Abstract
Patients with locally advanced non-small cell lung cancer infiltrating the left atrium (LA) or the intrapericardial base of the pulmonary veins (PVs) are generally not considered good candidates for surgery because of the poor long-term survival. In the last 10 years, 31 consecutive patients with non-small cell lung cancer directly invading the LA or the intrapericardial base of the PVs underwent surgery. Pneumonectomy was the operation performed most frequently. In-hospital mortality was 9.7% and overall morbidity was 52%. One-, 2- and 3-year survival rates were 64, 46 and 30%, respectively with a mean survival of 22 months. The systemic recurrence of disease was the major cause of death at follow-up. At statistical analyses, the N-factor and the type of operation were related to poor long-term survival. In these patients, surgery could be performed with an acceptable operative mortality and morbidity. Surgery should be considered whenever a complete resection is technically possible. A careful preoperative evaluation is mandatory to select good candidates for surgery.
Keywords: Lung cancer, Extended resection, Left atrium, Pulmonary veins
INTRODUCTION
T4-non-small cell lung cancer (NSCLC) represents a miscellany of locally advanced diseases with generally poor long-term survival [1, 2]. According to the latest version of the tumor node metastasis (TNM), an NSCLC invading the base of the pulmonary veins (PVs) or the left atrium (LA) wall is classified as T4 [1]. We retrospectively reviewed our 10-year surgical experience in the treatment of T4-NSCLC invading the LA or intrapericardial PVs.
METHODS
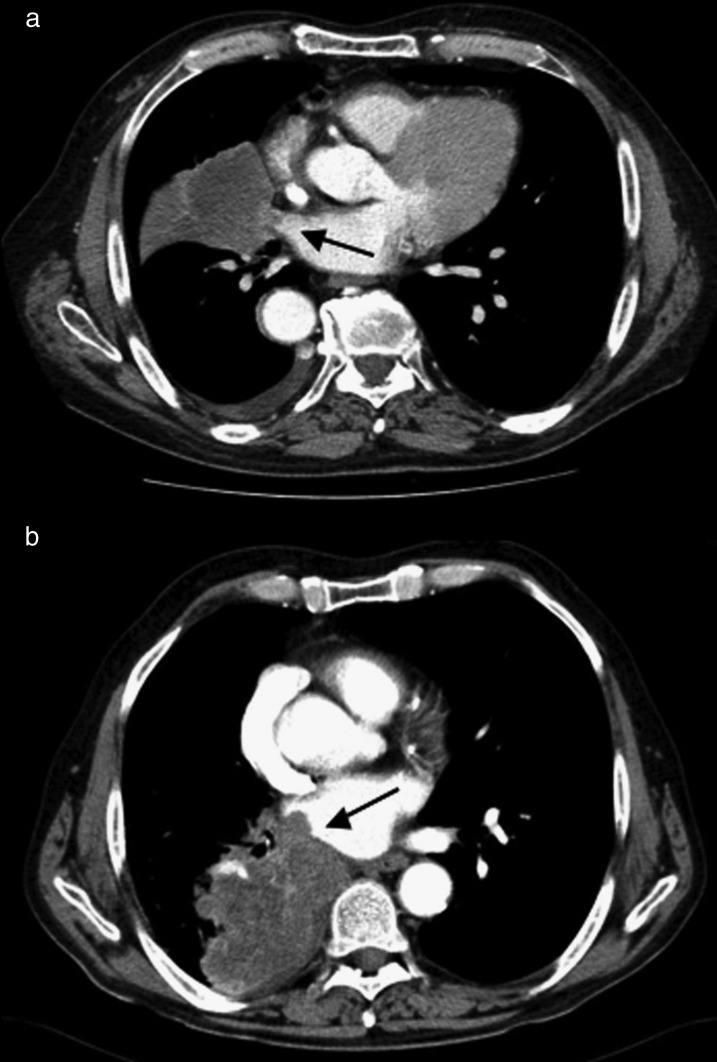
From January 2000 to December 2010, 31 consecutive patients with T4-NSCLC directly invading the LA (14 patients) or the intrapericardial base of the PVs (17 patients) underwent surgery (Fig. 1). The mean-age of the patients was 65.6 years (range 44–84 years), 27 patients were male (87%) and 4 were female (13%). The preoperative clinical profile of the population is summarized in Table 1. The patients were staged according to the 2009 TNM classification system [1].
