Abstract
Reduction in mediastinal adhesions is an issue in cardiac surgery. To evaluate a porcine-bioengineered collagen membrane (Cova™ CARD) intended to promote tissue regeneration, 18 sheep underwent a sternotomy and a 30 min period of cardiopulmonary bypass. They were divided into three equal groups: pericardium left open, placement of an e-polytetrafluoroethylene membrane (Preclude®) taken as a non-absorbable substitute comparator and placement of the absorbable Cova™ CARD membrane. Four months thereafter, the study animals underwent repeat sternotomy and were macroscopically assessed for the degree of material resorption and the intensity of adhesions. Explanted hearts were evaluated blindly for the magnitude of the inflammatory response, fibrosis and epicardial re-mesothelialization. The bioengineered membrane was absorbed by 4 months and replaced by a loosely adherent tissue leading to the best adhesion score. There was no inflammatory reaction (except for a minimal one in an animal). Fibrosis was minimal (P = 0.041 vs Preclude®). The highest degree of epicardial re-mesothelialization, albeit limited, was achieved by the bioengineered group in which five of six sheep demonstrated a new lining of mesothelial cells in contrast to two animals in each of the other groups. This collagen membrane might thus represent an attractive pericardial substitute for preventing post-operative adhesions.
Keywords: Pericardial adhesions, Biomaterials, Reoperation, Pericardium
INTRODUCTION
Despite continuous improvements, reoperations in cardiac surgery remain challenging because injury to the heart, great vessels or bypass conduits can be life-threatening. Several pericardial substitutes have been developed that broadly fall into two categories: non-absorbable materials, exemplified by the e-polytetrafluoroethylene (ePTFE) membrane, and absorbable membranes. The latter are expected to provide superior healing by guiding tissue regeneration [1]. However, they raise technical challenges as mechanical strength and suturability to provide good handling characteristics; and biological challenges because they need to be as biocompatible as possible and degrade according to a time frame matching the kinetics of the natural healing process without releasing pro-inflammatory by-products. The present study was designed to test a new bioengineered collagen pericardial substitute (Cova™ CARD, Biom'Up, Lyon, France) in a clinically relevant large animal model of sternotomy and cardiopulmonary bypass (CPB).
MATERIALS AND METHODS
Eighteen pre-Alpes sheep, weighing ∼60 kg, were used in this study. The study was approved by our Institutional Review Board and conducted in compliance with the European Convention on Animal Care.
Preparation of the absorbable pericardial membrane
Cova™ CARD is a CE-marked patented membrane made of purified porcine tendon type I collagen (Fig. 1) according to a preparation protocol described previously [2].
Figure 1:
Bioengineered collagen Cova™ CARD membrane.
Surgical protocol
After isoflurane-based anaesthesia and endotracheal intubation, a median sternotomy was performed, the pericardium was opened longitudinally and a 4 cm wide, 20 cm long strip of pericardium was removed. Extracorporeal circulation was instituted between the right atrium and the ascending aorta and kept on for 30 min at normothermia. The epicardial surface was strongly scratched with a saw blade to provoke a local blood oozing, autologous blood was poured on it and the sheep were then randomly allocated to one of the following three groups. In six animals, the pericardial edges were not re-approximated, thus leaving a central defect similar to what commonly occurs after cardiac operations (controls). In six sheep, the defect was closed by a e-PTFE membrane (Preclude®, Gore Medical, W.L. Gore & Associates, Inc., Flagstaff, USA), tailored at the 10 cm large × 15 cm long dimensions and anchored to the pericardial edges by 4–0 polypropylene sutures. In six other animals, the defect was closed with the Cova™ CARD membrane, tailored at the same size and similarly anchored to the pericardial edges. A pericardial drainage tube was inserted with high vacuum suction with a Drainobag® (B. Braun Medical, Boulogne-Billancourt, France) for 24 h and the chest was closed conventionally with steel wires.
