Abstract
Partial forms of Shone complex are rare. Surgical outcomes of the complete forms have generally been poor, whereas there is a lack of data on long-term follow-up of surgically treated adult partial complex. Between 2001 and 2011, nine patients (age: 38 ± 8 years; six males, 67%) were referred for valvular heart disease. Transthoracic and transoesophageal echocardiography was performed. Data were confirmed by intra-operative findings and reports. Patients were diagnosed as partial Shone complex and presented with mitral stenosis (MS) (45%) or mitral regurgitation (22%) or aortic regurgitation (22%). All but one patient (89%) reported previous surgery: coarctation of the aorta repair (87.5%) and aortic valvulotomy (12.5%). Redo intervention included: mitral valve replacement (25%), mitral repair (25%), aortic valve replacement (37.5%) and subvalvular aortic ridge resection (25%). One patient refused surgery. Patients surgically treated before the age of 5 (87.5%) showed favourable outcome (survival rate: 100%) and a 23.6 (± 4.6)-year follow-up free from events. The patient who underwent first intervention at the age of 50 and the patient with MS who refused surgery showed a 45 (± 7)-year follow-up free from major morbidity. Patients with partial Shone complex, properly diagnosed and treated, show favourable surgical outcome free from major clinical events.
Keywords: Parachute mitral valve, Aortic coarctation, Shone's syndrome, Congenital heart disease
INTRODUCTION
In 1963, Shone et al. originally described the developmental complex that includes parachute deformity of the mitral valve (PMV), supravalvular mitral ring (SVMR), subaortic ridge (membranous or muscular) and coarctation of the aorta (CoA) [1–4].
Complete Shone complex in children is well recognized and there are few case reports of the complex diagnosed in adults. Several series have reported poor outcomes from early aggressive surgical repair [5–9]. However, the partial forms, characterized by two or three of the obstructive components (Fig. 1), have been described rarely in adults [10–15]. Variability in presentation and severity of individual lesions has made appropriate management of the disease challenging. Objective of this work is to report the long-term follow-up of a small population affected by this rare syndrome undergone to early surgery and referred for symptomatic and apparently acquired valvular heart disease.
Figure 1:
Examples of the common inflow and outflow obstructive lesions in Shone complex. (a) supramitral shelf (arrows); (b) aortic coarctation; (c) subaortic ridge (open arrow), bicuspid aortic valve (arrows); (d) parachute mitral valve.
PATIENT POPULATION AND METHODS
Between 2001 and 2011, nine patients (age: 36 ± 10 years; six males, 67%) were referred at San Raffaele Hospital in Milan for progressive worsening of valvular heart disease. All data concerning the past clinical history were reviewed through medical reports, including preoperative invasive and non-invasive evaluations, surgical reports and outpatient follow-up notes. The clinical follow-up and the surgical outcome have been related to the period between the first intervention and the last clinical observation.
Assessment of cardiac morphology
All patients had been evaluated by new echocardiographic study at our institute. Transthoracic and transoesophageal echocardiography was performed for preoperative assessment of valvular heart disease responsible for clinical symptoms as well as for intra-operative monitoring of repair surgery. In the study population, the diagnosis of incomplete Shone complex was based on the presence of two or three of the obstructive lesions in adults (Fig. 1). Echocardioghraphic data obtained were confirmed by intra-operative findings and surgical reports.
RESULTS
Nine patients were diagnosed as having partial Shone complex by our cardiology team. Diagnosis was made, considering the anatomic findings in association to the previous surgical interventions (Table 1).
Table 1:
Echocardiographic patterns of incomplete Shone syndrome in the study population
| LV inflow lesions |
LVOT lesions |
||||
|---|---|---|---|---|---|
| SVMR | Mitral valve morphology | Subaortic obstruction | Aortic valve | Previous findings | |
| Patient 1 | No | PMV | Yes | Bicuspid | CoA repair |
| Patient 2 | No | PMV | No | Bicuspid | CoA repair |
| Patient 3 | No | PMV | Yes | Bicuspid | CoA repair |
| Patient 4 | No | PMV | No | Bicuspid | CoA repair |
| Patient 5 | No | PMV | No | Tricuspid | CoA repair PDA |
| Patient 6 | No | PMV | No | Bicuspid | Congenital Ao stenosis |
| Patient 7 | No | PMV | No | Bicuspid | Hyopoplastic ascending Ao |
| Patient 8 | Remnants of SVMR | PMV | No | Bicuspid | CoA repair PLSVC |
| Patient 9 | No | PMV | No | Tricuspid | CoA repair |
LV: left ventricle; LVOT: left ventricular outflow tract; SVMR: supravalvular mitral ring; PMV: parachute mitral valve; CoA: coarctation of the aorta; PLSVC: persistent left superior vena cava.