Figure 1:
Preoperative axial CT scan of two different patients; (a) invasion of the intrapericardial base of the superior right PV, a spread of the tumour was visible inside the lumen of the vein towards the LA chambers (black arrow); (b) direct invasion of the LA wall, the tumour protruding inside the LA chamber (black arrow).
Table 1:
Preoperative patient data
| Variables | No. of patients |
|---|---|
| Systemic hypertension | 18 (58.1) |
| COPD | 13 (41.9%) |
| Poly-vasculopathy | 7 (22.6%) |
| Chronic ischaemic cardiomyopathy | 6 (19.4%) |
| Previous CVE | 3 (9.7%) |
| Chronic renal failure (creatinine >2 mg/dl) | 1 (3.2%) |
| Low FEV1 (<70%) | 8 (25.8%) |
| Diabetes | 4 (12.9%) |
| Chronic AF | 2 (6.5%) |
| Obesity | 7 (22.6%) |
| Smoking history | 19 (61.3%) |
| Previous lung resection | 2 (6.5%) |
| Left-side disease | 18 (58%) |
| Right-side disease | 13 (42%) |
COPD: chronic obstructing pulmonary disease; FEV1: forced expiratory volume in 1 s; AF: atrial fibrillation.
All patients underwent preoperative evaluation with the chest, brain and abdominal computed tomography (CT) and positron-emission tomography (PET). Mediastinoscopy or endobronchial ultrasound (EBUS) were performed only in the case of PET-positive mediastinal lymph nodes. Globally, 13 patients (41.9%) underwent mediastinoscopy and 8 patients (25.8%) underwent EBUS before surgery. A cN2 disease was confirmed in three patients. Magnetic resonance imaging was performed in selected cases to exclude an extensive involvement of great vessels and/or cardiac chambers. The clinical stage was 8 patients with IIb (5 T3N0M0, 3 T2bN1M0), 21 with IIIa (12 T4N0M0, 8 T3N1M0, 1 T3N2M0) and 2 with IIIb (T4N2M0).
Seven patients (23%) had received preoperative treatment (four, induction chemoradiotherapy; three, neoadjuvant chemotherapy). Two patients had a previous lobectomy and were scheduled for completion pneumonectomy.
Statistical analysis
Statistics were performed using SPSS (Statistical Package for Social Science) version 11.0 for windows (SPSS, Inc, Chicago, IL, USA). All data were collected retrospectively from our institutional database.
Continuous variables were given as mean ± SD, and categorical variables were given as percentages. In-hospital risk factors for mortality and morbidity were analysed using a binary logistic regression model. The Cox multivariate regression was used to identify independent prognostic factors for long-term mortality using a stepwise model.
A P-value <0.05 was considered to be statistically significant. Survival analysis was conducted according to the Kaplan–Meier method, and the curves were compared by the long-rank test.
Operative technique
Intraoperative-TEE monitoring was used in all patients. Before beginning pulmonary resection, the pericardium is opened widely to evaluate the extent of tumour infiltration and provide a good access for atrial resection and/or intrapericardial pneumonectomy. The atrial resection was performed using a large Satinsky clamp even in the case of intrapericardial PVs involvement to obtain safe resection margins. On the right side before placing the clamp, the Sondergaard groove dissection is always performed. After clamp positioning, we observe the haemodynamic impact of the LA volume reduction using TEE. After resection, the stump of the LA was then doubly sutured using 4/0 or 5/0 polypropylene. Fresh-frozen pathological evaluation of the atrial and bronchial resection margins was performed in all patients. A complete lymphadenectomy was routinely performed.
