively.
Figure 1:
(A) Time-related prevalence of atrial fibrillation (solid line) and 68% CLs (broken lines) after a hybrid monolateral approach with a monopolar device. (B) Time-related prevalence of atrial fibrillation (solid line) and 68% CLs (broken lines) after a hybrid bilateral approach with bipolar device. (C) Time-related prevalence of atrial fibrillation by AF type after a hybrid monolateral approach with a monopolar device. (D) Time-related prevalence of atrial fibrillation by AF type after a hybrid bilateral approach with a bipolar device.
Fig. 1c and d shows the estimated prevalence of postoperative atrial fibrillation by AF type. At 1 year, it was 21.1 (17.6–24.9) and 8.2 (5.1–11.6) in long standing persistent (P < 0.001), 13.2 (10.6–17.8) and 3.8% (1.4–6.9) in persistent (P < 0.001) and 5.6 (2.8–8.3) and 3.2% (1.0–6.5) in paroxysmal AF (P = 0.64) in the monolateral and bilateral groups, respectively.
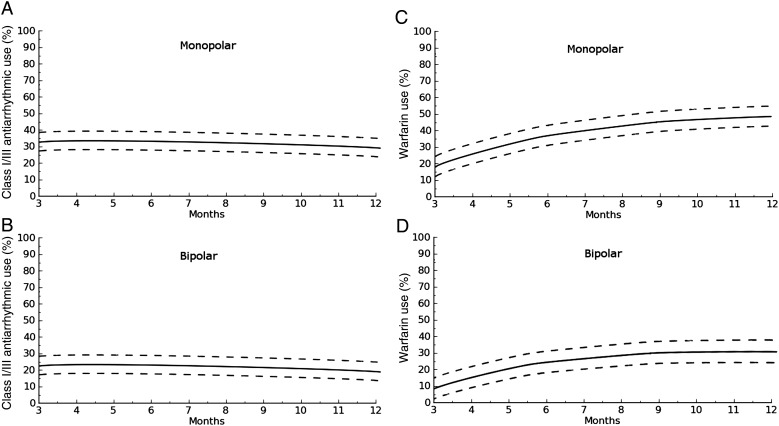
At 12 months, estimated prevalence of AAD (Fig. 2a and b) was 26 (22.4–30.1) and 18.0% (15.5–21.7, P = 0.04).
Figure 2:
(A) Time-related prevalence of class I/III anti-arrhythmic use after a hybrid monolateral approach with a monopolar device. (B) Time-related prevalence of class I/III anti-arrhythmic use after a hybrid bilateral approach with bipolar device. (C) Time-related prevalence of Warfarin use after a hybrid monolateral approach with a monopolar device. (D) Time-related prevalence of Warfarin use after a hybrid bilateral approach with bipolar device.
When comparing prevalence of AAD by AF type, it was significantly lower in the bilateral group in patients in long-standing persistent AF (26.2 [17.6–30.4] vs. 38.1% [34.3–43.4], P < 0.001) and in persistent (16.0 [13.4–20.6] vs. 26.3% [22.2–30.7], P < 0.001) where it was comparable in paroxysmal AF (12.2 [9.7–15.4] vs. 14.9% [9.9–17.8], P = 0.7).
One-year prevalence of Warfarin use was 48.2% (44.2–52.2) in the monolateral and 29.0% (26.2–33.1, P < 0.001) in the bilateral group, respectively (Fig. 2c and d).
This difference was still observed in long standing persistent (52.5 [48.8–55.2] vs. 35.5% [29.7–38.2], P < 0.001) and persistent (35.3 [32.5–39.2] vs. 22.7% [16.1–24.8], P < 0.001), whereas in paroxysmal AF data were comparable (19.4 [16.3–23.3] vs. 17.3% [15.4–21.2], P = 0.6).
Finally, the cumulative incidence of electrical cardioversion was 0.05 per patient in the monolateral group and 0.04 per patient in the bilateral group (P = 0.841).
