Abstract
Approximately 10% of patients with non-small cell lung cancer (NSCLC) have brain metastases at the time of diagnosis. When surgical resection is not possible, whole brain radiotherapy is the standard of care, with a cerebral response rate of approximately 30%. We report our experience with an upfront association of carboplatin and pemetrexed (areas under the curve, 5 and 500 mg/m2, respectively), every 3 weeks, in 30 patients presenting with newly diagnosed brain metastases and NSCLC. Cerebral MRIs were performed every 6–9 weeks. The radiologic response rates were assessed according to Response Evaluation Criteria in Solid Tumors. Overall survival was also determined. Twenty-six patients were evaluable for response, and the objective cerebral response rate (complete and partial response) in the intent-to-treat population was 40% (12 of 30 patients). Event-free survival was 31 weeks, and median overall survival was 39 weeks. The upfront association of carboplatin plus pemetrexed allows simultaneous treatment of cerebral and systemic disease in patients with NSCLC with newly diagnosed brain metastases and appears to be particularly interesting in terms of radiologic response and overall survival. Further clinical studies are warranted.
Keywords: brain metastasis, carboplatin, chemotherapy, lung cancer, pemetrexed
Up to 30% of patients with lung cancer develop metastases during the evolution of their disease,1–3 and 10% of patients have metastases at the time of diagnosis.4 Patients presenting with 1–3 metastases situated in a noneloquent area are eligible for surgical resection or radiosurgery because of the positive effect of these treatments on survival, local control, and quality of life.5–8 When surgical resection or radiosurgery are not possible, whole brain radiotherapy is currently the standard of care.
Current data suggest that platinum-based chemotherapy induces a 30% radiologic response rate,9–11 a rate similar to that obtained with whole brain radiotherapy.12–14
Pemetrexed is a new therapeutic agent that inhibits folate metabolism and is currently approved as a first-line treatment for nonsquamous non-small cell lung cancer (NSCLC), in association with platinum-based chemotherapy.15–20 Of interest, a recent trial has shown that a pemetrexed-based regimen can be effective against brain metastases.21
We report our experience with the association of carboplatin plus pemetrexed in a series of patients presenting with cerebral metastases secondary to nonsquamous NSCLC. All patients were administered chemotherapy only as a first-line therapy.
Materials and Methods
Patients
In this observational study, 30 consecutive patients with pathologically confirmed nonsquamous NSCLC and newly diagnosed brain metastases, treated in a single institution (Avicenne Hospital) with chemotherapy from April 2009 through July 2011, were studied. In these patients, surgery or radiosurgery of the brain metastases was not deemed to be appropriate, either because of the number (i.e., 3 or more) or the location of the metastatic lesions. These patient were not treated with whole brain radiotherapy upfront, either because the cerebral mass effect did not allow radiotherapy or because the patients were pauci-symptomatic, with no or minimum neurological symptoms that were easily controlled by corticosteroids. Patients had not received prior radiotherapy or chemotherapy.
In patients who did not have a brain biopsy, diagnosis of brain metastases was based on cerebral MRI and histologically documented lung carcinoma.
The patients were treated with carboplatin at a dose of area under curve (AUC) 5 and pemetrexed at a dose of 500 mg/m2, administered on a single day and repeated every 3 weeks until disease progression, unacceptable toxicity, or noncompliance. All patients received folic acid and B12 vitamin supplementation before chemotherapy.
All patients had documented radiological progression before changing the chemotherapeutic regimen.
Response Assessment and Toxicity Evaluation
The medical history, physical examination results, Eastern Cooperative Oncology Group performance status, and recursive partitioning analysis (RPA) were retrieved from the medical records. Objective tumor response was assessed using brain MRI and thoracic CT, according to the revised Response Evaluation Criteria in Solid Tumors, taking into account the largest lesions.22 A complete response was defined as the disappearance of all lesions. A partial response was defined as at least a 30% decrease in the sum of the longest baseline diameter and persistence of 1 or more nontargeted lesions. Progressive disease was defined as at least a 20% increase in the sum of the longest diameter, taking as reference the smallest sum of the longest diameter recorded after treatment or the appearance of 1 one or more new lesions, or the unequivocal progression of existing nontarget lesions. Stable disease was defined as the absence of significant shrinkage or enlargement qualifying a complete response, partial response, or progressive disease, taking as reference the smallest sum of the longest diameter recorded after treatment. Small lesions with diameter less than 10 mm are considered as nonmeasurable lesions. Cerebral and systemic responses were assessed every 6–9 weeks prospectively by the multidisciplinary staff and retrospectively by 1 author (O.B.). Response evaluation was the best response, calculated after 2 or more cycles. Toxicity was assessed according to Common Terminology Criteria for Adverse Events, version 3.0.23
The study was approved by the institutional review board and the local ethical committee.