Clinical presentation and morphological patterns
All patients presented with symptomatic valvular heart disease (NHYA: 2.5 ± 0.5); severe mitral stenosis (MS) (four patients, 45%) or mitral regurgitation (MR) (two patients, 22%) or severe aortic regurgitation (AR) (three patients, 33%). In all patients, the echocardiographic study revealed morphological patterns of incomplete adult Shone syndrome (Table 1). PMV was the prevalent anatomic finding (100%) (Figs 2a and 3), being associated with critical MS in four patients (45%) and with severe MR from flail anterior and posterior leaflets, respectively, in two patients (22%). The echocardiographic patterns of PMV are summarized in Table 2. Bicuspid aortic valve was the second most common anomaly (seven patients, 78%) and various degrees of AR were noted. Subaortic obstruction was present in two patients (22%); one patient (11%) presented hypoplastic ascending aorta without coarctation and one patient (11%) showed a remnant of SVMR (Fig. 3). No accurate data concerning cardiac size, pressure gradients and coronary anatomy were available.
Figure 2:
(a) Transthoracic short-axis views showing asymmetrical mitral orifice (white arrows) with unique papillary muscle. (b) Suprasternal views showing the residual narrowing of the aortic isthmus (yellow arrow). M: mitral valve; Ao: aorta.
Figure 3:
Transthoracic apical views illustrating a ‘parachute mitral valve’ associated with MS (yellow arrows) and remnants of SVMR (white arrows). LV: left ventricle; LA: left atrium; MS: mitral stenosis; SVMR: supravalvular mitral ring.
Table 2:
Echocardiographic patterns of parachute mitral valve
| Echocardiographic view | Morphological pattern |
|---|---|
| LV long-axis view | Shortened/thickened or elongated chordae converge into a single papillary muscle |
| LV short-axis view | Basal level: parachute leaflets |
| Mid-papillary level: single papillary muscle | |
| 4C view | Pear-shaped mitral configuration with diastolic dome shape |
LV: left ventricle; 4C: four-chamber.
Surgical interventions
Previous and current surgical procedures of the study population are shown in Table 3. All but one patient had a clinical history of previous surgical intervention; aortic coarctation repair in seven patients (87.5%) (Fig. 2b), and surgical valvulotomy for congenital aortic stenosis in one patient (12.5%). Current redo intervention included mitral valve replacement (two patients, 25%) and valve repair (two patients, 25%). Three patients (37.5%) had aortic valve replacement (AVR) and two patients (25%) underwent subvalvular aortic ridge resection. One patient with MS refused surgery (11%).
Table 3:
Clinical course of the population study and long-term follow-up
| Age at first intervention | Previous intervention | Reason for referral | Current intervention | Follow-up freedom from clinical events/reintervention | |
|---|---|---|---|---|---|
| Patient 1 | 4 months | CoA repair | MR | MVR | 228 months/19 years |
| Subvalvular aortic ridge resection | |||||
| Left atrial ablation | |||||
| Patient 2 | 5 years | CoA repair | MR | Mitral valve repair | 276 months/23 years |
| Ring annuloplasty | |||||
| Patient 3 | 50 years | CoA repair | AR | AVR | 24 months/2 years |
| Subvalvular aortic ridge resection | |||||
| Patient 4 | 4 months | CoA repair | AR | AVR | 348 months/29 years |
| Patient 5 | 6 months | CoA repair | MS | Mitral commissurotomy | 300 months/25 years |
| Reintervention/PTA | Papillary muscle splitting | ||||
| Posterior leaflet extension | |||||
| Ring annuloplasty | |||||
| Left atrial ablation | |||||
| Patient 6 | 5 months | Surgical Ao valvulotomy | MS + AR | MVR | 192 months/16 years |
| Percutaneous valvuloplasty | AVR | ||||
| Left atrial ablation | |||||
| Patient 7 | No | None | MS | None | 480 months/40 years |
| Patient 8 | 2 years | CoA repair | MS | None | 324 months/27 years |
| Patient 9 | 1 year | CoA repair | MS | None | 314 months/26 years |
CoA: coarctation of the aorta; MR: mitral regurgitation; AR: aortic regurgitation; MS: mitral stenosis; MVR: mitral valve replacement; AVR: aortic valve replacement.