In-hospital results
All patients were judged operable with radical intent after thoracotomy. Operative results are summarized in Table 2. The in-hospital mortality was 9.7% (3 of 31). The causes of death were: one acute myocardial infarction, one acute pulmonary embolism and one post-pneumonectomy acute lung injury. All these patients underwent right-side intrapericardial pneumonectomy. Pathological examination showed 15 patients with adenocarcinoma, 10 with squamous cell carcinoma and 6 with undifferentiated large cell carcinoma. An R0 resection was achieved in 29 patients (93.5%). In the remaining patients, the resection was not complete because of microscopic residual disease (R1) on the atrial margins in one patient and on the bronchial margin in another one. At the pathological staging, 8 patients were IIIb (T4N2M0) and 23 patients were IIIa (T4N0-1M0). Five patients were pN0, 18 patients were pN1 and 8 patients were pN2. The major post-operative complications are reported in Table 3. The mean hospital stay was 7.8 ± 4.3 days (range 6–21 days).
Table 2:
Operative results
| No. of patients | % | |
|---|---|---|
| Type of resection | ||
| Lobectomy | 2 (1—right, 1—Left) | 6.5 |
| Bilobectomy | 2 | 6.5 |
| Pneumonectomy | 24 (10—right, 14—left) | 77.4 |
| Completion pneumonectomy | 2 (left) | 6.5 |
| Sleeve lobectomy | 1 (left) | 3.2 |
| Associated procedures | ||
| SVC resection/reconstruction | 1 | 3.2 |
| Chest wall resection | 2 | 6.5 |
| Completeness of resection | ||
| R0 | 29 | 93.5 |
| R1 | 2 | 6.5 |
| Pathological N stage | ||
| N0 | 5 | 16 |
| N1 | 18 | 58 |
| N2 | 8 | 26 |
| Pathological TNM stage | ||
| IIIa | 23 | 74.2 |
| IIIb | 8 | 25.8 |
SVC: superior vena cava.
Table 3:
Post-operative complications
| Complications | No. of patients | % |
|---|---|---|
| Overall morbidity | 16 | 52 |
| Post-operative AF | 12 | 38.7 |
| Prolonged air-leak | 1 | 3.2 |
| Pneumonia | 1 | 3.2 |
| Stroke | 1 | 3.2 |
| Post-operative AMI | 2 | 6.5 |
| Acute pulmonary embolism | 1 | 3.2 |
| ALI/ARDS | 1 | 3.2 |
AF: atrial fibrillation; AMI: acute myocardial infarction; ALI/ARDS: acute lung injury/acute respiratory distress syndrome.