Four months later, the animals underwent repeat sternotomy under a similar anaesthesia protocol. The surgeon graded adhesion (from Grade 0: no adhesion to Grade 3: dense adhesions requiring sharp dissection) formation according to the score defined by Heydorn et al. [3]. One score was established for each animal. The sheep were then euthanized with an overdose of IV sodium pentobarbital.
Pathology
An in-block fragment was obtained from the anterior surface of the heart which comprised, from the inside to the outside, the right ventricular wall, the adhesion tissue and occasionally bone fragments. Specimens were fixed in 10% formaldehyde. For each animal, five fragments were systematically embedded in paraffin and cut into 5-µm-thick slices. The sections were stained with haematoxylin–eosin to assess the inflammatory reaction and fibrosis, identify potential remnants of the pericardial substitute and look for a mesothelial cell lining.
Sections were examined by a pathologist blinded to the treatment group. The severity of the inflammatory reaction was based on the quantification of inflammatory cells and inflammatory foci and graded using an inflammatory reaction score derived from that described by Lu et al. [4] (from Grade 0 (no cell infiltration) to Grade 3 (diffuse inflammatory cell infiltration)). Fibrosis was assessed with regard to its extent (0 = limited; 1 = extensive), density (0 = loose; 1 = dense) and thickness (0 = thin; 1 = thick) and a composite fibrosis score was created (from 0 to 3). The re-mesothelialization of the epicardial surface was assessed after immunostaining with an antibody against cytokeratins AE1/AE3 (Dako, Trappes, France), diluted at 1/100. Antigen retrieval used citrate buffer. The sections were revealed using the Avidin-Biotin Complex technique. The extent of immunolabeling was classified from 0 to 3 (0 = no cell marking; 1 = lining involving less than one-third of the section area; 2 = lining involving between one-third and two-thirds of the section area; 3 = almost complete lining marking). Five blocks were assessed for each animal leading to a score ranging from 0 to 15.
Statistical analysis
The Kruskall–Wallis test was used for assessing the statistical difference between the three groups. Statistical significance was set at the 0.05 level. If a difference was found significant, a Mann–Whitney–Wilcoxon test with exact P-values was subsequently performed within the groups.
RESULTS
Macroscopic findings
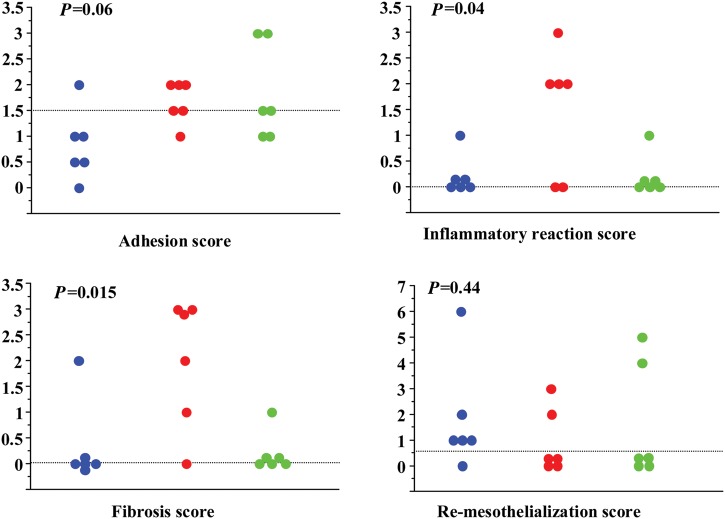
After 4 months, the bioengineered collagen membranes were absorbed and replaced by a loose regenerated tissue, leading to the best adhesion score. The surface of the heart was smooth and the coronary vessels were clearly identifiable. The ePTFE membrane was consistently associated with more adhesions either to the sternum or to the remainder of the pericardium, particularly at the borders of the membrane with the ability to slip out the whole membrane. The control group animals had consistently the worst adhesion score with moderate to strong adhesions (Fig. 2; Adhesion score).
