Abstract
In adults, primary malignant brain tumors (PMBT) are rare, but they have a devastating impact and the chances for survival are limited. UK clinical guidance on supportive care for patients with brain and central nervous system tumors was published in 2006 and relied on very limited evidence. The current article reviews literature from 2005 through 2011 on the psychosocial and supportive needs of patients with PMBT and their families or caregivers. Searches were conducted in PubMed, Web of Science, Psychinfo, Cochrane, Scopus, ASSIA, and Sciencedirect. The search initially yielded 6220 articles, of which 60 were found to be eligible (1%). Eleven qualitative and 49 quantitative studies are reviewed here and mapped onto the structure of the existing UK clinical guidance. Studies suggest rates of depression and anxiety up to 48% in patients and up to 40% in caregivers, with many unmet needs and dissatisfaction with health care provider communication and information. Cognitive deficits increase as the disease progresses, hampering communication and decision-making. A range of neurological and physical symptoms at the end of life need recognition. Some successful supportive and neuropsychological interventions are reported. Although the volume of available studies has increased since UK guidance, many remain observational in nature, with few trials of interventions. However, this review provides an up to date resource for clinicians involved with patients with PMBT, describing current knowledge on patients' psychosocial needs, the type of care which has been found to be beneficial, and highlighting areas where more research needs to be done.
Keywords: brain tumors, cognition, high-grade glioma, psychological health, rehabilitation, supportive care
High-grade glioma (HGG) is a malignant tumor that constitutes ∼75% of primary brain tumor diagnoses in adults.1 These tumors have an annual incidence of 3–4 cases per 100 000 population.2 Among adults, the chance of surviving these tumors is limited; they drastically shorten life expectancy, and treatments that can significantly increase survival remain hard to find.3 In patients with glioblastoma aged <70 years, median survival has been cited as 12.1 months with radiotherapy alone and 14.6 months with radiotherapy plus temozolomide treatment, and 5-year survival rates in these 2 groups are 1.9% (95% confidence interval [CI], 0.6–4.4) and 9.8% (95% CI, 6.4–14.0), respectively.4,5 Older patients and those with poor performance status have the worst prognosis. A recent phase III randomized study showed that those aged >70 have a median survival of 4 months with supportive care alone and 7 months with radiotherapy.6 With regard to grade 3 gliomas, prognosis is largely determined by histological subtype and molecular features. Patients with anaplastic glioma without codeletion of 1p and 19q chromosome regions have a median survival of 2–3 years, whereas patients with anaplastic oligodendroglial tumors with codeletion of 1p/19q have a median survival of at least 7 years.7,8
The unique effect of malignant brain tumors on patients' quality of life and relationships due to the rapidity of intellectual and physical decline has been documented. However, studies have frequently been in the clinical trial setting, thereby focusing on a specific group of high-performing patients and on the adverse effects of treatment.9,10 In contrast, this review focuses on wider research investigating how patients function in their daily lives and what types of care are beneficial to them and help to meet their psychosocial and supportive care needs.
In June 2006, the National Institute for Health and Clinical Excellence (NICE) produced guidance for health care professionals treating adult individuals with brain and other central nervous system (CNS) tumors.11 The section on supportive care covered communication, information provision, psychological support, rehabilitation services, palliative care, social support, and continuing care. In most cases, evidence (published up to 2005) was found to be sparse or missing, and only general guidance could be drawn from small, observational studies or from research on other cancer types. This current review of the literature from 2005 through 2011 aims to give updated guidance from recently published studies.
Methods
A literature search was conducted during 10–16 February 2011. The following databases were searched: PubMed, Web of Science, Psychinfo, Cochrane, Scopus, ASSIA, and ScienceDirect. For each database, 3 searches were conducted. Each search included the search terms: “glioma” or “primary brain tumor” or “brain cancer” or “malignant brain tumor” or “glioblastoma” or “GBM;” in addition, an “and” condition was specified for the following 3 groups of terms:
“quality of life” or “QOL” or “psychological” or “psychosocial” or “social” or “anxiety” or “depression” or “fatigue”,
“cognition” or “cognitive deficit” or “neuropsychological” or “rehabilitation,” and
“care needs” or “supportive care” or “follow-up care” or “nursing care” or “palliative care” or “communication” or “information” or “attitudes” or “awareness.”
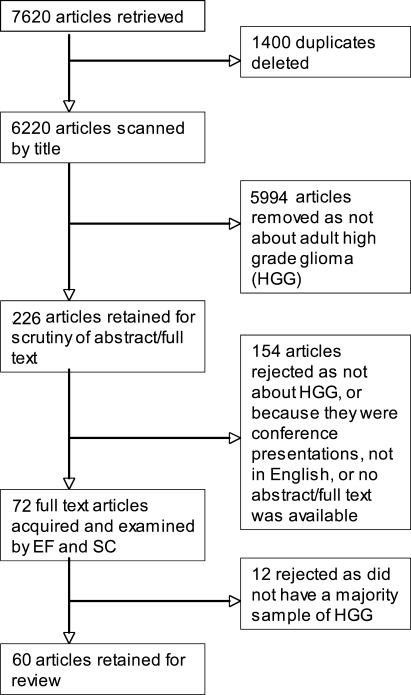
The latest dated reference included in the 2006 NICE guidelines was from August 2005, and the preceding reference was from 2004. All articles published from 1 January 2005 to the time of the search were therefore included in this search. In total, 7620 article citations were found and downloaded into EndNote, version X1 (Thomson Reuters, 2010). These were scanned using EndNote for duplicates, and 1400 were deleted, giving a final tally of 6220 articles.
Initially, the article titles were scrutinized for eligibility and included if the sample population was composed of adult patients with a diagnosis of primary malignant brain tumor (also specified as malignant glioma, HGG, anaplastic astrocytoma, glioblastoma multiforme, anaplastic oligodendroglioma, oligoastrocytoma, or World Health Organization tumor grade III and IV). In addition, articles were retained if they reported on any of the following: information and communication needs; psychological health and cognitive function; supportive care; psychological, cognitive, and rehabilitation interventions; palliative or end of life care; or experience or needs of caregivers.
If it was not clear from the title whether the article was eligible, it was retained for further examination. This first sift resulted in 226 articles for which abstracts and/or full text articles were obtained to check for content and eligibility. After the second inspection, 154 articles were rejected because they failed to meet the aforementioned eligibility criteria, were conference presentations, the main article was not in English, or no abstract and/or full text was available.
The 72 remaining articles were examined jointly by 2 authors (E.F. and S.C.), and both authors agreed on the elimination of 12 more articles in which the study samples did not have a majority (≥50%) of patients with high-grade or malignant tumors. However, in 4 cases, articles with a mixed sample were retained because the high-grade group results were reported separately. Articles were described as qualitative if they did not present any statistical analyses and quantitative if they presented results analyzed statistically in any way. Finally, 60 articles were reviewed and categorized according to NICE guideline sections given below. The entire process of searching and sifting is shown in Fig. 1.
Fig. 1.
Flow chart showing selection of studies.
Section 1: Communication and information
Section 2: Psychological health and supportive care services
Section 3: Cognitive symptoms and rehabilitation
Section 4: Palliative and end of life care
Section 5: Family and caregiver roles, stress, and needs.
Results
Section 1: Communication and Information
The NICE guidelines recommended that all health care professionals (HCPs) working with patients with brain tumors should have communication skills training and that communication should be face-to-face with patients and their family and/or caregivers, including discussion of diagnosis, prognosis, treatment options, recurrence, and end-of-life care. There were gaps in the evidence about how much patients wanted to know and how best to disclose diagnosis and prognosis. The guidance recognized that patients with brain tumors had specific information needs and that their understanding of information could be compromised by cognitive impairment. In addition, it was suggested that HCPs should elicit information on the use of complementary therapies from patients and their caregivers. The evidence review found no studies comparing different methods of providing information; however, it found studies in which many patients complained about a lack of information. The current review found 4 studies reporting on communication and information and an additional 4 reporting on complementary therapy use in patients with glioma.
