Abstract
The naked mole rat is an extremely long-lived (>31 years) small (35 g) rodent. Moreover, it maintains good health for most of its long life. We hypothesized that naked mole rats also show attenuated cardiac aging. With age, cardiac muscle can become less compliant, causing a decline in early diastolic filling (E) and a compensatory increase in atrial contraction-induced late filling (A). This results in decreased left ventricular E/A ratio. Doppler imaging showed no significant differences in E/A ratios (p = .48) among old (18–20 years) breeders and nonbreeders despite differences in estrogen levels. A cross-sectional study of 1- to 20-year-old naked mole rats (n = 76) revealed that E/A ratios declined with age in females (n = 40; p = .002) but not in males (n = 36; p = 0.45). Despite this, neither gender shows increased morbidity or mortality with age. These findings suggest that, notwithstanding the previously observed high lipid peroxidation in heart tissue, NMRs must possess mechanisms to stave off progression to fatal cardiac disease.
Keywords: Left ventricular function, Diastolic dysfunction, Naked mole rat, Hypogonadic, Cardiac imaging
Extraordinarily long-lived species may possess exceptional biological properties that defend against the vagaries of aging and age-associated diseases, like cardiovascular disease (CVD) and cancer (1). They therefore provide a powerful tool to elucidate evolved mechanisms that enable healthy aging and prolong a good quality of life. One such small mammal with extreme longevity is Heterocephalus glaber, the naked mole rat (NMR).
Hailing from northeast Africa, the NMR is the longest-lived rodent, outliving much larger rodents such as beavers, capybaras and porcupines (2). These miniature Methuselahs, in captivity weigh 20–110 g as adults with an average species mass of 35 g (3). NMRs exhibit a maximum life span potential (MLSP) of more than 31 years in captivity. This new record holder was an adult male, wild caught in 1980 that died in 2010, and lived in captivity for 30.5 years. After 25 years of age, he gradually lost weight and eventually developed sarcopenia and kyphosis. Despite this, he remained the alpha male of his colony, evidenced by the fact that he generally fed first and was observed mating in his last year of life.
In captivity, most NMRs live more than 15 years, although 80% of the original cohort of wild-caught animals lived more than 24 years in captivity (4). Even in their harsh natural subterranean milieu in sub-Saharan Africa, the reported maximum life span is more than 17 years (Braude; personal communication; [5]). These longevity records coupled with the allometric equation assessing the relationship between MLSP and body mass reveal that NMRs are exceptionally long lived for their size (6) with similar longevity quotients (observed/predicted MLSP) to those of humans. Whereas death is not always due to intrinsic mortality (ie, the failure of biological systems within an organism), MLSP nevertheless is a useful indicator of the rate of aging. Clearly, both humans and NMRs show attenuated aging. As such the NMR may provide pertinent insights into the mechanisms abrogating aging that may ultimately lead to interventions that extend human healthspan and quality of life.
Lifestyle and Eusociality of NMRs Favor Low Extrinsic Mortality
Because many readers of this special issue may be unfamiliar with this unusual rodent, we present a brief review of what is known about NMRs before addressing new and intriguing findings regarding cardiac function (for additional information see reviews) (5,7,8). NMRs belong to the rodent infraorder Hystricognathi, distinguished from other rodents by the angle of their jaws and the position of the incisors (9). This clade includes American and Old World porcupines as well as guinea pigs, chinchillas, and rock rats (10). Like the social insects (eg, bees), they are eusocial (11), living in large family groups with a strict division of labor. The role of breeding is monopolized by one female and one to four males that are often her brothers or sons. Most colony members are functionally sterile and participate in maintaining the burrows, foraging and caring for the young (12). These subordinates are sexually monomorphic and morphologically distinct from their breeding counterparts. Both nonbreeding males and females are hypogonadic with very low sex steroid levels (13,14); males have poor sperm quality with low counts, whereas females remain anovulatory (15,16). Urinary estrogen is undetectable in nonbreeding females (<80 pmol/L), whereas nonpregnant breeding females have measurable (100–300 pmol/mmol creatinine) levels (17).
Breeding females are capable of breeding throughout their long lives (18). Histological analysis revealed multiple large Graafian follicles in ovaries of a more than 28-year-old, wild-caught breeding female (19), indicating that she was about to ovulate. Many females continue to breed for more than 15 years. The most fecund breeding female was captive born; she lived 23.5 years and raised more than 900 pups in her 11-year reign. Despite pronounced differences in hormone profiles and the high energetic and physical demands of sustained breeding, we have documented similar life spans among breeders and subordinates (5).
Leaving the responsibility of reproduction to a few individuals has the obvious consequence of low genetic variability within a population. Rates of migration between NMR colonies are low (20), and DNA fingerprints from wild-caught animals unsurprisingly revealed that NMRs have the highest inbreeding coefficient of any known mammal. Additionally, a DNA fingerprint study between different NMR colonies reported greater genetic similarities than that observed in comparisons of other nonkin vertebrates (21).
NMR extraordinary longevity may be due in part to evolving in an environment with poor gas and heat exchange. As a consequence, NMRs have low body temperatures (32–34°C) (22) and are very tolerant of both hypoxia and hypercapnia (23). Their naturally low extrinsic mortality may also be an important contributing factor. Extrinsic, or externally caused, mortality is commonly due to predation, extreme changes in temperature and precipitation, as well as food and water availability (24). Living in underground burrows, NMRs are closeted from climatic extremes, most predators, and many pathogens (25). NMRs are notably xenophobic, vigorously attacking conspecifics from different colonies (26), although fatalities from intra-colony fighting are less common. This low species extrinsic mortality may contribute to the extreme longevity of NMRs, favoring the evolution of longevity assurance genes (3,25). Adult mortality rates in captivity are unchanged from 2 to 24 years. As such, NMRs do not show the typical age-associated acceleration in mortality risk that characterizes every other known mammalian species (18). Furthermore, longevity does not differ among genders or between breeders and subordinates. Determining the mechanisms reducing intrinsic mortality and extending healthspan can translate to a better understanding of healthy aging.