Follow-up results
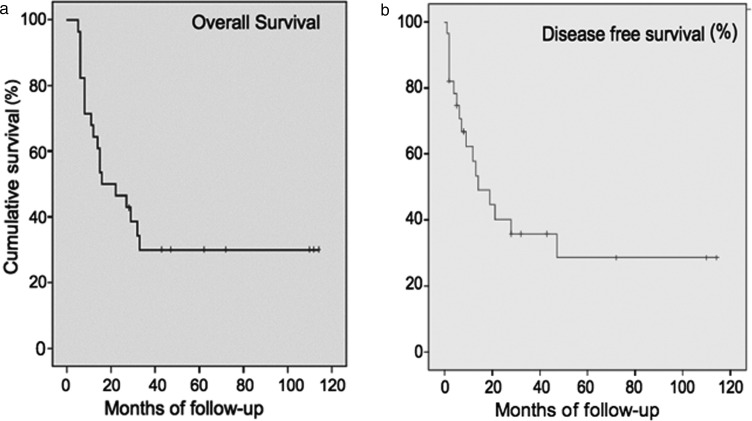
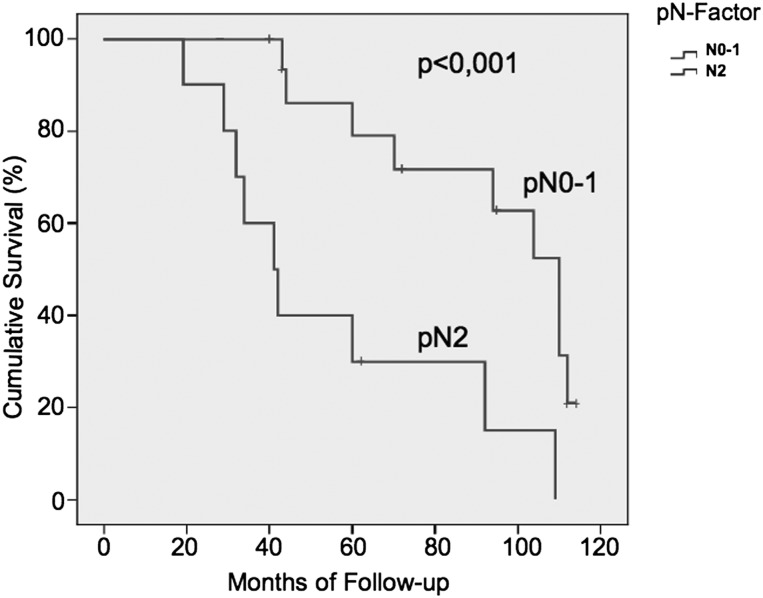
Twenty-eight patients were discharged from the hospital. The mean follow-up time was 32 months ranging from 5 to 114 months (median 19 ± 32 months, 100% complete). A total of 19 patients died during the follow-up. The overall mortality during the follow-up was 67.9%. Adjuvant postoperative chemotherapy was performed in 18 patients. Adjuvant chemoradiotherapy was performed in 6 patients. Six patients died due to causes not related with lung cancer. A late right-broncho-pleural fistula was the cause of death in one patient. One patient who had undergone bi-lobectomy 13 months earlier died as a result of local recurrence. The remaining 11 patients died due to systemic recurrence (92%). At the end of the study, 9 patients were still alive and 7 were free from cancer recurrence. The mean free-time from recurrence was 9.4 ± 8.5 months (range 2–32 months, median 3.6 ± 6.5). One-, 2-, 3- and 5-year survival rates were 64, 46, 30 and 30%, respectively, with a mean survival of 22 months (Fig. 2a). Disease-free survival was 58, 35 and 28% at 1, 3 and 5 year, respectively (Fig. 2b). At univariate analysis, a lower respiratory reserve, the type of resection (pneumonectomy) and the pN-factors (N2) were risk factors for poor prognosis. At multivariate analysis, the pN factors (Fig. 3) and the type of resection were risk factors for poor survival (Table 4). No statistical differences in terms of long-term survival and recurrence of disease were found by separately analysing patients with direct LA infiltration versus patients with infiltration of the intrapericardial portion of the PVs.
Figure 2:
Analyses of mean survivals using Kaplan–Meier curves and long-rank test. (a) The overall long-term survival curve; (b) disease-free survival curve.
Figure 3:
Kaplan–Meier curves stratified for pN0–1 or pN2 lymph node status.
Table 4:
Predictors of poor long-term survival at univariate and multivariate regression analyses (28 patients)
| Variables | RR | 95% CI | P-value |
|---|---|---|---|
| Univariate analyses | |||
| Pneumonectomy | 11,2 | 1.8–57.4 | 0.004 |
| Low FEV1 | 6.2 | 1.0–38,6 | 0.037 |
| N-factor (N2) | 14 | 2.0–94.2 | 0.003 |
| Multivariate analyses | |||
| Pneumonectomy | 2,1 | 0.6–3.7 | 0.03 |
| N-factor (N2) | 3,9 | 1.8–5.7 | <0.001 |
RR: relative risk; CI: confidence interval; FEV1: forced expiratory volume in 1 s.