Statistics
Event-free survival was defined as the time from the date of brain imaging before starting chemotherapy to the documented day of radiologic progression (cerebral or extracerebral progressions) or death. Living patients without progression were treated as censored. Overall survival was defined as the time from the date of brain imaging before starting chemotherapy to death from any cause in the intent-to-treat population. Event-free survival and overall survival were estimated using the Kaplan-Meier method.
Results
Patient Characteristics
From April 2009 through July 2011, 30 patients with pathologically confirmed nonsquamous NSCLC and newly diagnosed brain metastases were analyzed (over the same period, approximately500 patients with nonsquamous NSCLC were referred to our hospital). Upfront radiotherapy was not considered to be appropriate because of no or minimal neurological symptoms in 28 patients or because of poor performance status and mass effect on the third or the fourth ventricle in 2 patients. The characteristics of these 30 patients are shown in Table 1. All patients were smokers, and none were Asian. Four patients were not assessable for radiologic response because chemotherapy was interrupted prematurely because of adverse events: severe fatigue (grade 3) in a 75-year-old patient, severe respiratory distress syndrome (grade 4) in a 55-year-old patient, severe pancytopenia (grade 4) in an 85-year-old patient, and pulmonary embolism (grade 4) in a 61-year-old patient.
Table 1.
Patient demographic and baseline characteristics
| Characteristic | No. of patients (n= 30) | % |
|---|---|---|
| Age, years, median ± SD | 58 ± 10 | |
| Sex, M/F | 21/9 | 70/30 |
| Histologic subtype | ||
| Adenocarcinoma | 27 | 90 |
| Large cell carcinoma | 3 | 10 |
| Performance status (ECOG) | ||
| 0 | 7 | 23 |
| 1 | 20 | 67 |
| 2 | 3 | 10 |
| 3 | 0 | 0 |
| RPA | ||
| 1 | 8 | 27 |
| 2 | 20 | 67 |
| 3 | 2 | 6 |
| Metastases other than brain | 18 | 60 |
| Brain metastases/patient | ||
| 1 | 9 | 30 |
| 1–3 | 9 | 30 |
| >3 | 12 | 40 |
| Time between initial diagnosis and brain metastasis, (days), median ± SD | 18 ± 42 | |
| Brain metastasis revealed lung cancer | 22 | 73 |
| Corticosteroid (prednisone equivalent) | ||
| <30 mg | 11 | 37 |
| 30–60 mg | 13 | 43 |
| >60 mg | 6 | 20 |
Abbreviations: ECOG, Eastern Cooperative Oncology group; RPA, recursive partitioning analysis; SD, standard deviation.
Overall Cerebral Response Rate
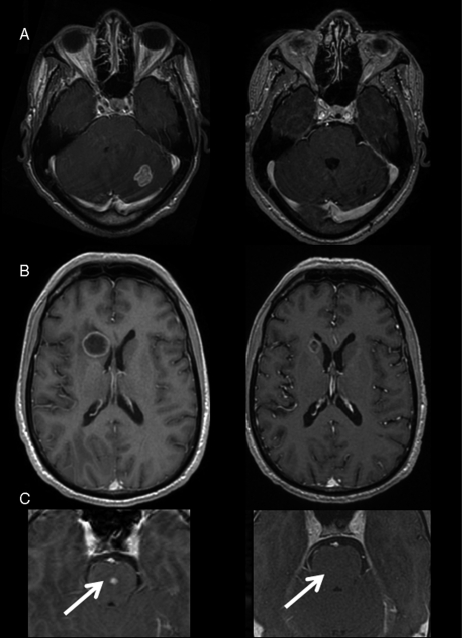
Of the 26 patients evaluable for response, 24 had measurable lesions (more than 1 cm). Ten of these 24 patients achieved a partial response (−50%, 55%, 40%, 35%, 50%, 40%, 30%, 40%, 30%, and 30%) (Fig. 1). Both patients with nonmeasurable lesions (less than 1 cm) had a complete response. The overall cerebral response rate was therefore 40% in the intent-to-treat population. In the 12 responder patients, the median time to best tumor response was 9.8 weeks after onset of chemotherapy. Although the radiologic responses outside the central nervous system (CNS) appeared to be less impressive (partial response in only 17%) than the cerebral responses, the primary sites of progression involved the brain in 16 of 21 patients (progressions limited to the brain in 7 patients). Of the 2 patients with a bad Karnofsky index, one had a cerebral partial response lasting 26 weeks, and the other showed a progressive disease. In the 12 patients who had a cerebral response, the mean steroid dose was reduced from 41 ± 39 mg to 24 ± 30 mg (equivalent prednisolone) at the time of best cerebral response.