Prolonged Healthy Aging in the NMR
NMRs display slowed and/or delayed aging in that they maintain good health and activity for at least 75% of their MLSP. This is accompanied by preserved body composition, bone mineral density, metabolic rate, and gastrointestinal function (18,27). Moreover, many biochemical traits also show no age-related changes (7,18,27,28). This sustained physiological and biochemical function may explain why many age-related pathologies are either markedly delayed in onset or just not exhibited by these rodents (15). At ages equivalent to 90 years and older in humans, NMRs often begin to show a steep decline in health with the most obvious signs of aging being changes in skin properties, sarcopenia, and osteoarthritis (27,28). Clearly, NMRs concur with the “Compression of Morbidity” paradigm, limiting the onset of frailty until near the end of life. Although we still have no clear indication as to the predominant cause of intrinsic mortality in NMRs, this late-onset frailty may reflect the loss of physiological organ reserve (29) for these rodents show pronounced resistance to most mid/late-life chronic diseases.
Although cancer is considered an inevitable byproduct of aging, to date we have never observed tumors, despite necropsies of more than 2,000 animals (18). In contrast, despite being short-lived for their body size, 70% of C57Bl/6 mice die of cancer and many more show signs of lesions and small nonlethal tumors (30). Further evidence of pronounced NMR cancer resistance was established by attempts at oncogenic transformation of NMR cells (31). Unlike cells from humans and laboratory rodents, transformed NMR cells do not form xenograft tumors but rather rapidly enter a “telomere-based” crisis. Additional transfection of NMR cells with human telomerase disables their resistance to tumorigenesis. NMRs are clearly better able to recognize abnormal cells and neutralize their tumorigenic properties. Given that cancer is one of the largest contributors to mortality in elderly humans along with CVD (32,33), sustained genomic maintenance and simultaneous invulnerability to cancer may contribute substantially to the extreme longevity of NMRs.
CVD and Aging
CVD is the leading cause of death in the United States (32). Although there are many kinds of CVD and various lifestyle and genetic factors impact CVD, age is still regarded as the largest risk factor. Age-related changes in the extracellular matrix and vasculature elasticity contribute significantly to CVD (34). Similar cardiac changes occur that may lead to the greater incidence of left ventricle (LV) hypertrophy in elderly humans (35). Postmitotic cardiomyocytes decline in number with age, forcing the remaining myocytes to stretch to fill vacated spaces. These cells consequently increase in size and lose their contractile ability. The extracellular matrix surrounding myocytes then proliferates and further decreases cardiac tissue contractility (34). Such changes are said to be exacerbated by pronounced alterations in sex steroids that also occur during aging (36–38). Currently, data on the ability of hormone replacement therapies to diminish the severity of CVD in postmenopausal women and elderly men are inconclusive (38–40). Some humans, notwithstanding age-related declines in cardiac tissue traits, can survive, delay, or escape the devastating effects of CVD and nevertheless attain extreme longevity as centenarians (41). Furthermore, it is likely that age-related cardiac protection is heritable, for offspring of centenarians also have a significantly reduced risk of CVD compared with the general population (42,43).
Declines in heart function with age may correspond to preclinical states that precede full-scale heart disease. Diastolic dysfunction is one such preclinical state that can contribute to age-related changes in healthspan. In diastole, the heart relaxes and the ventricles passively fill with blood. Due to increased stiffness and depressed relaxation of heart tissue, an individual with diastolic dysfunction will exhibit abnormal LV filling, as indicated by increased isovolumic relaxation time (IVRT) (44) and decreased early diastolic filling/atrial contraction-induced late filling (E/A) peak velocity ratio (45). Measurements of these variables can be acquired with pulsed-wave Doppler equipment. This ultrasound-based technique determines the speed and volume of blood flow through the LV mitral and aortic valves. IVRT represents the timing between the closing of the aortic valve and the opening of the mitral valve. As cardiac tissue stiffens, the time between these two events lengthens (46). E/A ratio is derived from the two peaks observed in the mitral flow with every heartbeat. The E peak corresponds to the blood flow through the mitral valve as the LV relaxes (early diastole), and the A peak corresponds to the blood pushed through the mitral valve by the left atrium during atrial contraction to complete LV filling to capacity (late diastole). When the LV is functioning normally, the E-peak velocity is significantly higher than that of the A peak. Reversal of normal patterns of mitral flow such that the A peak is greater than the E peak (ie, E/A ratio of <1.00) is indicative of elevated LV filling pressure and diastolic dysfunction (47). This can become progressively worse until LV function is so severely impaired that symptoms of diastolic heart failure are evident (45).
An asymptomatic decline in E/A ratio commonly occurs during human aging (48,49). Doppler measurements of the parameter from both men and women reportedly decline by 40% from 1.49 in adults aged 45 years or younger to 0.89 in adults aged 70 years or older (50). A recent study of patients undergoing nonrelated surgery found that patients with diastolic dysfunction were twice as likely to develop severe cardiac disease as those with normal LV function (51). However, it should be emphasized that diastolic dysfunction is not necessarily indicative of patient future mortality but rather an indicator of cardiac healthspan (52).
Noninvasive pulsed-wave Doppler LV measurements can be also made in small mammals (50,53). Additionally, M-mode echocardiography can be employed to make ultrasound measurements of cardiac tissue itself. Depending upon the physical dimensions of the ventricle walls and intraventricular chamber, cardiac function and health can be determined. This method can be particularly sensitive to hypertrophic cardiomyopathy in a rodent heart (53,54).