DISCUSSION
Operative mortality after resection of T4-NSCLC in literature ranges from 0 to 18% [3–7]. The mortality observed in our study was 9.7% similar to other series reported in the literature. Pneumonectomy contributed to all the operative deaths in our patients. If we consider patients who underwent a surgical resection other than pneumonectomy, the in-hospital mortality was 0% versus a mortality of 11.5% for patients who underwent pneumonectomy. The majority of studies regarding LA resection for T4-NSCLS failed to identify particular risk factors for operative mortality, probably due to the small population of patients. In a larger study on surgical resection of T4-NSCLC, right pneumonectomy was the only risk factor for in-hospital mortality. Three-year survival rates of T4-NSCLC invading the LA range from 8 to 21% [2–4, 6, 8]. Nevertheless, long-term results reported in the literature are not consistent. In 1997, Fukuse et al. [5] reported a 3-year survival of 0% in 14 patients operated on for NSCLC invading the LA. In 2004, Ratto et al. [6] reported on the contrary a 5-year survival of 14% with a mean survival time of 25 months in 19 patients with NSCLC invading the LA. Spaggiari et al. [9] reported a 3-year survival probability of 39% in 15 patients who underwent extended pneumonectomy with partial resection of the LA. In our experience, the overall 1- and 5-year survival rates were 64 and 30%, respectively, with a mean survival of 22 months (Fig. 2a). At statistical analysis, some factors for poor long-term survival were observed. The nodal status and thus the TNM stage had a great influence on the prognosis in the present study. The 5-year survival for N0–1 patients was 78 when compared with 29% in the N2 patients (Fig. 3b). Martini et al. [10] reported no long-term survival for operated patients with T4N2-NSCLC. Yildizeli et al. [3] reported a 5-year survival of 43% in cases of N0–1 when compared with that of 17.7% in cases of N2–3 or M1 for T4-NSCLC patients. Fukuse et al. [5] showed a median survival of 29 months in patients with T4N0 and of 9 months in those with T4N2-NSCLC. In our series, only three patients were cN2 and all of them were treated with preoperative chemotherapy. In two patients, we obtained the down-staging of the N-involvement documented by PET and medistinoscopy. Unfortunately, in the post-operative pathological evaluation, eight patients were pN2 reflecting an inaccurate preoperative staging. Five of these patients were operated before the advent of PET and they had no lymph node enlargement on the CT scan. The other three patients were PET negative. Given the poor results of patients with T4N2-NSCLC who have surgery, we have changed our staging protocol over the years, using EBUS or medistinoscopy more aggressively as advocated by other authors and guidelines [10, 11].
The role of induction or neoadjuvant chemotherapy/chemoradiotherapy in T4-NSCLC patients remains unclear. Some studies report an increase in operative mortality after preoperative chemotherapy or radiation therapy [4, 5, 8, 12, 13]. An increase in operative mortality from 6.7 to 13% after induction therapy in patients undergoing right carinal pneumonectomy has been reported [12], and an operative mortality as high as 24% after induction therapy in patients who underwent right pneumonectomy [13]. However, some studies on long-term results have reported that preoperative chemotherapy improves the survival in patients with T4-NSCLC [2, 4, 6]. Macchiarini et al. [4] reported a 3-year survival of 54% in stage IIIb T4-NSCLC patients after induction chemotherapy.