Qualitative Research
Two qualitative articles examined the information and communication needs of patients. Halkett et al12 found that patients expressed a need for information on prognosis and a need for diagnostic, prognostic, and treatment information to be provided in different formats (eg, written and verbal information, including reasons for various instructions). Patients found that decision-making was difficult because of receiving an unclear prognosis and being given a lack of alternatives to the treatments proposed. Patients appreciated being given time to ask questions and receive honest answers from HCPs, although they also found that cognitive and memory deficits, especially dysphasia, hampered effective communication. Lobb et al13 studied perceptions of the initial communication about diagnosis and prognosis of HGG. Patients and caregivers reported feeling a state of shock at the diagnosis, which made processing prognostic information very difficult at that time. The delivery of the prognosis took away hope and could be perceived as the HCPs giving up on the patient. One way of coping was for patients and caregivers to think of themselves as individuals and that statistics did not apply to them, preferring to remain positive. Only 2 of the 40 participants reported a positive experience of information giving by HCPs. Many wanted clinician communication to be more compassionate and empathic, to contain some positive messages, and to avoid treating patients as just numbers in the system.
Quantitative Research
Two studies reported quantitative data regarding information, communication, and decision-making. Diaz et al14 hypothesized that fear and anxiety may not be caused by brain tumor symptoms but by the perception of the threat posed by the symptoms. They suggested, therefore, that communication is fundamental to alleviating this anxiety, by improving predictability and feelings of control. Twenty-six patients with HGG reported their information preferences: 50% wanted all possible information, 23% wanted only important aspects, and 27% wanted only critical aspects. Fifteen percent of patients expressed a wish to ask their HCPs more questions. Younger patients (aged <65 years) wanted more information than did older patients. Anxiety was found to be lower in patients who wanted to know everything about their illness, understood the information better, and were more satisfied with the information that they received. These authors concluded that communication is a fundamental part of the care of patients with cancer and influences well-being and that information should be adapted to the needs of each patient.
Medical decision-making capacity is relevant for patients with HGG because they must make ongoing and challenging medical decisions about a disease that rapidly erodes cognition. Clinicians are legally and ethically required to ensure that their patients are capable of providing valid consent before initiating treatment. Triebel et al15 found that patients with HGG performed the same as controls (healthy adults aged >19 years, matched on age, education level, sex, and race) when expressing treatment choice but significantly worse than controls when providing reasons for a choice and understanding the treatment situation and risks and benefits. They showed a trend toward worse performance in appreciating the personal consequences of a treatment choice. Consent capacity was associated with neuropsychological performance in general and with verbal recall and semantic fluency in particular. These findings highlight the need for careful consideration of the capacity of the patient with HGG to consent to medical treatment. It may be necessary to increase the involvement of the patient's family in decision-making. Written formats may reduce verbal memory demands and support patients' medical decision-making capacity.
Complementary Therapies
Three studies reported on the use of complementary therapies by patients with brain tumor, and 1 study reported a massage therapy intervention. The NICE guidelines recommended that “healthcare professionals should discuss the use of complementary therapies with patients, their relatives, and caretakers and help identify possible side effects or interactions with conventional treatment,” (p. 104) but no evidence about use of complementary therapies was reported. In the studies identified by this review, rates of complementary and alternative medicine (CAM) use varied from 32% to 41%.16–18 The most commonly used CAM were homeopathy (39%), vitamin supplements (31%), psychological methods (29%),16 meditation (32%), herbs (22%), and faith healing (22%).17,18 Reasons given by patients for using CAM included “to do something for the treatment by myself,” “to build up body resistance,” “to support conventional therapy,” and “to have tried everything possible.”16 (p. 2232) Of patients using CAM, 88% found it to be helpful and believed that it contributed to tumor shrinkage. CAM use was associated with higher performance scores and well-being in one study,17 but a second study found that quality of life scores were not significantly different between users and nonusers of CAM and were not predicted by CAM use.18 Possible implications for HCPs of this high rate of CAM use by patients include the need to engage patients in dialogue about their use of CAM and to explore potential interactions of CAM with the patients' conventional treatments.
Massage therapy has been used in patients with cancer to reduce psychological stress and improve quality of life. In one study, 25 patients with HGG underwent massage twice weekly for 4 weeks.19 Stress levels decreased significantly during weeks 2–3. At the end of week 4, all stress scores were below the threshold and remained below baseline levels. After the massage course, patients reported improved emotional and social well-being and fewer brain tumor concerns, suggesting that the intervention is feasible and acceptable. However, because there was no control group, it is not possible to estimate the effect of the massage alone.
Section 2: Psychological Health and Supportive Care Needs and Services
The NICE guidance recognized that patients with brain tumors may experience psychological difficulties while adjusting to their life-threatening condition and can have cognitive and personality changes as a result of their illness. However, it was acknowledged that there was limited research on mood and personality changes. The current review found 1 qualitative study reporting on patients' adjustment to their diagnosis. An additional 5 studies reported on anxiety and depression in patients with brain tumor, including prevalence, causes, and consequences of anxiety and depression. In addition, 5 studies report on stress or distress in patients with brain tumor.
Qualitative Research
One study looked at personal and social processes of adjustment by patients with a brain tumor.20 Individuals perceived that they had been unprepared for what to expect after treatment, especially the ongoing tumor or treatment effects or long-term implications, and had difficulty making sense of the situation and its meaning for them. This may reflect a lack of communication, a reluctance to predict specific outcomes by HCPs, or a poor ability to recall conversations by patients.
Anxiety and Depression in Patients With HGG
Five studies reported on rates of anxiety and depression in populations of patients with primary malignant brain tumor. The quality of studies varied, and the use of different measurement tools contributed to the heterogeneity of rates of depression and anxiety.
The lowest reported rate of depression (5%) was generated by a retrospective review of oncology notes,21 whereas the highest rate (47%) resulted from applying the Beck Depression Inventory to a group of 15 patients with HGG.22,23 Rates of anxiety among patients ranged from 30%, using the Hospital Anxiety and Depression Scale,24 to 48%, using the Brief Patient Health Questionnaire.25 The data are summarized in Table 1. One study noted that patients who improved or remained functionally stable between surgery and radiotherapy did not report a high level of anxiety and depression. In contrast, patients who clinically deteriorated completed the scale less often and were under-represented.26
Table 1.
Rates of anxiety and depression in patients with primary brain tumor
| Study | N patients | Test used | Anxiety | Depression |
|---|---|---|---|---|
| Kilbride et al. 200726 | 51 (42 with high grade brain tumors) | HADS | 35% | 13% |
| Arnold et al. 200825 | 363 (266 with HGG) | Modified brief PHQ | 48% | 41% |
| Mainio et al, 2005a, 2005b, 200622,23,27 | 15 with HGG from full sample of 75 | BDI | – | 47% |
| Gathinji et al. 200921 | 1052 (all with malignant astrocytoma) | Review of oncology notes (diagnosis by physician) | – | 5% depressed in pre-operative period |
| Janda et al. 200724 | 75 (mixed; 44 malignant) | HADS | 30% | 17% |
Abbreviations: BDI, Beck Depression Inventory; HADS, Hospital Anxiety and Depression Scale; PHQ, patient health questionnaire.