Cardiovascular Function in the NMR
Oxidative stress reportedly plays a causative role in the pathogenesis of CVD (55). Despite high levels of oxidative damage evident in multiple tissues including the heart, and unexceptional antioxidant defenses (56–58), NMRs maintain good health and presumably cardiovascular function well into old age (59). Similarly, large clinical trials in humans have shown that supplementation with antioxidants to scavenge reactive oxygen species (ROS) and reduce oxidative damage accrual are ineffective treatments for CVD (60).
A negative association between life span and ROS production (hydrogen peroxide) in heart mitochondria was revealed in a study of 10 mammals and two bird species (59). Interestingly, within this data set, similar levels of ROS production were evident in mice and NMRs despite an order of magnitude difference in longevity. Levels of endogenous ROS production in carotid endothelial cells were also found to be similar between mice and NMRs (61). Although apoptotic cell death resulting from increased levels of oxidative stress is implicated in various age-associated diseases, such as atherosclerosis (62), clearly the high levels of endogenous ROS production in NMR heart and vascular mitochondria do not negatively impact NMR longevity. Experimentally induced oxidative stress in carotid blood vessels supports this premise. In sharp contrast to responses of mice, NMR vasculature only shows slight signs of DNA fragmentation and/or apoptotic activity when exposed to very high (mMol) concentrations of hydrogen peroxide (61). Overall, the NMR vasculature does not naturally deteriorate, despite high levels of endogenous ROS, and is actually more resistant to exogenous ROS than mice that have comparable in vivo production of ROS. Increased resistance of NMR vessels to ROS-induced DNA damage and apoptosis may mean that they are inherently better at escaping the ravages of CVD.
Age-related changes in both the heart and vasculature confirm that both cardiac oxidative stress and endothelial dysfunction are attenuated or substantially delayed. NMR hearts, even at a young age, have higher levels of isoprostanes (a marker of oxidative damage to lipids) than observed in kidney and liver as well as observed in shorter-lived mice (58). Although NMRs are more susceptible to oxidative stress, it does not appear to exert the ill effects commonly associated with high levels of oxidative damage. Despite this, expression of mitochondrial genes and NADPH oxidase remain unchanged until at least 26 years of age, unlike profiles of aged laboratory rodents (63). In both aged mice and rats, relaxation of the vasculature is significantly impaired, and this is attributed to increased oxidative insults with age (64). In contrast, NMRs maintain youthful vasculature (65). Delayed and attenuated vascular aging in the face of high ROS production seems counterintuitive (18). Current evidence, however, points to the existence of extremely effective repair mechanisms that allow NMRs to withstand damage (58) and better maintain cardiovascular health with age than do shorter-lived rodents.
One report does indicate several hallmarks of cardiac aging in NMR tissue, although the kinetics and impact thereof are unknown. A histological examination of two females (29 and 30 years old) has shown evidence of cardiac dysfunction. These include increased numbers of cardiomyocytes with large nuclei. There are also signs of fibrosis, common in cardiac dysfunction, whereby the myocytes are replaced by fibroblasts and connective tissue. Additionally, lipofuscin, a byproduct of oxidative damaged-induced failure of lysosomal digestion, is evident in the cardiomyocytes (27). Despite this preliminary evidence of age-related declines in cardiac health, the contribution of cardiac disease to mortality in NMRs remains unclear.
Age-Related Changes in Heart Function in the NMR
The NMR heart has not been studied since the initial description of its general morphology in 1955 (66). Here, cardiac function in the NMR is examined for the first time to determine the impact of the repression of sexual maturity on heart function and the kinetics of age-related changes. Despite the hints of decline in the extremely old NMR hearts outlined earlier, at present, we do not know if this leads to fatal cardiac disease. Rather, based upon the many indications of sustained organ and vasculature youthfulness with age (18,65), we hypothesize that NMRs display an attenuated age-dependent decline in cardiac function. In addition, given the pronounced differences in sex steroid profiles among breeders and subordinates, the NMR presents a unique circumstance, which can elucidate the role of these hormones on age-related changes in diastolic function.
METHODS
Animals
We compared age-related changes in cardiac function in 40 female and 36 male captive-born NMRs. Parental stock originated from animals captured in Kenya in 1980, and all animals used in this study were either first or second generation captive born. Animals were maintained at the University of Texas Health Science Center at San Antonio in multichambered burrow systems under constant climatic conditions, aimed to approximate their native habitat (30°C; 50% relative humidity). NMRs met all their nutrient and water needs through an ad-lib supply of fruit and vegetables, supplemented with a high protein and vitamin enriched cereal (Pronutro, South Africa). This study was approved by the Institutional Animal Care and Use Committee (#07089) at the University of Texas Health Science Center at San Antonio.
Heart Rate, Pulsed-Wave Doppler, and Echocardiography
Heart rates from awake unanesthetized NMRs were measured using a modified blood pressure tail cuff (Hatteras Instruments). For other in vivo analyses, the NMRs were anesthetized using 1%–2% isoflurane in a 100% oxygen mix. Pulsed-wave Doppler images were acquired using the Mouse Cardiovascular Research System (Indus Instruments) and were taken at heart rates of 150–190 beats per minute (bpm). The Doppler probe was placed at the inferolateral region in the radial short axis at the base of the LV for the assessment of E and A myocardial velocities. Echocardiographic measurements were acquired with the Vevo 770 TM Imaging System (Visual Sonics) and taken from the two-dimensional parasternal long-axis (M-mode) recordings from the midpapillary region. For each parameter, three images from three consecutive cardiac cycles were measured and averaged. Electrocardiograms were acquired from the 4-lead mousepad the animals were placed on during both techniques.