Four patients had a positive response to the induction regimen with a significant reduction in the primary tumour. In these patients, we achieved an R0 resection and, in two cases, we were able to avoid pneumonectomy. In our opinion, this is one of the major positive effects of induction therapy because pneumonectomy is a well-known risk factor for poor long-term survival [4–5, 13, 14]. Two patients with cN2 became cN0 after neoadjuvant chemotherapy and they were confirmed pN0 after the operation. These results confirm a trend towards positive effects of induction/neoadjuvant chemotherapy in terms of reduction in tumour dimension, sometimes avoiding the need for pneumonectomy and lymph node down-staging. Some authors consider a clarification of the definition of ‘left atrium resection’ mandatory for a correct analysis of the surgical results. Spaggiari et al. [9] and Doddoli et al. [7] consider a true LA resection as an extended resection involving the heart with a part of the muscular wall of the LA in the surgical specimen. The resection of the base of the PVs clamping the LA should not be considered a true LA resection in their opinion. In 2010, Riquet et al. [15] published a study in which 91 patients with NSCLC were divided on the basis of invasion of pericardium, pericardium and PV or pericardium and LA. The authors demonstrated that PV and LA invasions do not worsen the prognosis more than pericardial invasion alone. Instead, the need for pneumonectomy, lymph node involvement and incomplete resection (R1–2) and pericardial infiltration were the most important factors that can influence the long-term survival of patients. Similar results were reported by Yildizeli et al. [3] some years before. In our experience, the ‘true’ infiltration of the LA or the base of the PVs did not influence operative mortality and long-term results. No differences were found in terms of the degree of nodal involvement or type and completeness of resection between patients with LA versus PV infiltration. The major cause of death, in our, and other reported series, was disease recurrence, systemic or local, again independently of LA or intrapericardial PV infiltration. This gives the impression that the distinction between the true or false LA resection is more academic than prognostic.
The present study has several limitations that must be taken into account during the analysis of the results. First of all, it is subject to the bias of a retrospective study. Patients with locally advanced T4-NSCLC who underwent surgery represent a category of super-selected patients, particularly in terms of preoperative morbidity and performance status. Analysing data collected over a period of 10 years shows that the selection criteria changed on the basis of improvements in diagnostic tools, surgical techniques, anaesthesia management and post-operative care. In this study, all patients were re-staged using the last TNM system; this may have had implications for the treatment protocols used in these patients. Finally, the size of the population is small.
In conclusion, in patients with T4-NSCLC invading the LA or the intrapericardial portion of the PV surgery can be performed with an acceptable operative mortality and morbidity. A careful preoperative evaluation is mandatory to select good candidates for surgery. In patients with T4N2-NSCLC, surgery alone is inadequate and these patients should be managed with a multimodality protocol. Induction chemotherapy or chemoradiation therapy could be useful to reduce the need for pneumonectomy and to achieve a complete resection. Surgery should be considered whenever a complete resection is technically possible. In the future, further randomized studies are needed to clarify the role of surgery and multimodality protocols in the management of this category of patients.
Conflict of interest: none declared.
REFERENCES