Several factors were found to be associated with rates of depression and anxiety. The most common factor associated with depression was previous psychiatric illness,25–27 followed by being female.25,27 Tumor location did not appear to be associated with depression.27 In interviews, reasons given for low mood were hair loss, weight gain, tiredness, and poor functional state.26 Lower level of education and lower tumor grade were also associated with depression in a single study.25 Anxiety was also associated with previous psychiatric illness25 and female sex.25 Interviews revealed that anxiety was associated with uncertainty about when treatment would start and what it would involve and worries about what symptoms and effects the tumor would have. Only 44% of patients with anxiety and/or depression were taking psychiatric medication.25
Depression and anxiety have been found to have several consequences for patients with brain tumor. Anxiety and depression were negatively associated with all aspects of quality of life, as measured by the Functional Assessment of Cancer Therapy–Brain.24 An increase in depression over time was associated with decreased quality of life, suggesting a causal relationship.22 Functional status (measured by the Karnofsky Performance Status scale) was highly associated with depression both before surgery and in follow-up,27 although a causal direction could not be established in this study. In addition, although not significant, a trend toward faster death in those patients with current depression was found.27 A retrospective review of 1052 patients with astrocytoma21 showed that, after adjusting for all variables associated with survival (degree of disability, tumor grade, and treatment), preoperative depression was independently associated with decreased survival (relative risk, 1.41; 95% CI, 1.1–1.96).
Stress and Distress
Two articles studied stress in patients with brain tumor. Stress is defined as the psychological and/or physical response that occurs when one must adapt to changing conditions.28 In one study, 63% of patients reported elevated stress levels, and 86% indicated that they were at least somewhat interested in learning about programs to reduce stress.28 In a second study, it was found that stress did not reduce over time, because survivors of >18 months had the same stress levels as those who received a diagnosis more recently (<18 months). In both groups, 60%–61% of patients had stress levels above threshold on the Perceived Stress Scale.29
Three articles used the National Comprehensive Cancer Network (NCCN) Distress Thermometer to report distress levels in patients with brain tumor. Distress is defined as the experience of anxiety or depression symptoms that do not meet the full diagnostic criteria for these disorders. However, scores above the cutoff on distress measurement tools can be suggestive of a clinical case. One study found that 52% of patients scored above threshold on the NCCN Distress Thermometer.30 These scores were significantly correlated with other patient-reported emotional and physical sources of distress and concerns. In a second study of long-term surviving patients with HGG, 59% met the criteria for significant distress, as opposed to 49% of patients who had received a diagnosis less than 18 months ago.31 In the third study, 29% of patients scored above threshold for distress.32 In this study, distress was found to be negatively associated with social and emotional well-being, as measured by the Functional Assessment of Cancer Therapy–Brain. These researchers also found that many potential participants (33%) did not complete questionnaires adequately and, thus, could not be included; they noted that current tools for measuring quality of life may under-sample patients with the highest levels of distress.
Supportive Care
The NICE guidelines did not give specific recommendations for follow-up or supportive care for patients with brain tumor, instead focusing on neuro-psychological, rehabilitation, and palliative services. Since 2005, 5 studies have reported on aspects of follow-up and supportive care for patients. Two studies reviewed what is currently provided to patients with HGG in the United Kingdom, and one Australian study reported on the unmet needs of these patients. An additional 2 US studies reported on a specialist nurse support intervention. No studies were found that focused on efforts to reduce anxiety and depression in patients with brain tumor.
What Care Is Currently Available to Patients?
In a UK study, 102 clinicians from all UK neuro-oncology multidisciplinary teams were approached, of whom 86 replied to a survey on follow-up practices for HGG.33 All but one reported that regular follow-up services were available to patients with HGG after the end of radical treatment and that choices were conveyed verbally in consultations. Follow-up services included visits to the outpatient clinic, phone contact with a nurse, seeing the palliative care team, or appointments with the general practitioner. Most (98%) of these clinicians reported that their patients regularly attended outpatient clinics, which were mostly held in the oncology center. Brain imaging was a regular part of follow-up to detect recurrence and monitor treatment effects. More than 80% of respondents reported having referral access to neurologists, physiotherapy, speech therapy, and clinical trials. Fewer clinicians (60%–70%) were able to refer patients to an epilepsy nurse, social worker, counselor, neuro-psychologist or support group, or for rehabilitation, occupational therapy, or complementary therapies. The least accessible service was clinical psychology (50%). When asked what would improve supportive care for patients, the 5 top suggestions from clinicians were having well-resourced specialist nurse availability, providing better community support for families, having better access to physiotherapy, having more integrated services and/or team clinics, and having better access to psychologists or counselors.
In another UK study,34 a retrospective review of the case notes was conducted to understand what care patients had been given during the course of their illness. With regard to the care received, 28% of patients were admitted to a hospice inpatient unit and 15% to acute inpatient services. For outpatient care, 11% of patients attended oncologist outpatient appointments, 49% accessed community district nursing services, 7% accessed other voluntary-based services, 24% attended day hospice, and 36% were referred to social services for help with activities of daily living. Patients also accessed physiotherapy (35%) and occupational therapy (31%), and 34% of patients received financial benefits. Complementary therapies were used by 24% of patients, 35% used counseling services, and 13% accessed chaplains or church support. This study suggested that some patients were accessing specialist palliative care late, which may have implications for getting access to supportive care.
Unmet Patient Needs and What Patients Want
A study of patients with brain tumor in Queensland, Australia, reported unmet supportive care needs.35 Patients reported moderate to high requirements for help with physical needs (lack of energy and tiredness, not being able to do things they used to do), psychological issues (having a single HCP to talk to, anxiety and uncertainty about the future, trying to feel in control, concerns about those close to them, fears about the tumor spreading), and practical problems (easy car parking at the clinic, monetary needs for treatment and equipment). The top 5 unsupported tumor-specific needs reported by patients were the physical adverse effects of the tumor and treatment, changes in their mental abilities, feeling as if they were not the same person, wanting information on the latest developments in research and treatment, and changes in their ability to work. Patients and caregivers also expressed interest in services that could help them to improve physical activity and maintain healthier eating habits, achieve weight control, learn how to manage stress, and learn how to keep old friends and make new ones. Patients were also interested in learning how to return to their usual activities.
Interventions in Supportive Care
Two studies reported how Swedish patients with glioma and their families responded to intervention by a specialist nurse who was specifically instructed to address their needs.36,37 At the time of diagnosis, the nurse offered to serve as a resource to 16 patients and their families. Interactions between the nurse and the patient or family were analyzed qualitatively. Nurses were seen as a resource for the whole family in 9 cases, whereas in 3 cases, the nurse was solely used as a resource for the next of kin, and in 4 cases she was a resource for the patient only. Family members' initial contacts with the nurse were regarding subjects related to the patient's medical health and treatment, including health care and medical symptoms and their decline.37 Later contacts between family members and the nurse related to the family member's own needs and desires, including information about their partner's health and sharing their own emotions. Later, family members talked about topics that had nothing to do with the patient or the illness.
Having a specialist nurse to call and visit on demand was understood as a relational function rather than a service function, with the nurse acting as an active companion during the course of the disease. The nurse was conceptualized as providing a secure base for the caregivers throughout the illness of the loved one.
Section 3: Cognitive Symptoms and Rehabilitation
In the 2006 NICE guidelines, it was highlighted that patients may have cognitive and psychological changes and that clinical neuropsychologists with expertise in cognitive impairment should be involved in their treatment. This section reviews evidence on cognitive changes experienced by patients with primary brain tumor and rehabilitation interventions that have been trialed.