Figure 2:
Adhesion and pathology scores. Each sheep is represented by a dot. The horizontal dotted line is the median value for the score. Blue: Cova™ CARD; red: Preclude®; green: control.
Pathology findings
The bioengineered collagen and control groups only demonstrated a light inflammatory reaction in one case. This contrasted with the ePTFE group in which 4 sheep out of 6 exhibited an intense inflammatory reaction.
The fibrosis was minimal both in the bioengineered collagen and control groups (P = 0.015; control vs Preclude®: P = 0.028; Cova™ CARD vs Preclude®: P = 0.041; Cova™ CARD vs control: P = 1.0). In the control group, there was only one sheep with dense fibrosis. In the bioengineered collagen group, there was also only one sheep with thick and dense fibrosis. In contrast, the ePTFE group had the worst results for the fibrosis criteria, with an extensive (5 out of 6), dense (4 out of 6) and thick (3 out of 6) fibrosis underneath the membrane, impeding a straightforward visualisation of the underlying coronary arteries.
Regarding re-mesothelialization of the epicardium, 5/6 sheep of the bioengineered collagen group experienced a cell lining, albeit limited, with the best score of 6 (for a theoretical maximum of 15), whereas mesothelial cells were only identified in 2 sheep of each of the two other groups (Fig. 3). However, the difference was not significant.
Figure 3:
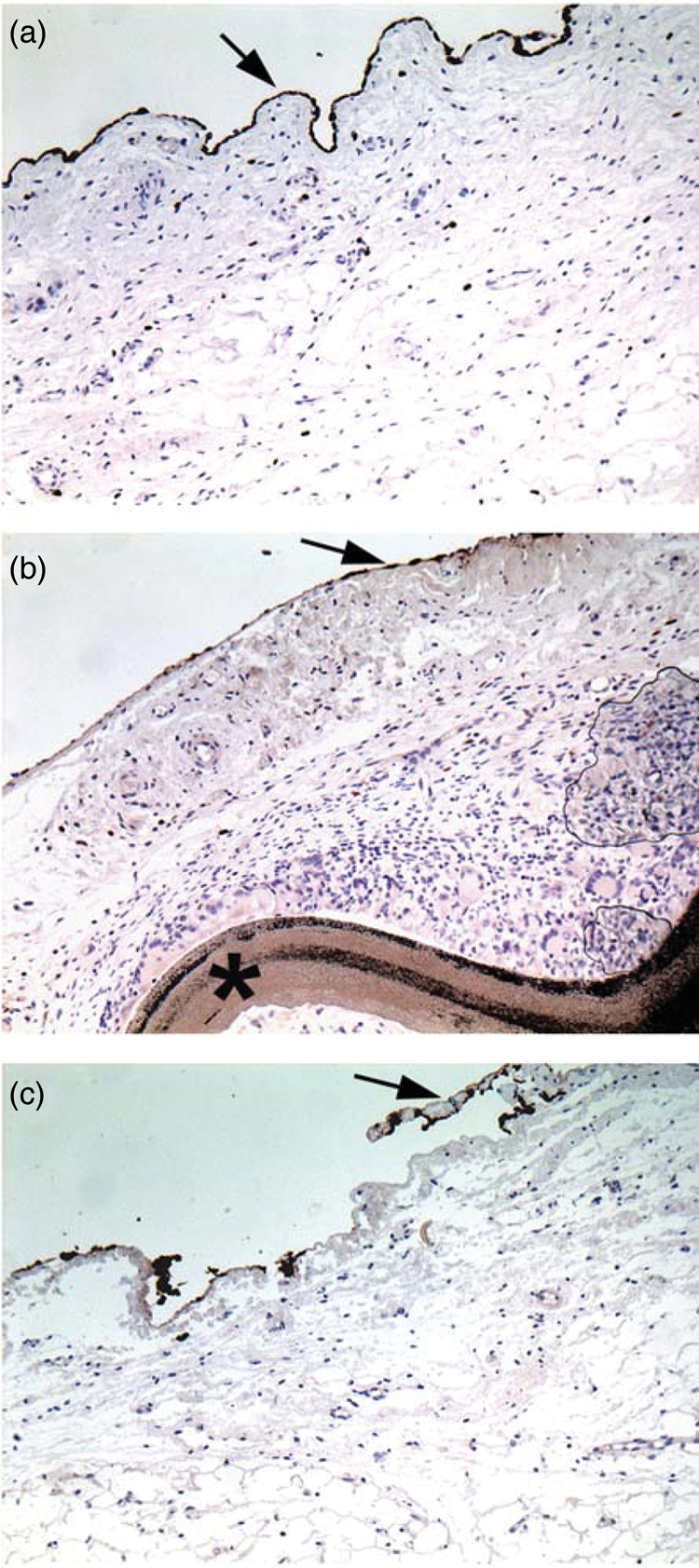
Representative picture of the epicardial coverage by mesothelial cells (arrow). (a) Cova™ CARD sheep; (b) Preclude® (star) sheep; (c) control sheep. Immunostaining of the mesothelial cells by an antibody against cytokeratins AE1/AE3. Original ×10. Note the continuous cell lining in the Cova™ CARD sheep whereas it is interrupted or absent in the Preclude® and control sheep, respectively.
Histological scores are summarized in Fig. 2.
DISCUSSION
The main finding of this study is that a resorbable collagen-based membrane (Cova™ CARD) yielded the best outcomes with regard to easiness to find a smooth cleavage plane, limitation of fibrosis and inflammation and promotion of re-mesothelialization. Conversely, the e-PTFE comparator resulted in dense fibrotic and inflammatory adhesions and its advantage as a safety barrier between the deep sternal aspect and the underlying heart was thus offset by the difficulty in dissection and identification of epicardial structures. The control group fell between the two material-treated groups with a high adhesion score contrasting with a relatively minor degree of inflammation and fibrosis, possibly reflecting species-specific natural tissue healing characteristics.