Fig. 1.
Cerebral MRI (T1-weighted axial images after gadolinium) showing a radiologic response in 3 patients (A, B, and C). Left: before treatment; right: after 2 or 3 cycles of chemotherapy alone.
Event-Free Survival and Overall Survival
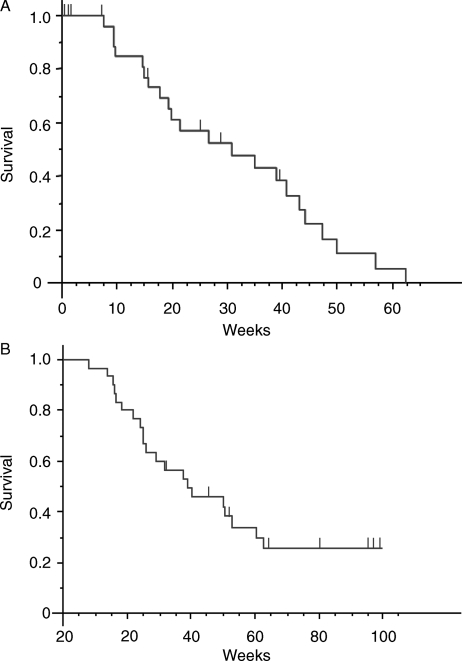
The median duration of follow-up was 46 weeks (range, 8–171 weeks). Median event-free survival was 31 weeks (Fig. 2A). In the intent-to-treat population (30 patients), median overall survival was 39 weeks (Fig. 2B). Eight patients (26%) were RPA class I, 20 (67%) were RPA class II, and only 2 (7%) were RPA class III. In these groups of patients, overall survival was 7.5 and 9.9 months for RPA class I and class II, respectively.
Fig. 2.
Event-free survival (A) and overall survival (B). Tick marks indicate censored data.
Discussion
Whole brain radiotherapy is the standard of care for cerebral metastases when surgery or radiosurgery is not possible. The place of chemotherapy in this context remains unclear. Pemetrexed in combination with cisplatin is recognized as a first-line treatment in advanced NSCLC,20,24,25 and preliminary data exist on its efficacy as an upfront treatment for brain metastases.21 We report a high cerebral response rate (40%) in a series of patients, with a good correlation between extracerebral and cerebral response. The radiologic responses appeared to be less impressive in the lung than in the brain, possibly because the extracerebral lesions were larger and were sometimes associated with lymphangitis carcinomatosis, bone metastases, or pleural lesions. Moreover, the primary sites of progression involved the brain in most patients, highlighting the different kinetics of radiologic response depending on the tumor site.
There are some limitations to our study. First, our population of patients was heterogeneous in terms of initial performance status. Second, this partially prospective study might have induced bias in patients’ selection. Third, our limited number of patients precludes any formal comparison between response rates with other series. However, the efficacy observed in our series is in line with 2 studies that reported similar cerebral response rates in patients with brain metastases treated with a similar regimen either as an upfront regimen21 or at recurrence after whole brain radiotherapy.26 Of interest, this good response rate translated into an overall survival that compares favorably with the figures reported in the RPA classification27 (7.5 and 9.9 months for RPA class I and class II, respectively). Again, these figures should be taken with caution, because our population was limited to patients with NSCLC with upfront metastases, contrary to the broader population (probably carrying a worse prognosis) from which the RPA classification was defined.
Classically, chemotherapy is not considered a standard of care for brain metastases because of the limited diffusion of most chemotherapeutic agents through the blood-brain barrier.28 However, contrast enhancement after gadolinium injection demonstrates that this barrier is not functional. Moreover, similar systemic and cerebral response rates (approximately30%) have been reported with platinum-based chemotherapy,9,11,29–32 demonstrating that this chemotherapy does reach brain metastases. Our cerebral response rate of 40% with carboplatin and pemetrexed confirms that the blood-brain barrier did not impede the effectiveness of intravenous chemotherapy. It is not possible to assess the respective efficacies of carboplatin and pemetrexed. Pemetrexed is a multitargeted antifolate cytotoxic chemotherapeutic agent that inhibits at least 3 target enzymes in the folate pathway (thymidilate synthase, dihydrofolate reductase, and glycinamide ribonucleotide formyl transferase). Thymidilate synthase is the main molecular target of pemetrexed. Overexpression of thymidilate synthase activity seems to be correlated with reduced activity.33 Correlation with thymidilate synthase activity in the tumor samples from our patients is ongoing.