Cognitive Impairments in Glioma
Thirteen studies delineated various aspects of research on cognitive impairments in patients with glioma. Four studies reported on changes in cognition that resulted from surgery, chemotherapy, and radiotherapy.38–42 There was little evidence supporting the hypothesis that cognitive decline differed according to the type of treatment given or the degree of surgical resection, but evidence did support an association between cognitive deficits and tumor characteristics, including histology, location, and size. For example, Mini-Mental State Examination (MMSE) scores did not differ depending on whether patients received only radiotherapy or chemotherapy in addition,38 cognitive decline did not correlate to the degree of surgical resection,39 and although one study found that cognition declined post-operatively, 79% of patients were showing deficits before surgery.40 Two studies reporting on cognitive decline over time suggested that clinically significant deterioration of MMSE scores was associated with more rapid time to tumor progression and death.43 These authors concluded that the cause of cognitive decline seems to be subclinical tumor progression that precedes radiographic changes. In a separate study, tumor recurrence was also associated with worse cognitive performance.44 Cognitive impairment was found to be associated with depression and low mood, fatigue, sleep disturbance, worse quality of life, and worse physical performance.45–47 Cognitive impairment was more likely to be observed in older patients and those with higher tumor grade.48 One study specifically examined aphasia in patients with malignant brain tumor who had undergone the resection of a left hemisphere tumor, which had resulted in speech pathology.49 Most patients had mild aphasia (63%), with the rest having moderate to severe aphasia. Anomic aphasia was the most common subtype (49%). Another study found that cognitive impairment interacted with caregiver ratings of patient mood, so that caregiver and patient ratings of mood agreed more strongly if patients had milder cognitive impairment.50
Rehabilitation
Three articles presented data on rehabilitation initiatives or interventions that were aimed at patients with primary brain tumors. Two of these presented data on neuro-cognitive rehabilitation51,52 and one on inpatient hospital rehabilitation.53
One study trialed a cognitive rehabilitation intervention in 19 patients with mild to moderate cognitive deficits.51 Patients and caregivers randomized to the intervention group (n = 12) were taught to use a calendar as an external aid to compensate for cognitive symptoms and a positive problem solving technique to manage behavioral symptoms. After 3 months, 50% of patients continued to use the strategies several times per week and 88% at least once per week. Participants and caregivers rated the intervention as helpful. No significant differences were found in quality of life ratings between the intervention and control group, but because participant numbers were low, this is unsurprising. In another study,52 11 patients attended weekly for 10 sessions of holistic mnemonic training, in which exercises were used to train perception, concentration, attention, memory, retentiveness, verbal memory, and creativity. Mean scores on cognitive tests improved from pre- to postintervention testing, but differences were not significant, partly because of the small group size. However, participants were satisfied with the training.
Fu et al53 compared the outcomes in patients with high- and low-grade conditions after inpatient hospital rehabilitation (n = 42). They found that patients with high-grade cases had longer stays in rehabilitation and higher total gain on a functional independence measure. However, no information was provided about the nature of the rehabilitation.
Section 4: Palliative and End-of-Life Care
The 2006 NICE guidelines recognized that for patients with primary brain tumor, a palliative approach may be needed from the time of diagnosis. However, generalist HCPs in the palliative care sector may have little experience of the particular care needs of these patients. The guidelines also recognized the increased decision-making burden placed on family and caregivers because of the cognitive and neurological decline in patients with brain tumor at the end of their life. Research evidence recognized difficulties in identifying patients' needs and that training programs for HCPs are needed.
This review identified 4 articles that retrospectively examined the records of patients with primary brain tumor and described their symptoms and care needs during the end of life phase.54–57 In the last weeks of life, patients experienced a wide range of symptoms, some of which appeared to be experienced by the majority of patients with primary malignant brain tumor. The rates of symptoms reported in each of the 4 studies are shown in Table 2. Drowsiness and loss of consciousness (referred to as loss of vigilance in one study) was the most common symptom among patients in the last week or weeks of their lives (85%–90%). Also commonly reported across studies were weakness (62%–80%), seizures (30%–56%), dysphagia (10%–79%), headache (33%–62%), and fatigue (25%–67%).
Table 2.
Rates of symptoms in patients with primary brain tumor at end of life
| Symptom | Sizoo et al. 201056 | Pace et al. 200954 | Faithfull et al. 200555 | Oberndorfer et al. 2008 (final 2 weeks of life)57 |
|---|---|---|---|---|
| Neurological | ||||
| Drowsiness, loss of consciousness | 87% | 85% | 90% | |
| Weakness/hemiparesis | 62% | |||
| Seizures/epilepsy | 45% | 30% | 56% | 48% |
| Focal neurological deficits e.g. motor/dysphasia | 51% | |||
| Poor mobility | 77% | |||
| Poor communication | 64% | |||
| Visual disturbance | 21% | |||
| Cognitive/Psychological | ||||
| Cognitive deficits/memory loss | 33% | 39% | ||
| Confusion | 29% | |||
| Anxiety/depression | 9% | |||
| Agitation/delirium/confusion | 15% | 31%/na/51% | ||
| Eating and Digestion | ||||
| Dysphagia | 71% | 85% | 10% | 79% |
| Nausea/vomiting | 20% | 33% | 28% | |
| Constipation | 9% | |||
| Pain | ||||
| Headache | 33% | 36% | 62% | 38% |
| Bodily pain | 25% | 13% | ||
| Respiratory | ||||
| Dyspnoea | 16% | |||
| Death rattle | 12% | |||
| Pneumonia | 24% | |||
| Urinary | ||||
| Incontinence | 40% | 28% | ||
| Urinary infection | 21% | |||
| Other | ||||
| Skin problems | 28% | |||
| Fever | 86% | |||
| Fatigue | 25% | 44% | ||
Several of the studies reported on end-of-life care given to patients in the weeks before their death. Pace et al54 reported that end-of-life decisions taken in study patients included tube feeding (13%), hydration (87%), steroid interruption (45%), and palliative sedation (13%). Only 6% of patients had established advance directives about end-of-life treatment, and progressive neurological deficits and loss of consciousness often meant that decisions had to be made on their behalf. Nevertheless, 82% of patients in this study experienced a peaceful death with progressive loss of consciousness, good symptom control, and no pharmacological sedation. With regard to services used by the end of life population, in one UK study of 39 patients,55 17 (44%) were admitted to hospice, 28 (72%) received district nursing, and 29 (74%) experienced acute hospital admission. In addition, 65% saw a speech therapist, 54% saw a social worker, 46% used social services, 26% used complementary therapy, and 31% spoke to chaplains. In this sample, 33% of patients died in a hospice, 33% died at home, 13% in a hospital, and 13% in a nursing home.
Section 5: Family and Caregiver Experience, Stress, and Needs
The role of the family or caregivers of patients with brain tumors was not covered in the NICE guidelines, except for a statement that they should be involved in communication and information giving. This review found 16 articles addressing the experiences of caregivers, their symptoms of stress or distress, their needs, and interventions helping caregivers to adjust.
The Experience of Caregivers
Four qualitative studies described some aspect of the caregivers' roles or experiences. One study highlighted that a role change occurs for loved ones after diagnosis of a brain tumor, from partner/child to caregiver. Loss of equality was identified as a shock, especially in spousal relationships.58 Mood and cognitive and physical decline in the patient contributed to the role change. Telling others of the diagnosis was especially difficult for caregivers and was distressing, although caregivers denied that caring for their loved one was a burden. In a second study, a central theme of “a time of rapid change” emerged, in which experiences involved changes occurring from diagnosis and throughout the illness, including after surgery.59 Loved ones suddenly went from being a partner or relative to being a caregiver and had to make decisions regarding work to have enough time to provide support for the patient. Two subthemes emerged that were “renegotiating relationships,” meaning taking on more responsibilities for care and decision-making and losing equality in the relationship, and “learning to be a caregiver,” such as providing personal care. In addition, the fear of seizures placed great limitations on what caregivers felt able to do with the patient.
In a study describing the views of caregivers and patients with primary brain tumor on what was important and meaningful in their lives,60 participants spoke about how brain cancer was unique in terms of the rapidity of intellectual and physical decline and the lack of any effective and long-lasting treatments. Caregivers talked about the lessons that they had learned, such as coping, fighting, and being strong. Most participants felt that quality of life was more important than prolonging life, and caregivers suggested that spirituality (but not religion) was helpful. Participants emphasized the importance of mental functioning. Caregivers commented that disabilities of the body were easier to manage than disabilities of the mind and that losing their loved one's identity, memory, and awareness was tantamount to their dying. Most caregivers directly supported the idea of euthanasia. Participants were grateful for the opportunity to speak to a member of the health care team about personal and distressing issues.
Bereaved relatives were interviewed after the death of their loved one due to HGG.61 Relatives described the quality of life of their loved ones, in which quality of life was considered to be good if the patient was fit and having a normal life and was considered to be poor if the patient had severe disabilities, loss of normal personality, constant deterioration, and existing in a state worse than death. A quantitative analysis of quality-of-life responses suggested that good quality of life was associated with lower cognitive or personality change, time lived free from physical disability, and the degree of the patient's initial distress.