Statistical Methods
E/A ratios are calculated according to the Indus Instruments Doppler Signal Processing Workstation algorithm. Multiple periods of flow through the mitral valve are analyzed over the course of one trace and then averaged so that E/A = (1/n)ΣrVEAn. The ratio for each period of flow is calculated as the velocity of rVEAn = vPE1/vPA1. The comparison of nonbreeding and breeding females was analyzed with a Student’s t test. We performed linear regression of E/A ratio onto age and gender and assessed whether the intercepts and slopes were gender specific by testing the gender main effect and the age by gender interaction. We considered that specific age groups (eg, the 10-year age group) might not fit the overall linear relationship between E/A ratio and age by creating a 0/1 factor variable for that age group, and we tested the significance of the age-group factor with a one degree of freedom F test for each gender. Analysis of the female cohorts’ mitral parameter averages was done by one-way analysis of variance with a Holm–Sidak adjustment.
RESULTS
The NMR Heart
The heart of an NMR is functionally different from that of a mouse. The average mass of a young NMR heart (139 ± 0.6 mg; n = 15) is similar to that of a young mouse (143 ± 0.3 mg; n = 10). However, when expressed as a function of the average body weight, NMR hearts are approximately 35% smaller (3.6 mg/g) than those (5.6 mg/g) of mice (58). Also, nonanesthetized heart rates of young mice (475–700 bpm) (67) are approximately double those of the young NMRs (202–370 bpm).
Based upon an allometric equation (log(heart rate [bpm]) = −0.251log(body mass [grams]) + 3.072), which predicts resting heart rate as a function of body size (68), average-sized young NMR (35 g), the expected heart rate is 483 bpm. This value is more than twofold higher than the observed average resting heart rate of 229.28 ± 7.89 bpm. Similarly, humans have a substantially lower resting heart rate than predicted (127 bpm) for a 70 kg person. Healthy human resting heart rates range from 40 to 100 bpm, depending on the person’s level of fitness (69). Well-trained athletes are at the low end of this range; this is attributed to better cardiac fitness that should lower the risk of CVD and is also associated with lower future mortality risk (70). As such, both long-lived humans and NMRs appear to have a lower resting heart rate than predicted.
Effects of Endogenous Steroid Hormones on NMR Diastolic Function
We ascertained whether there is a difference in E/A ratio between the hypogonadic nonbreeders of our oldest cohort and age-matched breeding females that still maintain normal levels of estrogen. Because decreased estrogen levels are thought to put postmenopausal women at risk for diastolic dysfunction (36–38), we compared breeders and sexually suppressed subordinates 18–20 years in age because we anticipated the greatest differences in diastolic function at this old age. The breeding females were clearly reproductively cycling at the time of measurements, as each had a vaginal mucus plug. We hypothesized that breeding females would have improved E/A ratios compared with the nonbreeders.
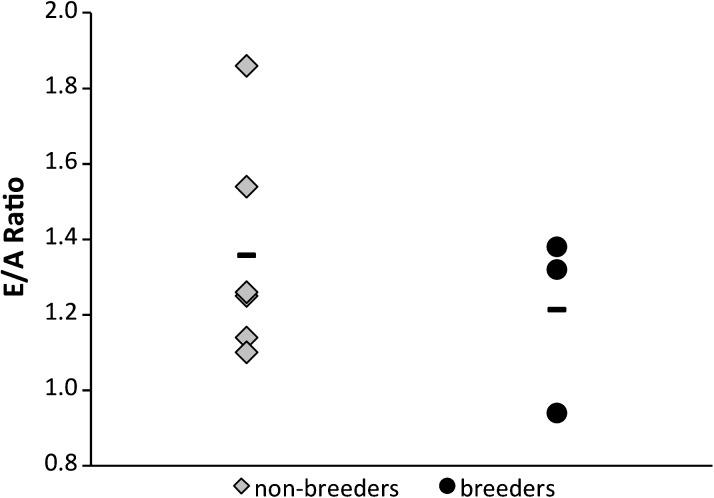
The data do not support our hypothesis, for the average E/A ratio for both nonbreeding (1.36 ± 0.29) and breeding (1.21 ± 0.24) females is similar (Figure 1; p = .48). Diastolic dysfunction is evident in one breeding female (E/A = 0.94 ± 0.03). Collectively, these data infer that the mechanisms by which aged female NMRs facilitate sustained diastolic function are likely not estrogen dependent. Because 18- to 20-year-old breeders and nonbreeders are not significantly different, they are therefore pooled in further analyses.
Figure 1.
Early diastolic filling/atrial contraction-induced late filling ratios of groups of nonbreeding (n = 6) and breeding (n = 3) 18- to 20-year-old females. Dashes represent the average value for each cohort. A Student’s t test was used to determine that there is no significance between cohorts (p = .48).
Age-Related Changes in Diastolic Function
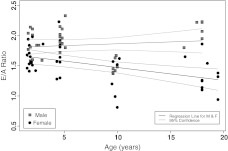
Assessment of LV mitral function was conducted cross-sectionally in both male and female NMRs ranging from 1 to 20 years of age (Figure 2). E/A ratio does not significantly change with age in males (p = .45), whereas the decline in diastolic function with age in females is highly significant (p = .002; Table 1). Interestingly, many 10-year-olds have E/A ratios that fall below the regression line (Figure 2). Because of the greater variability in the female cohort, these data are not significantly different from that predicted by the regression line, whereas the 10-year-old males are significant outliers (Table 2). We do not know if these statistically significant data are biologically meaningful, as these individuals appear to be asymptomatic. Surprisingly, E/A ratios of the oldest males do not differ significantly from the youngest males, contributing to the insignificant (p = .45), slightly positive (.006) regression slope in our cross-sectional study with age (Figure 2, Table 1). The apparent improvement in E/A ratio with age in males may be spurious, for it solely reflects a cohort of old males with well-maintained left diastolic function that were all from one colony. If successful cardiac aging is heritable in NMRs, this may explain the lack of a decrease in E/A ratio in the male sample.
Figure 2.