Clinical follow-up and surgical outcome
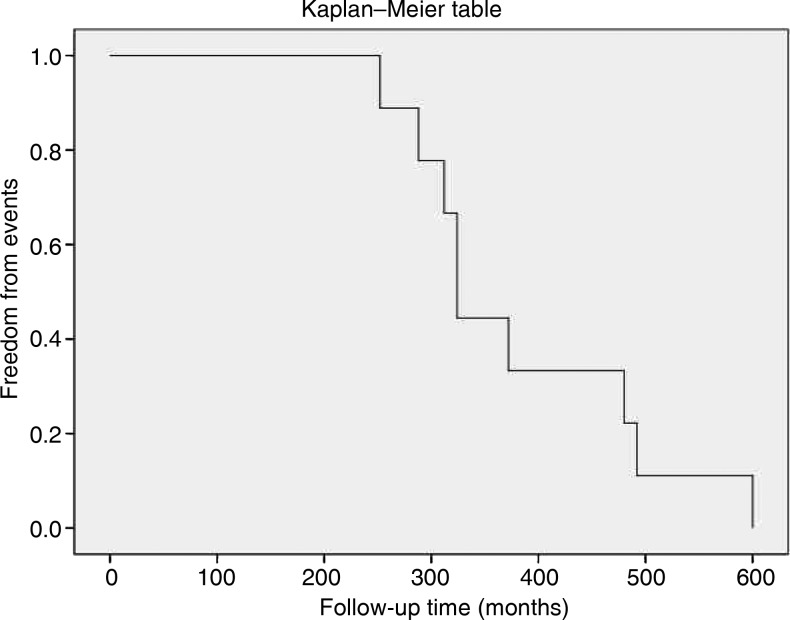
The entire clinical course of the study population is shown in Table 3. Among patients who underwent multiple interventions (Patients 5 and 6), since all treatments occurred in a short-period of time (12 months), the last procedure was considered the starting point of clinical follow-up. Most patients (seven patients, 87.5%) required their first intervention early in life (median age: 16 ± 20 months; range, 4 months to 5 years), whereas one patient (12.5%) had first intervention at the age of 50 years. Patients who had early surgery, before age of 5 years (seven patients, 87.5%), showed a favourable long-term surgical outcome, with the survival rate of 100% and a 23.6 (± 4.6)-year follow-up free from clinical events (Fig. 4). The patient who had undergone first intervention at age 50 (Patient 3) and the patient with severe MS who refused surgery (Patient 7) also showed a 45 (±7)-year clinical follow-up free from major morbidity.
Figure 4:
Surgical outcome and freedom from clinical events in the population study.
DISCUSSION
Partial Shone complex is rarely reported, and there is a general lack of data concerning the clinical follow-up in patients undergone to early surgery. The reports in the literature mainly address small groups of children affected by the complete form of the disease, showing that multiple operations are essential during infancy and childhood to correct left ventricular inflow and outflow obstructive lesions.
Other authors reported that most patients initially present for aortic coarctation repair [14]. Also in our report, most patients were treated during childhood for critical CoA associated with minimal or subclinical signs of left ventricular inflow pathology.
In the complete form, the severity of the obstructions in the left heart dictates the clinical outcome: SVMR and/or additional obstructive lesions usually lead to rapidly progressive pulmonary hypertension and cardiomyopathy. In this setting, ‘critical obstructive lesions’ may obscure other subclinical inflow lesions. In the incomplete forms diagnosed in our experience, after CoA repair had removed early symptoms, patients remained asymptomatic for a long period of time, until a progressive anatomic or valvular lesion triggers new clinical symptoms. Also in the partial form, the first surgical intervention is performed early in life and the following asymptomatic time-frame may be longer and better-tolerated until new worsening of valvular lesions (i.e. flail mitral leftlet, AR or MS), whose progression is favoured by the common congenital background. Because no previous sufficiently detailed echocardiographic data were available, the analysis of the disease progression resulted quite difficult in the population.
As far as our small population is concerned most patients required early surgical intervention and remained asymptomatic for an average time of a 23.6 (± 4.6) years, and were unaware of the rare disease affecting them. Moreover, prior to the appearance of symptoms that required CoA repair, Patient 3 had a 50-year clinical follow-up free from major events, while Patient 7 revealed a 40-year time-frame free from major morbidity before worsening of MS, confirming the variability and severity of the partial Shone complex. The incomplete form of the syndrome may therefore identify a mixed population in which children operated early in life and adults, with less obstructive lesion, can coexist.
Long-term follow-up clearly shows the good results in terms of clinical outcome following surgical treatment of early obstructive lesions. It should be therefore emphasized that appropriate assessments of the disease is undertaken in adult patients presenting with previous CoA repair and apparently acquired valvular heart disease [15]. Awareness of the partial form of the complex is pivotal in appropriate management of the disease.
CONCLUSIONS
Patients affected by partial Shone complex, if properly diagnosed and surgically treated and followed up, show a favourable surgical outcome from first intervention. Notwithstanding the need for reintervention with high rates of valve replacement surgery of one or both left-sided valves, a long-term clinical follow-up free from major events can be achieved.
Conflict of interest: none declared.