After cardiac operations, the ideal objective should be complete healing of the pericardium during the presence of a membrane substitute, with complete resorption of the latter afterwards, leading to the reconstitution of a single layer of flattened mesothelial cells resting on loose connective tissues.
It is documented that mesothelial cells, which can differentiate from multipotent cells present in the collagen basement, prevent adhesions through the synthesis of growth factors, lubricants, extracellular matrix proteins and the release of factors that promote the clearance of fibrin [5].
Local delivery of drugs or biologics has been proposed to prevent adhesions [6].
Among them, the keratinocyte growth factor topically applied [6] has generated interest because it might facilitate resumption of the pericardial fibrinolytic function by mesothelial cells. Most studies have focused on biologic or synthetic sheets designed as pericardial substitutes [7–10], among which the ePTFE patch is the most widely used. Despite its remarkable resistance to mechanical stretch, this material presents some disadvantages as it remains in situ as a permanent foreign body; it may form a fibrous capsule that negatively interferes with visualization of the cardiac architecture at the time of the reoperation [11]; it may cause an inflammatory reaction [9, 11] or even provoke a constrictive pericarditis [12]; and, as a permanent material, it potentially increases the risk of infection. Sakuma et al. [9] have reported that a bioabsorbable gelatin pericardial substitute generated less adhesions and inflammation compared with an ePTFE sheet and was ultimately replaced with regenerated tissue. However, a gelatin sheet has two substantial disadvantages. First, due to the physical nature of gelatin, the sheet swells once wet, which leads to a very malleable, hard to handle material. Second, for the same reason, its tensile strength is not high enough to steadily hold sutures to the native pericardium, hence the proposal to lattice the sheet with polyglycolic acid to improve its mechanical properties [10].