Regressions of early diastolic filling/atrial contraction-induced late filling (E/A) ratios of male (n = 36) and female (n = 40) naked mole rats ranging in age from 1 to 20 years. Pronounced gender differences are evident in that males show no significant age-related decline in E/A ratio (p = .45), whereas the slight decline (slope = −0.02) with age in females is significant (p = .002). There is no significant difference in the intercepts among males and females. Detailed statistical analysis of these regressions can be seen in Tables 1 and 2.
Table 1.
Statistical Analysis of the Age-Related Changes in E/A Ratios of Male and Female Naked Mole Rats as Shown in Figure 2
| Interpretation | Effect | SEM | p Value | |
| Male | Intercept | 1.80 | 0.07 | 1.38 × 10−23 |
| Slope | 0.006 | 0.01 | 0.45 | |
| Female | Intercept | 1.69 | 0.07 | 1.14 × 10−37 |
| Slope | −0.02 | 0.01 | 0.002 | |
| Comparison | Intercept test (M > F) | 0.12 | 0.1 | 0.23 |
| Difference in slope | 0.03 | 0.01 | 0.01 |
Note: While the intercepts for both males and females are significantly different from zero, only the slope of the females is significant from both zero and from the age-related slope of the males. E/A = early diastolic filling/atrial contraction-induced late filling.
Table 2.
Statistical Assessments of the 10-year-old Male and Female Naked Mole Rats With Respect to the Age-Related Changes in E/A Regression Analysis of Figure 2
| Interpretation | Effect | SEM | p Value | |
| Male | Intercept | 1.84 | 0.05 | 1.52 × 10−28 |
| Slope considering outlier | 0.02 | 0.01 | 0.01 | |
| 10-year effect | −0.50 | 0.08 | 4.30 × 10−7 | |
| Female | Intercept | 1.71 | 0.07 | 2.55 × 10−25 |
| Slope considering outlier | −0.02 | 0.01 | 0.004 | |
| 10-year effect | −0.23 | 0.12 | 0.06 |
Note: That the data from 10-year-old females is not significantly different from the regression analyses with those of 10-year-old males lie outside the 95% confidence interval were found to be statistical outliers. E/A = early diastolic filling/atrial contraction-induced late filling.
We further analyzed other mitral flow parameters from the female NMR Doppler traces in four age cohorts (1–3, 5, 10, and 18–20 years old; Table 3). A-peak velocity differs significantly between the 5- and 18- to 20-year-old females, which contributes to the significant difference between E/A ratios between these two groups. Average E/A ratios differ significantly (p = .004) between the two younger and the two older cohorts. IVRT significantly changes with age in the females. IVRT is clearly dependent on heart rate (46), but given that NMR heart rate does not change with age, changes in IVRT may be due to impaired LV relaxation.
Table 3.
Age-Related Changes in Pulsed-Wave Doppler Mitral Blood Flow Signals in Female Naked Mole Rats
| 1–3 y (n = 14) | 5 y (n = 10) | 10 y (n = 6) | 18–20 y (n = 9) | p Value | |
| Heart rate (bpm) | 170.45 ± 3.65 | 165.90 ± 3.76 | 158.23 ± 2.78 | 169.13 ± 2.31 | N.S. |
| R–R interval (ms) | 354.30 ± 8.12 | 363.33 ± 8.02 | 379.90 ± 6.68 | 355.34 ± 5.03 | N.S. |
| E-peak velocity (cm/s) | 56.00 ± 2.00 | 49.29 ± 1.91 | 49.71 ± 2.83 | 56.88 ± 5.68 | N.S. |
| A-peak velocity (cm/s) | 34.14 ± 1.13 | 30.57 ± 1.47* | 41.89 ± 6.81 | 46.00 ± 6.34* | .03 |
| E/A ratio | 1.65 ± 0.05*† | 1.65 ± 0.10‡§ | 1.29 ± 0.13*‡ | 1.31 ± 0.09†§ | .004 |
| Isovolumic contraction time (ms) | 34.33 ± 1.85 | 37.07 ± 2.62 | 34.49 ± 3.45 | 27.69 ± 3.12 | N.S. |
| Isovolumic relaxation time (ms) | 67.18 ± 2.41*† | 73.66 ± 2.17‡ | 88.07 ± 1.60*‡ | 79.12 ± 4.43† | <.001 |
Note: All values are M ± SEM. The data were statistically analyzed using one-way analysis of variance (df = 3,38). Symbols denote significant differences in cohort values for A-peak velocity (F = 3.46), E/A ratio (F = 5.34), and isovolumic relaxation time (F = 8.05). Other parameters were not significantly different (N.S.) among the different age cohorts. E/A = early diastolic filling/atrial contraction-induced late filling.