Caregiver Well-Being
Seven studies report on well-being, stress, or distress in caregivers of patients with a primary malignant brain tumor. Two observational studies used a range of instruments to measure caregiver strain and stress but had small samples. Caregivers had high levels of stress (72%; Perceived Stress Scale),62 with 40% scoring above cutoff on depressive measures (Hospital Anxiety and Depression Scale)24 and the average caregiver exhibiting a significant reduction in their quality of life (>0.5 standard deviations lower on the Functional Assessment of Cancer Therapy–General, compared with norms).24
Factors associated with caregiver strain, burden, or distress included the caregiver being younger and female.63 Patient factors were also associated with caregiver well-being, including patient well-being,63 neuro-psychiatric status,64 declining health,65 and tumor grade (although trends were found in both directions).24,62 One study found a direct effect of patients' problem behaviors on caregiver depression and an indirect effect of operating through caregiver mastery.66 One study found that perception of economic hardship predicted caregiver depression scores and was associated with a trend for higher anxiety 4 months after diagnosis. Economic hardship was also related to caregiver burden because of feelings of abandonment.67
Caregiver Needs
Only one study described caregivers' needs.68 Caregivers provided “extraordinary, uncompensated care,” (p. 61) involving significant amounts of time and energy for months or years. Caregivers described constantly solving problems and making decisions but being completely untrained and unprepared in this role. Because of the heavy burdens of patient care, their own needs were neglected. This study made recommendations for helping caregivers. These involved giving the caregiver clear communication and information about the patient's diagnosis and treatment, especially for transition from hospital to home-based care. It was suggested that home-based visits from specially trained health care staff would promote better patient care at home. It was recommended that caregivers may benefit from an educational plan to develop communication skills to describe their needs and get the information that they require and that a social worker or other HCP could act as a bridge to access services. A family consultation in the crisis phase, in addition to internet-based or telephone support groups in the chronic phase, were suggested as methods for supporting caregivers' emotional needs.
Interventions for Caregivers
Two studies were published on stress reduction preferences in caregivers,69,70 of whom 86% believed that stress could be reduced by using stress-reduction techniques. Participants had already used exercise (77%), massage (32%), and meditation (23%) to reduce their stress. Overall, 81% expressed an interest in learning new stress-reducing techniques. Male participants ranked exercise, massage, meditation, and deep soft-belly breathing as their preferred interventions, whereas women preferred exercise, massage, coping skills, and progressive muscle relaxation. Those who reported higher levels of stress also reported increased interest in stress-reduction techniques.
A study evaluated an educational program to caregivers of patients with malignant glioma.71 Program content included brain tumor biology and treatment, symptom and adverse effect management, safety in the home, the role of palliative care, brain behavior relationships, understanding and coping with cognitive changes, and obtaining psychosocial support. Twenty-four caregivers participated, and assessment showed that knowledge was significantly improved; the program was very favorably evaluated by participants. Participants also appreciated the opportunity to interact with other caregivers.
Discussion
Although much new research has emerged since 2005 regarding the psychosocial health, experience, and needs of patients with primary malignant brain tumor and their caregivers, much of it is observational in nature and few new interventions have been trialed. There were several recurring themes in the studies reviewed, one of which was problems with communication. Patients were, overall, dissatisfied with communication experiences with their health care providers. Several reasons for this emerged. Some patients mentioned a lack of discussion of alternatives to the treatments proposed and a lack of positive messages. Others mentioned feeling unprepared for what to expect after treatment. These examples highlight the difficult balance that clinicians must strike between truth-telling, preparation for dying, and giving some positive messages or hope. Little research evidence is currently available to guide clinical practice, although one article makes the suggestions that clinicians can emphasize what can still be done (eg, controlling physical symptoms, sourcing emotional and practical support), they can explore realistic goals (eg, going on a special trip, spending time with family, or settling their affairs), and they can discuss how to cope with day-to-day living.72 This qualitative study also suggested that sources of hope change over the course of the illness, but some hope can remain even at the end of life for patients (eg, hope of living longer than expected, having special times with family, and feeling well cared for and having a peaceful death).
It was found that disease-related cognitive impairment interfered with communication, understanding, and decision-making. In studies examining the unmet needs of patients, more information and having access to a single, dedicated HCP were high on the list. Lack of information and uncertainty about treatment and prognosis were linked to anxiety. In addition, the importance of effective communication between HCPs and caregivers was prominent, because caregivers became the primary decision-makers or facilitators of communication as patients' cognition declined. Few patients had formulated advance directives for end-of-life treatment, and therefore, caregivers were required to be involved in decision-making with the palliative team.
A picture emerged that cognitive decline in patients with primary malignant brain tumors may be linked to tumor progression as much as it is to treatment modality. Indeed, the evidence suggested that cognitive deterioration may be an early marker of disease progression. Neurological and cognitive deficits were commonplace in the end-of-life phase. There was some evidence that scores on cognitive tests could be improved by training interventions, but sample sizes were very small and more work needs to be done in this area. The value of such training interventions will also depend on the prognosis for the individual patient. For a patient who has mild cognitive deficits and has a life expectancy of several months or years, training interventions will be of use. Conversely, for those patients who are in the final stages of their illness, time may be spent more productively in other ways.
A body of mainly qualitative evidence was found that documented the experience and needs of caregivers of patients with primary malignant brain tumors (PMBTs). Studies suggested that family caregivers experience high rates of stress and anxiety and that the economic burden of coping with disease may contribute to depression and emotional burden. Descriptive studies showed that family members are required to give “extraordinary uncompensated care” for months or even years. Loved ones also described that the role change from partner or child to caregiver and the loss of equality, especially in spousal relationships, was a shock. Caregivers reported that mental decline and loss of identity were the most difficult aspects of their loved one's deterioration. In addition, caregivers had to constantly solve problems and make decisions for their loved one without any training or preparation for this role. Although few caregiver interventions have been trialed, recommendations to emerge from this literature include having a dedicated HCP, such as a nurse, to provide supportive care to families; having educational programs for caregivers to prepare them for changes in their loved one and to increase understanding of treatment processes; teaching caregivers stress reduction techniques; involving caregivers more in communication with HCPs; and having family consultations in the crisis phase.
The clinical implications to emerge from these studies are that communication surrounding the imparting of diagnostic and prognostic information is still not meeting patient or caregiver needs. Specifically, communication style must take into account potential cognitive deficits, mental health issues, the dynamics of shifting personal identity, and the importance of caregivers in patient decision-making. Communication skills training tailored to address these issues would be beneficial and should be a primary focus of new research in this area. One study was identified in which a documentary film about the experience of family caregiver was trialed as an education tool for physicians.73 This kind of intervention, which reminds HCPs of the wider social circumstances of their patients, may be helpful in developing training programs for specialist communication skills. It may be of use to clinicians to have some training in alternative communication tools, such as writing, prompt cards, or pictures, for when speaking and understanding becomes more difficult for the patient. It also requires great sensitivity to discuss with a patient the possibility that they may experience a decline in the ability to communicate. However, this discussion may help patients to state their wishes about dying while they are still fully able to communicate and help them to prepare ways of making their wishes felt in the event of cognitive decline or reduced conscious awareness. As shown by this review, there is no current literature on strategies for persons with communication problems after PMBT, but speech and language pathologists may successfully use strategies developed for use in stroke or brain injury populations.74
In addition, systematic evaluation of cognitive status or deterioration would not only help HCPs to deliver information appropriately but may also give an early indication of tumor progression. Both patients and caregivers appear to prize longevity of mental capacity over retaining physical function and report mental rather than physical decline as being the most difficult hardship. There seems to be no universal format for testing neuropsychological status in this population, with some studies favoring the MMSE and others administering a full battery of cognitive tests. Future research could address the best way of testing cognitive status in an acceptable way to patients and within the constraints of health services and could investigate the most useful follow up, rehabilitation, or support interventions that meet the needs of patients.