In human cardiac surgery, asides from the ePTFE patch, the most used resorbable pericardial substitutes are the Repel CV® membrane (SyntheMed, Iselin, USA) made out of polylactic acid, and the Seprafilm® membrane [7], composed of carboxymethyl cellulose and hyaluronic acid. The two main disadvantages of these membranes are their composition and lack of mechanical resistance. In a 142-patient study, Repel CV® has been shown effective in the prevention of pericardial adhesions [8] but inflammatory reactions have been reported with polylactic acid [13]. Two limitations of the Seprafilm® for cardiac operations must be noted: first, its biological composition does not allow it to be applied on wet sutures, which is the common situation in cardiac surgery; secondly, sutures can easily be displaced or broken by the heartbeats [14]. Furthermore, the resorption of its main component does not involve naturally present endogenous enzymes (like collagenases) but occurs by hydrolysis, which causes the release of non-metabolized fragments into the mediastinal cavity.
To try overcoming these hurdles, the Cova™ CARD membrane, made out of purified porcine type I collagen, was tested in the present study on the basis of encouraging results reported with its use in other surgeries [15]. Collagen has an extensive safety record in cardiovascular applications [2]. In the particular setting of adhesion prevention, collagen has the additional advantage of providing a substrate for cell adhesion and growth, thereby promoting wound healing. Finally, shaping of the collagen is relatively easy to produce elastic and suturable membranes.
We acknowledge some limitations of the current protocol. First, it entailed the assessment of a single comparator (ePTFE). Indeed, we did not include the only other clinically approved Seprafilm® membrane because a previous study showed that its use did not significantly reduce adhesions compared with a control group [2]. Secondly, although the dissecting surgeon necessarily identified the ePTFE membrane at the time of surgery, the pathologist was totally blinded to the treatment group. Thirdly, reoperation entailed a new sternotomy, which is more adequate than lateral thoracotomy to induce tight adhesions between the heart and the sternum, but we acknowledge that the tested material is designed to make dissection of adhesions easier and not to constitute a safety barrier for avoiding cardiac injury at the time of re-entry into the chest cavity, which is efficiently achieved by an ePTFE membrane [11]. Fourthly, a 4-month reoperation delay was chosen to assess the outcomes at the expected time of maximal inflammatory reactions, which does not exclude that additional changes may occur at earlier or later time points. Of note, the characteristics of the membrane (thickness and reticulation) have been designed to achieve a full resorption by 3 months. Finally, one cannot exclude that re-mesothelialization was partly underestimated in all groups because this frail epicardial lining is particularly prone to be teared off at the time of heart explantation even when care is taken to be minimally injurious.
In conclusion, the present data support the effectiveness of the new bioengineered collagen Cova™ CARD membrane in reducing postoperative adhesions following sternotomy and may warrant its clinical evaluation.
FUNDING
The sponsor, Biom'Up, Lyon, France, funded the animal experiments but had no role in data collection, analyses, interpretation or writing the report. All authors had full access to all the data and had the final responsibility for the decision to submit for publication.
Conflict of interest: none declared.