Echocardiography Confirms Naturally Impaired Diastolic Function
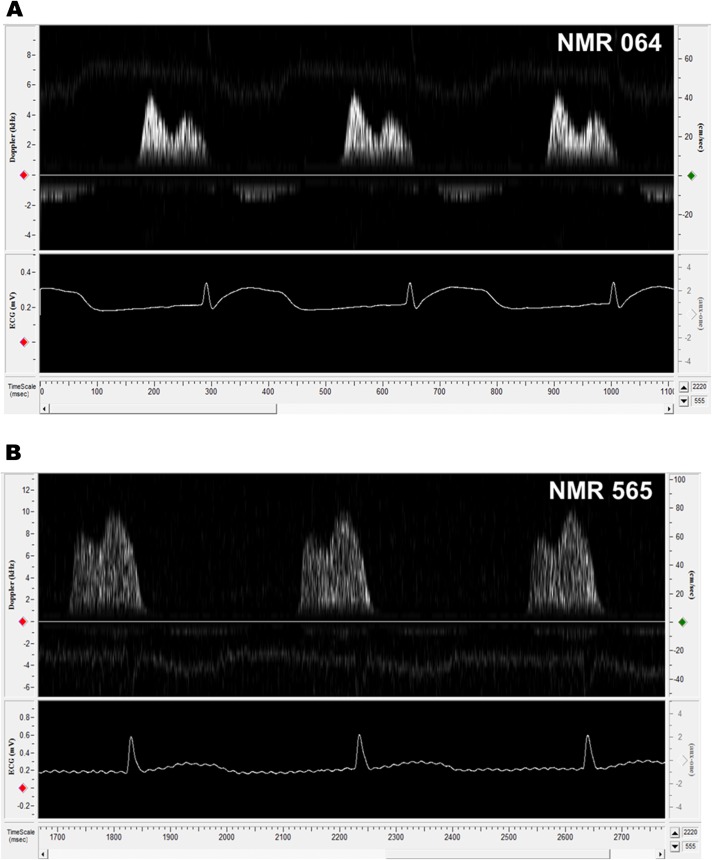
E/A ratios of several females were less than 1.00. We examined in more detail the cardiac function of the10-year-old female with the lowest E/A ratio in the study and compared her with a healthy 5-year-old female (Figure 3A) to better understand what may be occurring in the 10-year-old cohort. Doppler traces of mitral valve blood flow revealed that the 10-year old had reversed E and A peaks (Figure 3B) resulting in an E/A ratio of 0.81 ± 0.03. Her IVRT was 90.67 ± 5.22, whereas that of the healthy 5-year old was 71.14 ± 3.72. IVRT has the highest sensitivity of all Doppler indices in detecting abnormal relaxation because it is the first variable to become compromised (46,71). These values would signify a considerable impairment of LV relaxation in humans (50). Despite her low E/A ratio, she maintained normal activity levels and appeared asymptomatic. Although, clearly we cannot evaluate whether she is “clinically normal” because we cannot assess common diagnostic traits associated with this type of cardiac disease (eg, shortness of breath upon exertion). We speculate that this low E/A ratio in the NMR represents preclinical diastolic dysfunction.
Figure 3.
Pulsed-wave Doppler mitral flow patterns comparing two nonbreeding females. Naked mole rat (NMR) 064 (A) was 5 years of age at the time of Doppler. Her mitral flow is representative of a healthy young adult animal (E/A ratio = 1.30 ± 0.02). NMR 565 (B) was 10 years old at the time of Doppler and exhibits a reversal of E and A peaks (E/A ratio = 0.81 ± 0.03). This reversal is indicative of diastolic dysfunction whereby left ventricle relaxation is impaired (resulting in a lower early diastolic filling peak), causing a decrease in the amount of blood, which can passively fill the ventricle. The left atrium must then pump more blood into the ventricle during late diastole (atrial contraction), resulting in a higher atrial contraction-induced late filling peak, so as to ensure that cardiac output is not diminished.
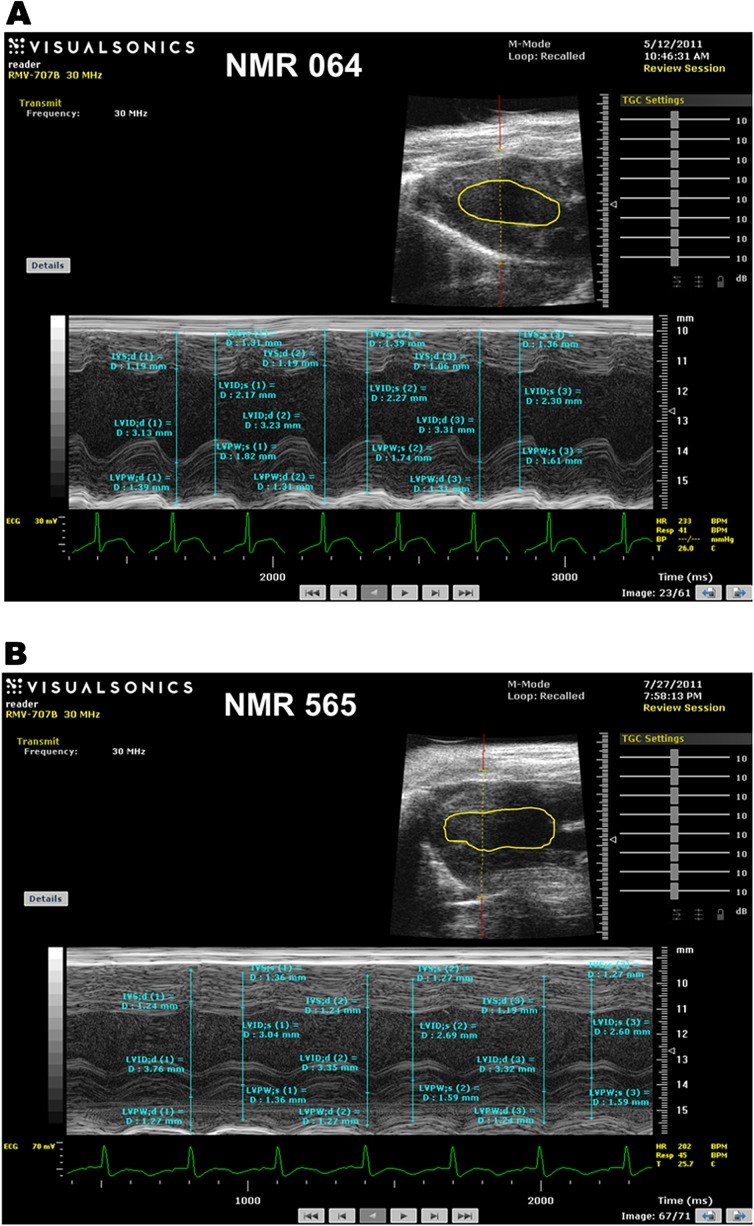
Other indications of diastolic dysfunction were also evident from the analyses of M-mode echocardiograms (Figure 4A and B). LV end diastolic and systolic dimensions were both higher than in the healthy 5-year-old animal. The 10-year old also showed reduced LV posterior wall thickness in systole and diastole (Table 4). These changes signify ventricular dilation, wherein the LV literally has become stretched out. Additionally, fractional shortening, an index of contractility, is decreased by 10%, and ejection fraction, a measure of the proportion of blood that is pumped out of a ventricle during systole, is 20% lower than that of her healthy counterpart (Table 4). Collectively, imaging data support a diagnosis of LV dilation leading to diastolic dysfunction. These data illustrate that NMRs can naturally develop diastolic dysfunction. Furthermore, if this individual and others with reduced E/A ratios continue to survive, they may provide insight into the mechanisms employed to stave off serious cardiac events in spite of LV dysfunction.