Echocardiographic results
Table 3 shows echocardiographic data. At 3-month follow-up, biplane LAVI was significantly reduced only in patients who underwent the bipolar hybrid procedure (P = 0.05 and P = 0.03 in monopolar and bipolar, respectively). It further decreased in both groups without reaching statistical significance, but it was lower in the bilateral group. Based on the cut-off value (≥15% decrease in LAVI), LARR occurred in 47.3% (n = 9) of patients in the monolateral group and in 85.7% (n = 30) of patients in the bilateral group (P < 0.001).
Table 3:
Hybrid procedures: echocardiographic results (n = 54)
| Monopolar (n = 19) | Bipolar (n = 35) | |
|---|---|---|
| LAVI (ml/m2) | ||
| 3 months | 45 ± 10 | 35 ± 8a |
| 12 months | 40 ± 11 | 32 ± 7a |
| P-value | 0.09 | 0.1 |
| LAMax (ml/m2) | ||
| 3 months | 46 ± 15 | 40 ± 12a |
| 12 months | 45 ± 13 | 36 ± 11a |
| P-value | 0.7 | 0.6 |
| LAMin (ml/m2) | ||
| 3 months | 27 ± 10 | 22 ± 6a |
| 12 months | 25 ± 8 | 19 ± 5a |
| P-value | 0.86 | 0.8 |
| LAEF (%) | ||
| 3 months | 41 ± 14 | 49 ± 15a |
| 12 months | 43 ± 14 | 54 ± 14a |
| P-value | 0.56 | 0.9 |
| LA A-P (cm) | ||
| 3 months | 3.9 ± 0.4 | 3.7 ± 0.4a |
| 12 months | 3.7 ± 0.4 | 3.4 ± 0.3a |
| P-value | 0.73 | 0.04 |
| LA S-I (cm) | ||
| 3 months | 5.9 ± 0.6 | 5.7 ± 0.5a |
| 12 months | 5.8 ± 0.6 | 5.2 ± 0.3a |
| P-value | 0.81 | 0.008 |
Normal data were presented as mean ± 1 standard deviation (SD).
LAVI: (biplane) left atrial volume index; LAMax: maximum left atrial volume; LAMin: minimum left atrial volume; LAEF: left atrial emptying fraction; LA A-P: left atrial antero-posterior diameter; LA S-I: left atrial antero-posterior diameter.
aSignificance vs. baseline.
LAEF rose only in patients who underwent the bipolar hybrid procedure (P = 0.6 and P = 0.02 in monopolar and bipolar, respectively). It further increased, but not significantly in both groups at 12-month control. Nonetheless, LAEF was significantly higher in the bilateral group at each control. Both LA diameters decreased significantly at 3 months (LA A-P, P = 0.02; LA S-I, P = 0.03) and 1 year (LA A-P, P = 0.04; LA S-I, P = 0.008) in patients who underwent the bipolar hybrid procedure. In patients who underwent monopolar hybrid ablation, LA diameters decreased significantly at 3 months (LA A-P, P = 0.02; LA S-I, P = 0.03), whereas at 1 year there was a further reduction (LA A-P, P = 0.73; LA S-I, P = 0.81) which was not statistically significant.
DISCUSSION
Minimally invasive, off-pump, video-assisted thoracoscopic surgical techniques are being employed worldwide, but success rates reported in the literature are highly variable [9] and complications are not infrequent [10]. The hybrid approach was introduced to overcome these limitations [1]. Nonetheless, no information exists as to whether, in hybrid procedures, the theoretical advantages of a less invasive monopolar right thoracoscopic approach outweigh the clear advantages of employing bipolar RF clamps (transmural and continuous lesions, ligation/excision of the left atrial appendage, LAA) in the bipolar/bilateral thoracoscopic approach.