There are several limitations to the research reported in this review, and these restrict the extent to which the findings can be generalized. Most studies were small and observational. The larger samples tended to be part of clinical trials or retrospective reviews of case notes. The fact that HGG is a rare cancer that is characterized by rapid decline will always hamper recruitment efforts for psychosocial studies, which do not attract the same levels of funding as do more clinically focused research. In addition, as was discussed in 2 studies, the poorer performing patients tended to drop out as time goes on, meaning that they may have been under-represented in the findings. This suggests that true rates of distress, mental disorders, and cognitive deficits may be higher than reported.
Patients with PMBTs often receive a sudden diagnosis, followed by deteriorating health, multiple symptoms, and cognitive impairments. It may therefore be necessary to develop methodologies for testing psychosocial interventions that do not rely on the application of normal trials methods. Other authors have written extensively on research methods suitable for palliative care.75,76 Observational studies will, of necessity, form the bulk of the research, but it should be noted that trials of interventions need to take into account the fact that dropout rates are high and these patients do not drop out at random. Future research should find a way to distinguish the effect of the intervention on quality of life or function from the effect of disease progression.
Findings from this review may also be able to inform the practice of HCPs who care for patients with brain metastases originating from other primary sites. Although the psychological experience of the diagnosis of brain tumors is different for this population, because they will already have experienced cancer for some time, they may share some of the same care needs as the disease progresses. For example, needs related to cognitive decline and rehabilitation,77 difficulties with communication, and palliative care78 may overlap. Other reviews draw attention to similarities and differences in the needs of patients with primary versus secondary brain tumor.78
Conclusions
Although new research on the psychosocial needs of patients with PBMT has been conducted, much of it has still involved small samples or is limited in scope. Research still focuses on describing patient and caregiver experience rather than establishing the best methods of providing care or information or developing and trialing new supportive care interventions. This review suggests that profitable avenues for future research include developing communication skills training packages for HCPs, standardizing cognitive testing, and providing more support and education for caregivers.
Conflict of interest statement. None declared.
Funding
This work was supported by a Cancer Research UK Program Grant (C54/A7374: Supportive Interventions in Cancer Care).
References
- 1.Polednak AP, Flannery JT. Brain, Other Central Nervous System, and Eye Cancer. Cancer. 1995;75:330–337. doi: 10.1002/1097-0142(19950101)75:1+<330::aid-cncr2820751315>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 2.DeAngelis LM. Brain Tumours. New England Journal of Medicine. 2001;344(2):114–123. doi: 10.1056/NEJM200101113440207. [DOI] [PubMed] [Google Scholar]
- 3.Mirimanoff R-O, Gorlia T, Mason W, et al. Radiotherapy and Temozolomide for Newly Diagnosed Glioblastoma: Recursive Partitioning Analysis of the EORTC 26981/22981-NCIC CE3 Phase III Randomized Trial. Journal of Clinical Oncology. 2006;24(16):2563–2569. doi: 10.1200/JCO.2005.04.5963. [DOI] [PubMed] [Google Scholar]
- 4.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. New England Journal of Medicine. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 5.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. The Lancet Oncology. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 6.Keime-Guibert F, Chinot O, Taillandier L, et al. Radiotherapy for Glioblastoma in the Elderly. New England Journal of Medicine. 2007;356:1527–1535. doi: 10.1056/NEJMoa065901. [DOI] [PubMed] [Google Scholar]
- 7.van den Bent MJ, Carpentier AF, Brandes AA, et al. Adjuvant Procarbazine, Lomustine, and Vincristine Improves Progression-Free Survival but Not Overall Survival in Newly Diagnosed Anaplastic Oligodendrogliomas and Oligoastrocytomas: A Randomized European Organisation for Research and Treatment of Cancer Phase III Trial. Journal of Clinical Oncology. 2006;24:2715–2722. doi: 10.1200/JCO.2005.04.6078. [DOI] [PubMed] [Google Scholar]
- 8.Cairncross G, Berkey B, Shaw E, et al. Phase III Trial of Chemotherapy Plus Radiotherapy Compared With Radiotherapy Alone for Pure and Mixed Anaplastic Oligodendroglioma: Intergroup Radiation Therapy Oncology Group Trial 9402. Journal of Clinical Oncology. 2006;24:2707–2714. doi: 10.1200/JCO.2005.04.3414. [DOI] [PubMed] [Google Scholar]
- 9.Catt S, Chalmers A, Fallowfield L. Psycho-social and supportive-care needs in high-grade glioma. Lancet Oncology. 2008;9:884–891. doi: 10.1016/S1470-2045(08)70230-4. [DOI] [PubMed] [Google Scholar]
- 10.Heimans JJ, Taphoorn MJB. Impact of brain tumour treatment on quality of life. Journal of Neurology. 2002;249:955–960. doi: 10.1007/s00415-002-0839-5. [DOI] [PubMed] [Google Scholar]
- 11.NICE. Improving Outcomes for People with Brain and Other CNS Tumours. London: National Institute of Health and Clinical Excellence; 2006. http://www.nice.org.uk/csgbraincns . [Google Scholar]
- 12.Halkett GKB, Lobb EA, Oldham L, Nowak AK. The information and support needs of patients diagnosed with High Grade Glioma. Patient Education and Counseling. 2010;79(1):112–119. doi: 10.1016/j.pec.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 13.Lobb EA, Halkett GK, Nowak AK. Patient and caregiver perceptions of communication of prognosis in high grade glioma. J Neurooncol. 2011;104(1):315–322. doi: 10.1007/s11060-010-0495-z. [DOI] [PubMed] [Google Scholar]
- 14.Diaz JL, Barreto P, Gallego JM, Barbero J, Bayes R, Barcia JA. Proper information during the surgical decision-making process lowers the anxiety of patients with high-grade gliomas. Acta Neurochir (Wien) 2009;151(4):357–362. doi: 10.1007/s00701-009-0195-7. [DOI] [PubMed] [Google Scholar]
- 15.Triebel KL, Martin RC, Nabors LB, Marson DC. Medical decision-making capacity in patients with malignant glioma. Neurology. 2009;73(24):2086–2092. doi: 10.1212/WNL.0b013e3181c67bce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heese O, Schmidt M, Nickel S, et al. Complementary therapy use in patients with glioma An observational study. Neurology. 2010;75(24):2229–2235. doi: 10.1212/WNL.0b013e31820202c6. [DOI] [PubMed] [Google Scholar]
- 17.Armstrong T, Cohen MZ, Hess KR, et al. Complementary and alternative medicine use and quality of life in patients with primary brain tumors. Journal of Pain and Symptom Management. 2006;32(2):148–154. doi: 10.1016/j.jpainsymman.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 18.Fox S, Laws ER, Jr, Anderson F, Jr, Farace E. Complementary therapy use and quality of life in persons with high-grade gliomas. J Neurosci Nurs. 2006;38(4):212–220. doi: 10.1097/01376517-200608000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Keir ST. Effect of massage therapy on stress levels and quality of life in brain tumor patients-observations from a pilot study. Support Care Cancer. 2011;19(5):711–715. doi: 10.1007/s00520-010-1032-5. [DOI] [PubMed] [Google Scholar]