Figure 4.
M-mode echocardiograms demonstrating left ventricle (LV) dimensions and wall thicknesses for two nonbreeding females. Naked mole rat (NMR) 064 (A) is a healthy 5-year-old female, whereas NMR 565 (B) is a 10-year-old female with diastolic dysfunction. The long axis image is shown in the upper right inset with the LV chamber dimensions traced. Measurements shown on these figures are used to calculate the values listed in Table 2.
Table 4.
Echocardiography Confirms Left Ventricular Dysfunction in a 10-Year-Old Female NMR and Highlights Differences Between That Individual and a Healthy 5-Year-Old
| NMR 064 (5-year-old) | NMR 565 (10-year-old) | |
| Heart rate (bpm) | 233 | 202 |
| Diastolic LV posterior wall thickness (mm) | 1.30 ± 0.06 | 1.26 ± 0.01 |
| Systolic LV posterior wall thickness (mm) | 1.72 ± 0.06 | 1.51 ± 0.08 |
| LV end diastolic dimension (mm) | 3.22 ± 0.09 | 3.48 ± 0.14 |
| LV end systolic dimension (mm) | 2.25 ± 0.07 | 2.78 ± 0.13 |
| Ejection fraction (%) | 59.0 | 42.4 |
| Fractional shortening (%) | 30.3 | 20.3 |
Note: Data are presented as M ± SEM. Measurements were made in triplicate from each NMR’s echocardiogram (see Figure 4). LV = left ventricle; NMR = naked mole rat.
DISCUSSION
This first study of in vivo cardiac measurements on NMRs has yielded rather intriguing insights into cardiac aging in an animal model of extreme longevity. Despite differences in circulating sex steroid levels among breeders and nonbreeders, we found that circulating estrogen was not cardioprotective. Given the low sex steroid levels, we were surprised to find pronounced gender differences among subordinates. Whereas female NMRs showed a significant decline in E/A ratio with age, males did not. Indeed the lack of a significant change in E/A ratio in males with age concurs with previous findings of “negligible senescence” in this species (18). Despite the observed decline in female diastolic function, there is no impact upon the morbidity or mortality, for both males and females, as well as breeders and nonbreeders, have similar life spans (18). These findings suggest that female NMRs can tolerate changes in diastolic function and foil progression to pathological states of CVD.
NMRs: Thwarting Diastolic Disease
Although diastolic dysfunction can occur in both men and women, women reportedly are more susceptible to age-related diastolic dysfunction than are men (72,73). Our data corroborate this, for females show a significant decline in E/A ratio and also an increase in IVRT with age (Figure 2, Table 3). Both of these changes are established markers of diastolic functional impairment in humans (44,45).
We found that certain NMRs, like some humans (72,73), show signs of age-associated diastolic dysfunction. In women, this decline is usually attributed to the postmenopausal decrease in sex steroids (36). Although old breeding female NMRs maintain ovarian function and breed throughout their lives, they do not exhibit the purported cardioprotective effect of sustained circulating estrogen levels. The lowest observed E/A ratio in this age group belonged to a breeding female (Figure 1), whereas the remaining breeders had similar E/A ratios to nonbreeders. As such, declines in sex steroids cannot be responsible for the observed age-related decline in diastolic function. Similarly, equivocal reports on the efficacy of hormone replacement therapy on cardiac health in women (36,39) suggest that age-related changes in sex steroids are merely correlated with cardiac dysfunction and are not causally related.
Given that NMRs are not experimentally inbred, the observed large variability in E/A ratios is not unexpected. Interestingly, the 10-year-old animals (equivalent to 40-year-old humans) generally appear to be outliers in this study (Figure 2, Table 2). This may be evidence of a period of selection against middle-aged individuals who are progressing toward a cardiac event and may die rather than attain their MLSP, or this decline may be biologically irrelevant. We have previously noted that the 10-year age cohort of our colony is often an outlier in age-related physiological characteristics (28) and that this age cohort appears to have a slightly higher incidence of mortality than do either older or younger age groups above 2 years.
Three of the 40 females (two 10-year-old nonbreeders and one 20-year-old breeder) examined in this study had E/A ratios of less than 1.00, yet they showed no outward obvious manifestations of declines in healthspan. We do not know if these three animals will progress to debilitating or fatal heart disease, or if like some centenarians, they will survive well into old age with this preclinical phenotype of diastolic dysfunction. The 18- to 20-year-old females, in keeping with what is known about extremely long-lived humans, likely represent NMRs that may have either escaped diastolic dysfunction, delayed its onset, or survived its onslaught (41). Although we have only measured one aspect of heart function and one type of CVD, given the extreme longevity of NMRs, it is likely, although not assured, that this rodent exhibits successful cardiac aging. The recently sequenced NMR genome coupled with transcriptome analyses reveal a multitude of genes whose expression appear to be linked to mammalian longevity. These include an upregulation of genes encoding products that protect protein and DNA functions (74). NMRs, like the offspring of centenarians, may inherit longevity-ensuring cardioprotective genes and thereby reduce the risk of CVD. The oldest males were all brothers in the same colony and collectively exhibited high E/A ratios (an average of 2.08 ± 0.07). Thus their preserved diastolic function relative to the older females may be reflective of their genetic relatedness and not necessarily of the entire population of older male NMRs. Further studies will assess if the diastolic function of males 16 years or older from multiple colonies to verify if the unchanged E/A ratios with age in males remains true and whether the decline in E/A ratio with age is a female-specific phenomenon.