- 1.Detterbeck FC, Boffa DJ, Tanoue LT. The new lung cancer staging system. Chest. 2009;136:260–71. doi: 10.1378/chest.08-0978. [DOI] [PubMed] [Google Scholar]
- 2.Bernard A, Bouchot O, Hangry O, Favre JP. Risk analysis and long-term survival in patients undergoing resection of T4 lung cancer. Eur J Cardiothorac Surg. 2001;20:344–9. doi: 10.1016/s1010-7940(01)00788-6. [DOI] [PubMed] [Google Scholar]
- 3.Yildizeli B, Dartevelle PG, Fadel E, Mussot S, Chapelier A. Results of primary surgery with T4 non-small cell lung cancer during a 25-year period in a single center: the benefit is worth the risk. Ann Thorac Surg. 2008;86:1065–75. doi: 10.1016/j.athoracsur.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Macchiarini P, Chapelier A, Monnet I, Vannetzel JM, Rebischung JL, Cerrina J, et al. Extended operation after induction therapy for stage IIIB (T4) non-small cell lung cancer. Ann Thorac Surg. 1994;57:966–73. doi: 10.1016/0003-4975(94)90215-1. [DOI] [PubMed] [Google Scholar]
- 5.Fukuse T, Wada H, Hitomi S. Extended operation for non-small cell lung cancer invading great vessels and the left atrium. Eur J Cardiothorac Surg. 1997;11:664–9. doi: 10.1016/s1010-7940(96)01140-2. [DOI] [PubMed] [Google Scholar]
- 6.Ratto GB, Costa R, Vassallo G, Alloisio A, Mainieri P, Bruzzi P. Twelve-year experience with left atrial resection in the treatment of non-small cell lung cancer. Ann Thorac Surg. 2004;78:234–7. doi: 10.1016/j.athoracsur.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 7.Doddoli C, Rollet G, Thomas P, Ghez O, Serée Y, Giudicelli R, et al. Is lung cancer surgery justified in patients with direct mediastinal invasion. Eur J Cardiothorac Surg. 2001;20:339–43. doi: 10.1016/s1010-7940(01)00759-x. [DOI] [PubMed] [Google Scholar]
- 8.Yang H, Hou X, Lin P, Rong T, Yang H, Fu J. Survival and risk factors of surgically treated mediastinal invasion T4 non-small cell lung cancer. Ann Thorac Surg. 2009;88:372–9. doi: 10.1016/j.athoracsur.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 9.Spaggiari L, D'aiuto M, Veronesi G, Pelosi G, de Pas T, Catalano G, et al. Extended pneumonectomy with partial resection of the left atrium, without cardiopulmonary bypass, for lung cancer. Ann Thorac Surg. 2005;79:234–40. doi: 10.1016/j.athoracsur.2004.06.100. [DOI] [PubMed] [Google Scholar]
- 10.Martini N, Yellin A, Ginsberg RJ, Bains MS, Burt ME, McCormack PM, et al. Management of non-small cell lung cancer with direct mediastinal involvement. Ann Thorac Surg. 1994;58:1447–51. doi: 10.1016/0003-4975(94)91933-x. [DOI] [PubMed] [Google Scholar]
- 11.De Leyn P, Lardinois D, Van Schil PE, Rami-Porta R, Passlick B, Zielinsky M, et al. ESTS guidelines for preoperative lymph node staging for non-small cell lung cancer. Eur J Cardiothorac Surg. 2007;32:1–8. doi: 10.1016/j.ejcts.2007.01.075. [DOI] [PubMed] [Google Scholar]
- 12.de Perrot M, Fadel E, Mercier O, Mussot S, Chapelier A, Dartevelle P. Long-term results after carinal resection for carcinoma: does the benefit warrant the risk? J Thorac Cardiovasc Surg. 2006;131:81–9. doi: 10.1016/j.jtcvs.2005.07.062. [DOI] [PubMed] [Google Scholar]
- 13.Martin J, Ginsberg RJ, Abolhoda A, Bains MS, Downey RJ, Korst RJ, et al. Morbidity and mortality after neoadjuvant therapy for lung cancer: the risks of right pneumonectomy. Ann Thorac Surg. 2001;72:1149–54. doi: 10.1016/s0003-4975(01)02995-2. [DOI] [PubMed] [Google Scholar]
- 14.Meacci E, Cesario A, Cusumano G, Lococo F, D'Angelillo R, Dall'Armi V, et al. Surgery for patients with persistent pathological N2 IIIA stage in non-small-cell lung cancer after induction radio-chemotherapy: the microscopic seed of doubt. Eur J Cardiothorac Surg. 2011;40:656–63. doi: 10.1016/j.ejcts.2010.12.062. [DOI] [PubMed] [Google Scholar]
- 15.Riquet M, Grand B, Arame A, Pricopi CF, Foucault C, Dujon A, et al. Lung cancer invading the pericardium: quantum of lymph nodes. Ann Thorac Surg. 2010;90:1773–8. doi: 10.1016/j.athoracsur.2010.07.039. [DOI] [PubMed] [Google Scholar]