- 20.Ownsworth T, Chambers S, Hawkes A, Walker DG, Shum D. Making sense of brain tumour: a qualitative investigation of personal and social processes of adjustment. Neuropsychol Rehabil. 2011;21(1):117–137. doi: 10.1080/09602011.2010.537073. [DOI] [PubMed] [Google Scholar]
- 21.Gathinji M, McGirt MJ, Attenello FJ, et al. Association of preoperative depression and survival after resection of malignant brain astrocytoma. Surg Neurol. 2009;71(3):299–303. doi: 10.1016/j.surneu.2008.07.016. discussion 303. [DOI] [PubMed] [Google Scholar]
- 22.Mainio A, Hakko H, Niemela A, Koivukangas J, Rasanen P. Gender difference in relation to depression and quality of life among patients with a primary brain tumor. Eur Psychiatry. 2006;21(3):194–199. doi: 10.1016/j.eurpsy.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 23.Mainio A, Hakko H, Timonen M, Niemela A, Koivukangas J, Rasanen P. Depression in relation to survival among neurosurgical patients with a primary brain tumor: a 5-year follow-up study. Neurosurgery. 2005;56(6):1234–1241. doi: 10.1227/01.neu.0000159648.44507.7f. discussion 1241–1232. [DOI] [PubMed] [Google Scholar]
- 24.Janda M, Steginga S, Langbecker D, Dunn J, Walker D, Eakin E. Quality of life among patients with a brain tumor and their carers. J Psychosom Res. 2007;63(6):617–623. doi: 10.1016/j.jpsychores.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 25.Arnold SD, Forman LM, Brigidi BD, et al. Evaluation and characterization of generalized anxiety and depression in patients with primary brain tumors. Neuro Oncol. 2008;10(2):171–181. doi: 10.1215/15228517-2007-057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kilbride L, Smith G, Grant R. The frequency and cause of anxiety and depression amongst patients with malignant brain tumours between surgery and radiotherapy. J Neurooncol. 2007;84(3):297–304. doi: 10.1007/s11060-007-9374-7. [DOI] [PubMed] [Google Scholar]
- 27.Mainio A, Hakko H, Niemela A, Koivukangas J, Rasanen P. Depression and functional outcome in patients with brain tumors: a population-based 1-year follow-up study. J Neurosurg. 2005;103(5):841–847. doi: 10.3171/jns.2005.103.5.0841. [DOI] [PubMed] [Google Scholar]
- 28.Keir ST, Guill AB, Carter KE, Friedman HS. Stress and intervention preferences of patients with brain tumors. Support Care Cancer. 2006;14:1213–1219. doi: 10.1007/s00520-006-0087-9. [DOI] [PubMed] [Google Scholar]
- 29.Keir ST, Swartz JJ, Friedman HS. Stress and long-term survivors of brain cancer. Supportive Care in Cancer. 2007;15(12):1423–1428. doi: 10.1007/s00520-007-0292-1. [DOI] [PubMed] [Google Scholar]
- 30.Keir ST, Calhoun-Eagan RD, Swartz JJ, Saleh OA, Friedman HS. Screening for distress in patients with brain cancer using the NCCN's rapid screening measure. Psychooncology. 2008;17(6):621–625. doi: 10.1002/pon.1271. [DOI] [PubMed] [Google Scholar]
- 31.Keir ST, Farland MM, Lipp ES, Friedman HS. Distress persists in long-term brain tumor survivors with glioblastoma multiforme. J Cancer Surviv. 2008;2(4):269–274. doi: 10.1007/s11764-008-0069-7. [DOI] [PubMed] [Google Scholar]
- 32.Kvale EA, Murthy R, Taylor R, Lee JY, Nabors LB. Distress and quality of life in primary high-grade brain tumor patients. Support Care Cancer. 2009;17(7):793–799. doi: 10.1007/s00520-008-0551-9. [DOI] [PubMed] [Google Scholar]
- 33.Catt SL, Anderson JL, Chalmers AJ, Fallowfield LJ. A UK-wide survey of follow-up practices for patients with high-grade glioma treated with radical intent. J Eval Clin Pract. 2011;17(1):1–6. doi: 10.1111/j.1365-2753.2009.01292.x. [DOI] [PubMed] [Google Scholar]
- 34.Arber A, Faithfull S, Plaskota M, Lucas C, De Vries K. A study of patients with a primary malignant brain tumour and their carers: Symptoms and access to services. International Journal of Palliative Nursing. 2010;16(1):24–30. doi: 10.12968/ijpn.2010.16.1.46180. [DOI] [PubMed] [Google Scholar]
- 35.Janda M, Steginga S, Dunn J, Langbecker D, Walker D, Eakin E. Unmet supportive care needs and interest in services among patients with a brain tumour and their carers. Patient Educ Couns. 2008;71(2):251–258. doi: 10.1016/j.pec.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 36.Spetz A, Henriksson R, Bergenheim AT, Salander P. A specialist nurse-function in neurooncology: a qualitative study of possibilities, limitations, and pitfalls. Palliat Support Care. 2005;3(2):121–130. doi: 10.1017/s1478951505050200. [DOI] [PubMed] [Google Scholar]
- 37.Spetz A, Henriksson R, Salander P. A specialist nurse as a resource for family members to patients with brain tumors: an action research study. Cancer Nurs. 2008;31(4):E18–E26. doi: 10.1097/01.NCC.0000305741.18711.8f. [DOI] [PubMed] [Google Scholar]
- 38.Wang MH, Cairncross G, Shaw E, et al. Cognition and quality of life after chemotherapy plus radiotherapy (RT) vs. RT for pure and mixed anaplastic oligodendrogliomas: Radiation Rherapy Oncology Group trial 9402. International Journal of Radiation Oncology Biology Physics. 2010;77(3):662–669. doi: 10.1016/j.ijrobp.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshii Y, Tominaga D, Sugimoto K, et al. Cognitive function of patients with brain tumor in pre- and postoperative stage. Surg Neurol. 2008;69(1):51–61. doi: 10.1016/j.surneu.2007.07.064. discussion 61. [DOI] [PubMed] [Google Scholar]
- 40.Talacchi A, Santini B, Savazzi S, Gerosa M. Cognitive effects of tumour and surgical treatment in glioma patients. J Neurooncol. 2011;103(3):541–549. doi: 10.1007/s11060-010-0417-0. [DOI] [PubMed] [Google Scholar]
- 41.Hilverda K, Bosma I, Heimans JJ, et al. Cognitive functioning in glioblastoma patients during radiotherapy and temozolomide treatment: initial findings. Journal of Neuro-Oncology. 2010;97:89–94. doi: 10.1007/s11060-009-9993-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corn BW, Wang MH, Fox S, et al. Health related quality of life and cognitive status in patients with glioblastoma multiforme receiving escalating doses of conformal three dimensional radiation on RTOG 98–03. Journal of Neuro-Oncology. 2009;95:247–257. doi: 10.1007/s11060-009-9923-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brown PD, Jensen AW, Felten SJ, et al. Detrimental effects of tumor progression on cognitive function of patients with high-grade glioma. J Clin Oncol. 2006;24(34):5427–5433. doi: 10.1200/JCO.2006.08.5605. [DOI] [PubMed] [Google Scholar]
- 44.Bosma I, Vos MJ, Heimans JJ, et al. The course of neurocognitive functioning in high-grade glioma patients. Neuro-Oncology. 2007;9(1):53–62. doi: 10.1215/15228517-2006-012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Giovagnoli AR, Silvani A, Colombo E, Boiardi A. Facets and determinants of quality of life in patients with recurrent high grade glioma. J Neurol Neurosurg Psychiatry. 2005;76(4):562–568. doi: 10.1136/jnnp.2004.036186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fox SW, Lyon D, Farace E. Symptom clusters in patients with high-grade glioma. J Nurs Scholarsh. 2007;39(1):61–67. doi: 10.1111/j.1547-5069.2007.00144.x. [DOI] [PubMed] [Google Scholar]