REFERENCES
- 1.Schwartzmann M. Use of collagen membranes for guided bone regeneration: a review. Implant Dent. 2000;9:63–6. doi: 10.1097/00008505-200009010-00011. [DOI] [PubMed] [Google Scholar]
- 2.Bel A, Kachatryan L, Bruneval P, Peyrard S, Gagnieu C, Fabiani JN, et al. A new absorbable collagen membrane to reduce adhesions in cardiac surgery. Interact CardioVasc Thorac Surg. 2010;10:213–6. doi: 10.1510/icvts.2009.215251. [DOI] [PubMed] [Google Scholar]
- 3.Heydorn WH, Daniel JS, Wade CE. A new look at pericardial substitutes. J Thorac Cardiovasc Surg. 1987;94:291–6. [PubMed] [Google Scholar]
- 4.Lu JH, Chang Y, Sung HW, Chiu YT, Yang PC, Hwang B. Heparinization on pericardial substitutes can reduce adhesion and epicardial inflammation in the dog. J Thorac Cardiovasc Surg. 1998;115:1111–20. doi: 10.1016/S0022-5223(98)70411-8. [DOI] [PubMed] [Google Scholar]
- 5.Mutsaers SE. Mesothelial cells: their structure, function and role in serosal repair. Respirology. 2002;7:171–91. doi: 10.1046/j.1440-1843.2002.00404.x. [DOI] [PubMed] [Google Scholar]
- 6.Lopes JB, Dallan LA, Campana-Filho SP, Lisboa LA, Gutierrez PS, Moreira LF, et al. Keratinocyte growth factor: a new mesothelial targeted therapy to reduce postoperative pericardial adhesions. Eur J Cardiothorac Surg. 2009;35:313–8. doi: 10.1016/j.ejcts.2008.09.046. [DOI] [PubMed] [Google Scholar]
- 7.Walther T, Rastan A, Dähmert I, Falk V, Jacobs S, Mohr FW, et al. A novel adhesion barrier facilitates reoperations in complex congenital cardiac surgery. J Thorac Cardiovasc Surg. 2005;129:359–63. doi: 10.1016/j.jtcvs.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 8.Lodge AJ, Wells WJ, Backer CL, O'Brien JE, Jr, Austin EH, Bacha EA, et al. A novel bioresorbable film reduces postoperative adhesions after infant cardiac surgery. Ann Thorac Surg. 2008;86:614–21. doi: 10.1016/j.athoracsur.2008.04.103. [DOI] [PubMed] [Google Scholar]
- 9.Sakuma K, Iguchi A, Ikada Y, Tabayashi K. Closure of the pericardium using synthetic bioabsorbable polymers. Ann Thorac Surg. 2005;80:1835–40. doi: 10.1016/j.athoracsur.2005.04.078. [DOI] [PubMed] [Google Scholar]
- 10.Yoshioka I, Saiki Y, Sakuma K, Iguchi A, Moriya T, Ikada Y, et al. Bioabsorbable gelatin sheets latticed with polyglycolic acid can eliminate pericardial adhesion. Ann Thorac Surg. 2007;84:864–70. doi: 10.1016/j.athoracsur.2007.04.029. [DOI] [PubMed] [Google Scholar]
- 11.Salminen JT, Mattila IP, Puntila JT, Sairanen HI. Prevention of postoperative pericardial adhesions in children with hypoplastic left heart syndrome. Interact CardioVasc Thorac Surg. 2011;12:270–2. doi: 10.1510/icvts.2010.241448. [DOI] [PubMed] [Google Scholar]
- 12.Bergoend E, Marchand M, Casset-Senon D, Cosnay P. Localized constrictive pericarditis after Gore-Tex pericardial substitution. Interact CardioVasc Thorac Surg. 2010;10:813–5. doi: 10.1510/icvts.2009.225763. [DOI] [PubMed] [Google Scholar]
- 13.Dawes E, Rushton N. Response of macrophages to poly(l-lactide) particulates which have undergone various degrees of artificial degradation. Biomaterials. 1997;18:1615–23. doi: 10.1016/s0142-9612(97)00095-1. [DOI] [PubMed] [Google Scholar]
- 14.Noishiki Y, Shintani N. Anti-adhesive membrane for pleural cavity. Artif Organs. 2010;3:224–9. doi: 10.1111/j.1525-1594.2009.00787.x. [DOI] [PubMed] [Google Scholar]
- 15.Masmejean E, Schlur C, Chetouani M, Dana C. Evaluation of a new anti-adhesion collagen membrane (Cova™ ORTHO) for upper limb nerve and/or tendon surgery – preliminary results. e-Mémoires de l'Académie Nationale de Chirurgie. 2011;10:33–7. [Google Scholar]