In our experience, time-related prevalence of AF, AAD and Warfarin use at 1 year was significantly lower in the bipolar group in long-standing persistent and persistent AF, whereas in paroxysmal AF there was no statistically significant difference. Longer duration of AF is known to cause progressive remodelling and increased LA volume and long-standing persistent and persistent AF may be considered to be advanced stages of the arrhythmia characterized by significant changes in the atrial tissue and muscle (substrate modification) that leads to chaotic electrical activity. Thus, these patients might need more areas in the heart ablated compared with what is done in the monopolar approach and they might benefit much more from the bilateral/bipolar procedure which increases the likelihood that ablation lines are transmural. Indeed, it has been demonstrated that monopolar sources are unable to create transmural lesions when used from the epicardial surface on the beating heart especially when the wall thickness is >4 mm [11, 12]. Because of the absence of transmural lesions with this monopolar device (temperature setting at 60°C and timing at 120 s), there is a necessity to complete the lesions endocardially. This is easily achieved at the level of the PVs, but much more difficult at the roof and inferior line. This explains the acceptable results in patients with paroxysmal (where PV isolation is mostly the treatment of choice), but insufficient for patients with non-paroxysmal. With this current knowledge, we have stopped using a monopolar device for the surgical treatment of AF, even for patients where a monolateral approach has a potential advantage because of reduced pulmonary function. In these patients, we now perform a monolateral approach from the right or left side, depending on the wish to close the LAA, using bipolar devices and isolating the opposed PVs endocardially with RF or cryo energy.
However, gap lesions occurred with the bipolar device located at the tip of the bipolar clamp at the RSPV or in the roof or inferior line. The roof and inferior line were created with a bipolar unidirectional device (Coolrail) positioned on the epicardial side of the left atrium. This catheter does not have the advantage of a bipolar bidirectional clamp where the ablated tissue is squeezed between two jaws (no heat-sink effect) and the energy is driven from one jaw to the other. The bipolar unidirectional devices have limitations regarding the lesion depth and do not guarantee transmural lesions. The gaps at the level of the tip of the bipolar clamp at the RSPV can be explained by the anatomy of the patient and the rigidity of the bipolar clamp. It is not always possible to get the tip of the clamp sufficiently beyond the superior border of the RSPV because of the vicinity of the right PA and the risk of perforation of this structure. We always overlap this area with multiple applications from a bipolar unidirectional device while creating the roof line, but apparently this does not guarantee a transmural lesion.
Finally, only patients who underwent the bilateral approach showed a significant improvement in LA function as well as a significant reduction in LA dimensions and LAVI and, based on the cut-off value (≥15% decrease in LAVI) LARR occurred in 47.3% of patients in the monolateral group and in 85.7% of patients in the bilateral group (P < 0.001). Due to the small number of patients in the monolateral group, we could not compare LAEF, LA diameters and LA volumes by AF type. However, a high percentage of patients (73.7%) in this group had a long-standing persistent or persistent AF and a greater extent of substrate modifications and atrial structural remodelling, and this could explain the lack of LARR in this group. Indeed, all the five patients with paroxysmal AF in the monopolar group showed a ≥15% decrease in LAVI.
Limitations of the study
This study has some limitations which have to be pointed out. The small patient population and the retrospective nature of the study do not allow us to draw any conclusion about the effectiveness of this technique. Furthermore, the follow-up was limited. Larger series with long-term follow-up are needed to confirm the effectiveness of the hybrid approach and to establish whether the monopolar right-sided thoracoscopic hybrid procedure may represent a suitable less-invasive choice for all LAF patients referred to surgery.
Furthermore, while data regarding postoperative AF prevalence were obtained from a large number of observations and this allowed us to compare prevalence by AF type, we could not compare echocardiographic parameters in a subgroup of patients.
Finally, our study did not compare results of the hybrid approach to catheter ablation. However, this will be the subject of ongoing studies.
CONCLUSIONS
Even with the above-mentioned limitations, we can conclude that the hybrid bilateral approach with a bipolar device for the treatment of LAF showed a good 1-year success rate independently of the AF type and a seems to be the better choice for LAF fibrillation referred to surgery. In contrast, the hybrid monolateral approach was less effective in long-standing persistent and persistent LAF.
ACKNOWLEDGEMENTS
We gratefully acknowledge Pol Chambille and Monique De Jong for their invaluable help. We thank Dr Orlando Parise for statistical analysis and James Douglas for the English revision of the manuscript.
Conflict of interest: M.L.M. is consultant/advisor for Atricure and Estech. Other co-authors have no conflict of interest.