- 47.Brown PD, Ballman KV, Rummans TA, et al. Prospective study of quality of life in adults with newly diagnosed high-grade gliomas. J Neurooncol. 2006;76(3):283–291. doi: 10.1007/s11060-005-7020-9. [DOI] [PubMed] [Google Scholar]
- 48.Cheng JX, Liu BL, Zhang X, et al. Health-related quality of life in glioma patients in China. BMC Cancer. 2010;10:305. doi: 10.1186/1471-2407-10-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davie GL, Hutcheson KA, Barringer DA, Weinberg JS, Lewin JS. Aphasia in patients after brain tumour resection. Aphasiology. 2009;23(9):1196–1206. [Google Scholar]
- 50.Brown PD, Decker PA, Rummans TA, et al. A prospective study of quality of life in adults with newly diagnosed high-grade gliomas - Comparison of patient and caregiver ratings of quality of life. American Journal of Clinical Oncology-Cancer Clinical Trials. 2008;31(2):163–168. doi: 10.1097/COC.0b013e318149f1d3. [DOI] [PubMed] [Google Scholar]
- 51.Locke DEC, Cerhan JH, Wu W, et al. Cognitive rehabilitation and problem-solving to improve quality of life of patients with primary brain tumors: a pilot study. Journal of Supportive Oncology. 2008;6(8):383–391. [PubMed] [Google Scholar]
- 52.Hassler MR, Elandt K, Preusser M, et al. Neurocognitive training in patients with high-grade glioma: a pilot study. J Neurooncol. 2010;97(1):109–115. doi: 10.1007/s11060-009-0006-2. [DOI] [PubMed] [Google Scholar]
- 53.Fu JB, Parsons HA, Shin KY, et al. Comparison of functional outcomes in low- and high-grade astrocytoma rehabilitation inpatients. Am J Phys Med Rehabil. 2010;89(3):205–212. doi: 10.1097/PHM.0b013e3181ca2306. [DOI] [PubMed] [Google Scholar]
- 54.Pace A, Di Lorenzo C, Guariglia L, Jandolo B, Carapella CM, Pompili A. End of life issues in brain tumor patients. J Neurooncol. 2009;91(1):39–43. doi: 10.1007/s11060-008-9670-x. [DOI] [PubMed] [Google Scholar]
- 55.Faithfull S, Cook K, Lucas C. Palliative care of patients with a primary malignant brain tumour: case review of service use and support provided. Palliat Med. 2005;19(7):545–550. doi: 10.1191/0269216305pm1068oa. [DOI] [PubMed] [Google Scholar]
- 56.Sizoo EM, Braam L, Postma TJ, et al. Symptoms and problems in the end-of-life phase of high-grade glioma patients. Neuro-Oncology. 2010;12(11):1162–1166. doi: 10.1093/neuonc/nop045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oberndorfer S, Lindeck-Pozza E, Lahrmann H, Struhal W, Hitzenberger P, Grisold W. The end-of-life hospital setting in patients with glioblastoma. J Palliat Med. 2008;11(1):26–30. doi: 10.1089/jpm.2007.0137. [DOI] [PubMed] [Google Scholar]
- 58.Schmer C, Ward-Smith P, Latham S, Salacz M. When a family member has a malignant brain tumor: the caregiver perspective. J Neurosci Nurs. 2008;40(2):78–84. doi: 10.1097/01376517-200804000-00006. [DOI] [PubMed] [Google Scholar]
- 59.McConigley R, Halkett G, Lobb E, Nowak A. Caring for someone with high-grade glioma: a time of rapid change for caregivers. Palliat Med. 2010;24(5):473–479. doi: 10.1177/0269216309360118. [DOI] [PubMed] [Google Scholar]
- 60.Lipsman N, Skanda A, Kimmelman J, Bernstein M. The attitudes of brain cancer patients and their caregivers towards death and dying: a qualitative study. BMC Palliat Care. 2007;6:7. doi: 10.1186/1472-684X-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Davies E, Clarke C. Views of bereaved relatives about quality of survival after radiotherapy for malignant cerebr glioma. Journal of Neurology Neurosurgery and Psychiatry. 2005;76(4):555–561. doi: 10.1136/jnnp.2004.035931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Keir ST, Guill AB, Carter KE, Boole LC, Gonzales L, Friedman HS. Differential levels of stress in caregivers of brain tumor patients - observations from a pilot study. Supportive Care in Cancer. 2006;14(12):1258–1261. doi: 10.1007/s00520-006-0090-1. [DOI] [PubMed] [Google Scholar]
- 63.Keir ST, Farland MM, Lipp ES, Friedman HS. Family Appraisal of Caregiving in a Brain Cancer Model. Journal of Hospice & Palliative Nursing. 2009;11(1):60–66. [Google Scholar]
- 64.Sherwood PR, Given BA, Given CW, et al. Predictors of distress in caregivers of persons with a primary malignant brain tumor. Res Nurs Health. 2006;29(2):105–120. doi: 10.1002/nur.20116. [DOI] [PubMed] [Google Scholar]
- 65.Munoz C, Juarez G, Munoz ML, et al. The quality of life of patients with malignant gliomas and their caregivers. Soc Work Health Care. 2008;47(4):455–478. doi: 10.1080/00981380802232396. [DOI] [PubMed] [Google Scholar]
- 66.Sherwood PR, Given BA, Given CW, et al. The influence of caregiver mastery on depressive symptoms. J Nurs Scholarsh. 2007;39(3):249–255. doi: 10.1111/j.1547-5069.2007.00176.x. [DOI] [PubMed] [Google Scholar]
- 67.Bradley SE, Sherwood PR, Kuo J, et al. Perceptions of economic hardship and emotional health in a pilot sample of family caregivers. J Neurooncol. 2009;93(9):333–342. doi: 10.1007/s11060-008-9778-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schubart JR, Kinzie MB, Farace E. Caring for the brain tumor patient: family caregiver burden and unmet needs. Neuro Oncol. 2008;10(1):61–72. doi: 10.1215/15228517-2007-040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Keir ST. Levels of stress and intervention preferences of caregivers of brain tumor patients. Cancer Nurs. 2007;30(6):E33–E39. doi: 10.1097/01.NCC.0000300174.18584.f9. [DOI] [PubMed] [Google Scholar]
- 70.Swartz JJ, Keir ST. Program preferences to reduce stress in caregivers of patients with brain tumors. Clin J Oncol Nurs. 2007;11(5):723–727. doi: 10.1188/07.CJON.723-727. [DOI] [PubMed] [Google Scholar]
- 71.Cashman R, Bernstein LJ, Bilodeau D, et al. Evaluation of an educational program for the caregivers of persons diagnosed with a malignant glioma. Can Oncol Nurs J. 2007;17(1):6–15. doi: 10.5737/1181912x171610. Winter. [DOI] [PubMed] [Google Scholar]
- 72.Clayton JM, Butow PN, Arnold RM, Tattersall MHN. Fostering coping and nurturing hope when discussing the future with terminally ill cancer patients and their caregivers. Cancer. 2005;103:1965–1975. doi: 10.1002/cncr.21011. [DOI] [PubMed] [Google Scholar]
- 73.Rabow MW, Goodman S, Chang S, Berger M, Folkman S. Filming the family: a documentary film to educate clinicians about family caregivers of patients with brain tumors. J Cancer Educ. 2010;25(2):242–246. doi: 10.1007/s13187-010-0105-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wallace T, Bradshaw A. Technologies and strategies for people with communication problems following brain injury or stroke. NeuroRehabilitation. 2011;28:199–209. doi: 10.3233/NRE-2011-0649. [DOI] [PubMed] [Google Scholar]
- 75.Field D, Clark D, Corner J, Davis C. Researching Palliative Care. Buckingham: Open University Press; 2001. [Google Scholar]
- 76.Addington-Hall JM, Bruera E, Higginson IJ, Payne S. Research Methods in Palliative Care. Oxford University Press; 2007. [Google Scholar]
- 77.Tang V, Rathbone M, Dorsay JP, Jiang S, Harvey D. Rehabilitation in primary and metastatic brain tumours - Impact of functional outcomes on survival. Journal of Neurology. 2008;255(6):820–827. doi: 10.1007/s00415-008-0695-z. [DOI] [PubMed] [Google Scholar]
- 78.Ostgathe C, Gaertner J, Kotterba M, et al. Differential palliative care issues in patients with primary and secondary brain tumours. Support Care Cancer. 2010;18(9):1157–1163. doi: 10.1007/s00520-009-0735-y. [DOI] [PMC free article] [PubMed] [Google Scholar]