Our results underline the potential role of upfront chemotherapy in the management of brain metastases, allowing the treatment of both cerebral and extracranial disease at the same time. Such a strategy advantageously delays radiotherapy until the time of cerebral progression and reduces cerebral toxicity, because radiotherapy may induce cognitive dysfunction. Withholding whole brain radiotherapy did not appear to affect overall survival in 2 randomized trials using platinum-based chemotherapy.9,10 According to the literature, whole brain radiotherapy does not induce a higher radiologic response rate or cerebral progression-free survival12–14 than that observed in our study. In a recent study, a combination of pemetrexed and cisplatin followed by whole brain radiotherapy (in 63% of cases) gave a cerebral and systemic response rate in 42% and 35% of patients, respectively, and a cerebral progression-free survival of 5.7 months,21 a figure similar to that found in our series.
A randomized clinical trial comparing whole brain radiotherapy with an association of platinum-based chemotherapy and pemetrexed is warranted.
Funding
This work was supported by the Association Oligocyte, the Association pour le développement des neurosciences à Avicenne, and the Assistance Publique-Hôpitaux de Paris.
Acknowledgments
Conflict of interest statement. None declared.
References
- 1.Klos KJ, O'Neill BP. Brain metastases. Neurologist. 2004;10:31–46. doi: 10.1097/01.nrl.0000106922.83090.71. doi:10.1097/01.nrl.0000106922.83090.71. [DOI] [PubMed] [Google Scholar]
- 2.Posner JB. Neurologic complications of systemic cancer. Dis Mon. 1978;25:1–60. doi: 10.1016/s0011-5029(78)80010-8. doi:10.1016/S0011-5029(78)80010-8. [DOI] [PubMed] [Google Scholar]
- 3.Takakura K, Keji S, Shuntaro H, Asao H. Treatment. In: Takakura K, Sano K, Hojo S, editors. Metastatic Tumors of the Central Nervous System. Tokyo: Igaku Shoin; 1982. pp. 195–257. [Google Scholar]
- 4.Sanchez de Cos J, Gonzalez MAS, Montero MV, et al. Non small cell lung cancer and silent brain metastasis survival and prognostic factors. Lung Cancer. 2009;63:140–145. doi: 10.1016/j.lungcan.2008.04.013. doi:10.1016/j.lungcan.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 5.Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322:494–500. doi: 10.1056/NEJM199002223220802. doi:10.1056/NEJM199002223220802. [DOI] [PubMed] [Google Scholar]
- 6.Vecht CJ, Haaxma-Reiche H, Noordijk EM, et al. Treatment of single brain metastasis: radiotherapy alone or combined with neurosurgery? Ann Neurol. 1993;33:583–590. doi: 10.1002/ana.410330605. doi:10.1002/ana.410330605. [DOI] [PubMed] [Google Scholar]
- 7.Schöggl A, Kitz K, Reddy M, et al. Defining the role of stereotactic radiosurgery versus microsurgery in the treatment of single brain metastases. Acta Neurochir (Wien) 2000;142:621–626. doi: 10.1007/s007010070104. doi:10.1007/s007010070104. [DOI] [PubMed] [Google Scholar]
- 8.O'Neill BP, Iturria NJ, Link MJ, Pollock BE, Ballman KV, O'Fallon JR. A comparison of surgical resection and stereotactic radiosurgery in the treatment of solitary brain metastases. Int J Radiat Oncol Biol Phys. 2003;55:1169–1176. doi: 10.1016/s0360-3016(02)04379-1. doi:10.1016/S0360-3016(02)04379-1. [DOI] [PubMed] [Google Scholar]
- 9.Robinet G, Thomas P, Breton JL, et al. Results of a phase III study of early versus delayed whole brain radiotherapy with concurrent cisplatin and vinorelbine combination in inoperable brain metastasis of non-small-cell lung cancer: Groupe Français de Pneumo-Cancérologie (GFPC) Protocol 95-1. Ann Oncol. 2001;12:59–67. doi: 10.1023/a:1008338312647. doi:10.1023/A:1008338312647. [DOI] [PubMed] [Google Scholar]
- 10.Slotman B, Faivre-Finn C, Kramer G, et al. EORTC Radiation Oncology Group and Lung Cancer Group. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med. 2007;357:664–672. doi: 10.1056/NEJMoa071780. doi:10.1056/NEJMoa071780. [DOI] [PubMed] [Google Scholar]