REFERENCES
- 1.Shone JD, Sellers RD, Anderson RC, Adams P, Jr, Lillehei CW, Edwards JE. The developmental complex of ‘parachute mitral valve,’ supravalvular ring of left atrium, subaortic stenosis, and coarctation of aorta. Am J Cardiol. 1963;11:714–25. doi: 10.1016/0002-9149(63)90098-5. doi:10.1016/0002-9149(63)90098-5. [DOI] [PubMed] [Google Scholar]
- 2.Purvis JA, Smyth S, Barr SH. Multi-modality imaging of an adult parachute mitral valve. J Am Soc Echocardiogr. 2010;24:351. doi: 10.1016/j.echo.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Becker AE, Becker MJ, Edwards JE. Anomalies associated with coarctation of aorta; particular reference to infancy. Circulation. 1970;41:1067–75. doi: 10.1161/01.cir.41.6.1067. [DOI] [PubMed] [Google Scholar]
- 4.Davachi F, Moller JH, Edwards JE. Diseases of the mitral valve in infancy. An anatomic analysis of 55 cases. Circulation. 1971;43:565–79. doi: 10.1161/01.cir.43.4.565. [DOI] [PubMed] [Google Scholar]
- 5.Hakim FA, Kendall CB, Alharthi M, Mancina JC, Tajik JA, Mookadam F. Parachute mitral valve in adults-a systematic overview. Echocardiography. 2010;27:581–86. doi: 10.1111/j.1540-8175.2009.01143.x. doi:10.1111/j.1540-8175.2009.01143.x. [DOI] [PubMed] [Google Scholar]
- 6.Moustafa SE, Lesperance J, Rouleau JL, Gosselin G. A forme fruste of Shone's anomaly in a 65 year-old patient. Mcgill J Med. 2008;11:19–21. [PMC free article] [PubMed] [Google Scholar]
- 7.Prunier F, Furber AP, Laporte J, Geslin P. Discovery of a parachute mitral valve complex (Shone's anomaly) in an adult. Echocardiography. 2001;18:179–82. doi: 10.1046/j.1540-8175.2001.00179.x. doi:10.1046/j.1540-8175.2001.00179.x. [DOI] [PubMed] [Google Scholar]
- 8.Da Silva CL, Edwards JE. Parachute mitral valve in an adult. Arq Bras Cardiol. 1973;26:149–53. [PubMed] [Google Scholar]
- 9.Popescu BA, Jurcut R, Serban M, Parascan L, Ginghina C. Shone's syndrome diagnosed with echocardiography and confirmed at pathology. Eur J Echocardiogr. 2008;9:865–7. doi: 10.1093/ejechocard/jen200. doi:10.1093/ejechocard/jen200. [DOI] [PubMed] [Google Scholar]
- 10.Brown JW, Ruzmetov M, Vijay P, Hoyer MH, Girod D, Rodefeld MD, et al. Operative results and outcomes in children with Shone's anomaly. Ann Thorac Surg. 2005;79:1358–65. doi: 10.1016/j.athoracsur.2004.09.013. doi:10.1016/j.athoracsur.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 11.Bolling SF, Iannettoni MD, Dick M, II, Rosenthal A, Bove EL. Shone's anomaly: operative results and late outcome. Ann Thorac Surg. 1990;49:887–93. doi: 10.1016/0003-4975(90)90861-y. doi:10.1016/0003-4975(90)90861-Y. [DOI] [PubMed] [Google Scholar]
- 12.Brauner RA, Laks H, Drinkwater DC, Scholl F, McCaffery S. Multiple left heart obstructions (Shone's anomaly) with mitral valve involvement: long-term surgical outcome. Ann Thorac Surg. 1997;64:721–29. doi: 10.1016/s0003-4975(97)00632-2. doi:10.1016/S0003-4975(97)00632-2. [DOI] [PubMed] [Google Scholar]
- 13.Ikemba CM, Eidem BW, Fraley JK, Eapen RS, Pignatelli R, Ayres NA, et al. Mitral valve morphology and morbidity/mortality in Shone's complex. Am J Cardiol. 2005;95:541–3. doi: 10.1016/j.amjcard.2004.10.030. doi:10.1016/j.amjcard.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 14.St Louis JD, Bennan MM, Lutin WA, Wiles HB. Surgical strategies and outcomes in patients with shone complex: a retrospective review. Ann Thorac Surg. 2007;84:1357–63. doi: 10.1016/j.athoracsur.2007.05.003. doi:10.1016/j.athoracsur.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Grimaldi A, Vermi AC, Maisano F, Sacco F, Castiglioni A, Zangrillo A, et al. Echocardiographic patterns of incomplete Shone's syndrome in adults. J Heart Valve Dis. 2011;20:552–6. [PubMed] [Google Scholar]