REFERENCES
- 1.Pison L, La Meir M, van Opstal J, Blaauw Y, Maessen JG, Crijns HJ. Hybrid thoracoscopic surgical and transvenous catheter ablation of atrial fibrillation. J Am Coll Cardiol. 2011 doi: 10.1016/j.jacc.2011.12.055. in press. [DOI] [PubMed] [Google Scholar]
- 2.Krul SP, Driessen AH, van Boven WJ, Linnenbank AC, Geuzebroek GS, Jackman WM, et al. Thoracoscopic video-assisted pulmonary vein antrum isolation, ganglionated plexus ablation and periprocedural confirmation of ablation lesions. First results of a hybrid surgical-electrophysiological approach for atrial fibrillation. Circ Arrhythm Electrophysiol. 2011;4:262–70. doi: 10.1161/CIRCEP.111.961862. [DOI] [PubMed] [Google Scholar]
- 3.Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines; European Society of Cardiology Committee for Practice Guidelines; European Heart Rhythm Association; Heart Rhythm Society. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:257–354. doi: 10.1161/CIRCULATIONAHA.106.177292. [DOI] [PubMed] [Google Scholar]
- 4.Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, et al. European Heart Rhythm Association; European Association for Cardio-Thoracic Surgery. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Europace. 2010;12:1360–420. doi: 10.1093/europace/euq350. ESC Committee for Practice Guidelines. Document Reviewers. [DOI] [PubMed] [Google Scholar]
- 5.Calkins H, Brugada J, Packer DL, Cappato R, Chen SA, Crijns HJ, et al. European Heart Rhythm Association (EHRA); European Cardiac Arrhythmia Society (ECAS); American College of Cardiology (ACC); American Heart Association (AHA); Society of Thoracic Surgeons (STS) HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. Heart Rhythm. 2007;4:816–61. doi: 10.1016/j.hrthm.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Shemin RJ, Cox JL, Gillinov AM, Blackstone EH, Bridges CR Workforce on Evidence-Based Surgery of the Society of Thoracic Surgeons. Guidelines for reporting data and outcomes for the surgical treatment of atrial fibrillation. Ann Thorac Surg. 2007;83:1225–30. doi: 10.1016/j.athoracsur.2006.11.094. [DOI] [PubMed] [Google Scholar]
- 7.Kuppahally SS, Akoum N, Burgon NS, Badger TJ, Kholmovki EG, Vijayakumar MS, et al. Left atrial strain and strain rate in patients with paroxysmal and persistent atrial fibrillation: relationship to left atrial structural remodeling detected by delayed enhancement-MRI. Circ Cardiovasc Imaging. 2010;3:231–9. doi: 10.1161/CIRCIMAGING.109.865683. [DOI] [PubMed] [Google Scholar]
- 8.Jiamsripong P, Honda T, Reuss CS, Hurst RT, Chaliki HP, Grill DE, et al. Three methods for evaluation of left atrial volume. Eur J Echocardiogr. 2008;9:351–5. doi: 10.1016/j.euje.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Tops LF, Delgado V, Bertini M, Marsan NA, Den Uijl DW, Trines SA, et al. Left atrial strain predicts reverse remodeling after catheter ablation for atrial fibrillation. J Am Coll Cardiol. 2011;57:324–31. doi: 10.1016/j.jacc.2010.05.063. [DOI] [PubMed] [Google Scholar]
- 10.Nathell L, Nathell M, Malmberg P, Larsson K. COPD diagnosis related to different guidelines and spirometry techniques. Respir Res. 2007;8:89. doi: 10.1186/1465-9921-8-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gelsomino S, La Meir M, Lucà F, Lorusso R, Crudeli E, Vasquez L, et al. Treatment of lone atrial fibrillation: a look at the past, a view of the present and a glance at the future. Eur J Cardiothorac Surg. doi: 10.1093/ejcts/ezr222. doi:10.1093/ejcts/ezr222. [DOI] [PubMed] [Google Scholar]
- 12.Oh YS, Yoon JS, Jo KH, Kim HW. Total occlusion of both right-sided pulmonary veins after radiofrequency ablation for atrial fibrillation. Eur J Cardiothorac Surg. 2011;40:769–70. doi: 10.1016/j.ejcts.2010.12.007. [DOI] [PubMed] [Google Scholar]