- 11.Lee DH, Han JY, Kim HT, et al. Primary chemotherapy for newly diagnosed nonsmall cell lung cancer patients with synchronous brain metastases compared with whole-brain radiotherapy administered first: result of a randomized pilot study. Cancer. 2008;113:143–149. doi: 10.1002/cncr.23526. doi:10.1002/cncr.23526. [DOI] [PubMed] [Google Scholar]
- 12.Mehta MP, Rodrigus P, Terhaard CH, et al. Survival and neurologic outcomes in a randomized trial of motexafin gadolinium and whole-brain radiation therapy in brain metastases. J Clin Oncol. 2003;21:2529–2536. doi: 10.1200/JCO.2003.12.122. doi:10.1200/JCO.2003.12.122. [DOI] [PubMed] [Google Scholar]
- 13.Stea B, Suh JH, Boyd AP, Cagnoni PJ, Shaw E REACH Study Group. Whole-brain radiotherapy with or without efaproxiral for the treatment of brain metastases: Determinants of response and its prognostic value for subsequent survival. Int J Radiat Oncol Biol Phys. 2006;64:1023–1030. doi: 10.1016/j.ijrobp.2005.10.004. doi:10.1016/j.ijrobp.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Suh JH, Stea B, Nabid A, et al. Phase III study of efaproxiral as an adjunct to whole-brain radiation therapy for brain metastases. J Clin Oncol. 2006;24:106–114. doi: 10.1200/JCO.2004.00.1768. doi:10.1200/JCO.2004.00.1768. [DOI] [PubMed] [Google Scholar]
- 15.Manegold C, Gatzemeier U, von Pawel J, et al. Front-line treatment of advance non-small-cell lung cancer with MTA (LY231514, pemetrexed disodium, ALIMTA) and cisplatin: a multicenter phase II trial. Ann Oncol. 2000;11:435–440. doi: 10.1023/a:1008336931378. doi:10.1023/A:1008336931378. [DOI] [PubMed] [Google Scholar]
- 16.Shepherd FA, Dancey J, Arnold A, et al. Phase II study of pemetrexed disodium, a multitargeted antifolate, and cisplatin as first-line therapy in patients with advanced nonsmall cell lung carcinoma: a study of the National Cancer Institute of Canada Clinical Trials Group. Cancer. 2001;92:595–600. doi: 10.1002/1097-0142(20010801)92:3<595::aid-cncr1359>3.0.co;2-d. doi:10.1002/1097-0142(20010801)92:3<595::AID-CNCR1359>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 17.Zinner RG, Fossella FV, Gladish GW, et al. Phase II study of pemetrexed in combination with carboplatin in the first-line treatment of advanced nonsmall cell lung cancer. Cancer. 2005;104:2449–2456. doi: 10.1002/cncr.21480. doi:10.1002/cncr.21480. [DOI] [PubMed] [Google Scholar]
- 18.Ma CX, Nair S, Thomas S, et al. North Central Cancer Treatment Group; Mayo Clinic; Eli Lilly & Company. Randomized phase II trial of three schedules of pemetrexed and gemcitabine as front-line therapy for advanced non-small-cell lung cancer. J Clin Oncol. 2005;23:5929–5937. doi: 10.1200/JCO.2005.13.953. doi:10.1200/JCO.2005.13.953. [DOI] [PubMed] [Google Scholar]
- 19.Bogart JA, Govindan R. A randomized phase II study of radiation therapy, pemetrexed, and carboplatin with or without cetuximab in stage III non-small-cell lung cancer. Clin Lung Cancer. 2006;7:285–287. doi: 10.3816/CLC.2006.n.009. doi:10.3816/CLC.2006.n.009. [DOI] [PubMed] [Google Scholar]
- 20.Scagliotti GV, Park K, Patil S, et al. Survival without toxicity for cisplatin plus pemetrexed versus cisplatin plus gemcitabine in chemonaïve patients with advanced non-small cell lung cancer: a risk-benefit analysis of a large phase III study. Eur J Cancer. 2009;45:2298–2303. doi: 10.1016/j.ejca.2009.04.033. doi:10.1016/j.ejca.2009.04.033. [DOI] [PubMed] [Google Scholar]
- 21.Barlesi F, Gervais R, Lena H, et al. Pemetrexed and cisplatin as first-line chemotherapy for advanced non-small-cell lung cancer (NSCLC) with asymptomatic inoperable brain metastases: a multicenter phase II trial (GFPC 07-01) Ann Oncol. 2011;11:2466–2470. doi: 10.1093/annonc/mdr003. doi:10.1093/annonc/mdr003. [DOI] [PubMed] [Google Scholar]
- 22.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. doi:10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 23.Cancer Therapy Evaluation Program, Common Terminology Criteria for Adverse Events (CTCAE) Version 3.0, DCTD, NCI, NIH, DHHS. March 31, 2003.
- 24.Ricciardi S, Tomao S, de Marinis F. Pemetrexed as first-line therapy for non-squamous non-small cell lung cancer. Ther Clin Risk Manag. 2009;5:781–787. doi: 10.2147/tcrm.s3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rossi A, Ricciardi S, Maione P, de Marinis F, Gridelli C. Pemetrexed in the treatment of advanced non-squamous lung cancer. Lung Cancer. 2009;66:141–149. doi: 10.1016/j.lungcan.2009.06.006. doi:10.1016/j.lungcan.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Bearz A, Garassino I, Tiseo M, et al. Activity of Pemetrexed on brain metastases from Non-Small Cell Lung Cancer. Lung Cancer. 2010;68:264–268. doi: 10.1016/j.lungcan.2009.06.018. doi:10.1016/j.lungcan.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 27.Gaspar LE, Scott C, Murray K, Curran W. Validation of the RTOG recursive partitioning analysis (RPA) classification for brain metastases. Int J Radiat Oncol Biol Phys. 2000;47:1001–1006. doi: 10.1016/s0360-3016(00)00547-2. doi:10.1016/S0360-3016(00)00547-2. [DOI] [PubMed] [Google Scholar]
- 28.Kortmann RD, Jeremic B, Weller M, Plasswilm L, Bamberg M. Radiochemotherapy of malignant glioma in adults. Clinical experiences. Strahlenther Onkol. 2003;179:219–232. doi: 10.1007/s00066-003-1027-y. doi:10.1007/s00066-003-1027-y. [DOI] [PubMed] [Google Scholar]
- 29.Minotti V, Crinò L, Meacci ML, et al. Chemotherapy with cisplatin and teniposide for cerebral metastases in non-small cell lung cancer. Lung Cancer. 1998;20:93–98. doi: 10.1016/s0169-5002(98)00021-x. doi:10.1016/S0169-5002(98)00021-X. [DOI] [PubMed] [Google Scholar]
- 30.Crinò L, Scagliotti GV, Ricci S, et al. Gemcitabine and cisplatin versus mitomycin, ifosfamide and cisplatin in advanced non-small cell lung cancer: a randomized phase III study of the Italian lung cancer project. J Clin Oncol. 1999;17:3522–3530. doi: 10.1200/JCO.1999.17.11.3522. [DOI] [PubMed] [Google Scholar]
- 31.Fujita A, Fukuoka S, Takabatake H, Tagaki S, Sekine K. Combination chemotherapy of cisplatin, ifosfamide, and irinotecan with rhG-CSF support in patients with brain metastases from non-small cell lung cancer. Oncology. 2000;59:291–295. doi: 10.1159/000012185. doi:10.1159/000012185. [DOI] [PubMed] [Google Scholar]
- 32.Cortot AB, Gerinière L, Robinet G, et al. Groupe Lyon-Saint-Etienne d'Oncologie Thoracique; Groupe Français de Pneumo-Cancérologie. Phase II trial of temozolomide and cisplatin followed by whole brain radiotherapy in non-small-cell lung cancer patients with brain metastases: a GLOT-GFPC study. Ann Oncol. 2006;17:1412–1417. doi: 10.1093/annonc/mdl146. doi:10.1093/annonc/mdl146. [DOI] [PubMed] [Google Scholar]
- 33.Giovannetti E, Mey V, Nannizzi S, et al. Cellular and pharmacogenetics foundation of synergistic interaction of pemetrexed and gemcitabine in human non-small-cell lung cancer cells. Mol Pharmacol. 2005;68:110–118. doi: 10.1124/mol.104.009373. [DOI] [PubMed] [Google Scholar]