A New Model for Cardiac Aging
Recent advances in specialized imaging technology have opened the door for the use of small rodents use in cardiac research, making the laboratory mouse the mainstay of cardiovascular research. These animals are chosen primarily for their relatively short life spans, which provide the opportunity for experimental manipulations and rapid assessment of age-related declines. However, they clearly have poor defenses against aging (75), as indicated by an MLSP, half that predicted by their body size (76). As such, they may not possess mechanisms pertinent to successful cardiac aging (75).
Reported E/A ratios of young mice are more than twofold greater than those of physiologically age-matched NMRs. Although this may reflect an inherent species difference in cardiac function, it could also be due to interstudy differences in equipment sensitivity. Laboratory mice are known to develop diastolic dysfunction with age (77). The 55% drop in E/A ratios from 4 months (E/A ratio = 3.47) to about 30 months (E/A ratio = 1.55) in female wild-type mice (78) is considerably greater than the ∼25% decline observed between young (1–3 years) and old (18–20 years) female NMRs. Surprisingly, E/A ratios of young NMRs regardless of gender are similar to those of senescent mice, yet the former are still able to function normally for an additional three decades, whereas senescent mice have a high probability of dying within less than a year. Intriguingly, both E/A ratio and IVRT measurements for NMRs are in the same range as those reported for humans, another extremely long-lived species (46,50). These assessments of diastolic function are not only a useful gauge for cardiovascular risk (45), but we speculate that they could also be a biomarker of aging. Further supporting this premise is a study that found an attenuated decline in diastolic function in people practicing caloric restriction compared with those who were not (79).
Extended longevity in genetically manipulated mouse models (eg, Ames dwarf and Little mice) share some common cardiac features with NMRs. Like NMRs, these genetic mutant models have lower heart rates, smaller heart sizes, and diminished unstressed E/A ratios compared with wild-type mice (80,81). We postulate that NMRs, like Ames and Little mice, maintain cardiac function when faced with various stressors, and this may be a significant piece in the puzzle of the astounding longevity of NMRs. NMRs also display improved protein maintenance and resistance to unfolding stressors (82), enhanced genomic stability (31), and superior detoxification (83).
Cytoprotective mechanisms facilitating this broad-based stress resistance are regulated by the nuclear factor erythroid 2-related factor 2 (Nrf2) signaling pathway, considered by some “the guardian of healthspan and gatekeeper to longevity” (84) because of its multifaceted roles in biological systems that influence aging. Nrf2 activates the transcription of a myriad of cytoprotective genes that safeguard against oxidative stress and inflammation and produce molecular chaperones to stabilize proteins as well as remove damaged cell components via upregulated autophagy or proteasomal degradation (58). Interestingly, there is cross talk between Nrf2 and the insulin signaling pathway (85), and the latter is widely accepted as an integral component of aging. Nrf2 appears to be a potential mediator of some of the beneficial effects of caloric restriction in various diseases (86). Similarly, there is some evidence to suggest that Nrf2 signaling may also be a factor in a longevity pathway that is triggered when insulin signaling is low, perhaps functioning in parallel to the forkhead box protein pathway (87).
When compared with wild-type mice, Nrf2 is constitutively expressed at higher levels in all NMR tissues, including the heart (84,88). It is possible that such mechanisms function in the NMR heart to allow these animals to delay the progression of diastolic dysfunction to heart failure. Our findings support further study of cardiovascular mechanisms of aging in long-lived animal models such as the NMR and may ultimately help elucidate mechanisms of human longevity and healthspan.
FUNDING
This study was supported by grants to M.L.L. (the Max and Minnie Tomerlin Voelcker Fund and the Veteran’s Administration [Merit]) and R.B. (American Federation for Aging Research Breakthroughs in Gerontology, the Glenn Foundation, and the National Institute on Aging/National Institutes of Health [AG022891-01]).
Acknowledgments
Rogelio Zamilpa and Wesley Lowell are thanked for their help with acquiring and analyzing the echocardiographs. Megan Smith and the Laboratory Animal Resources staff at the University of Texas Health Science Center at San Antonio provided excellent care of the animals. Yael Edrey lent her editorial skills.
References
- 1.Barzilai N, Shuldiner AR. Searching for human longevity genes: the future history of gerontology in the post-genomic era. J Gerontol A Med Sci. 2001;56(2):M83–M87. doi: 10.1093/gerona/56.2.m83. [DOI] [PubMed] [Google Scholar]
- 2.Weigl R. Longevity of Mammals in Captivity; From the Living Collections of the World. Stuttgart, Germany: Stuttgart Kleine Senckenberg-Reihe; 2005. [Google Scholar]
- 3.Jarvis J, Bennett N. Ecology and behavior of the Family Bathyergidae. In: Sherman P, Jarvis J, Alexander R, editors. The Biology of the Naked Mole-Rat. Princeton, NJ: Princeton University Press; 1991. pp. 66–96. [Google Scholar]
- 4.Sherman PW, Jarvis JUM. Extraordinary life spans of naked mole-rats (Heterocephalus glaber) J Zool. 2002;258:307–311. [Google Scholar]
- 5.Buffenstein R. The naked mole-rat? A new long-living model for human aging research. J Gerontol A Biol Sci Med Sci. 2005;60(11):1369–1377. doi: 10.1093/gerona/60.11.1369. [DOI] [PubMed] [Google Scholar]
- 6.Hulbert AJ, Pamplona R, Buffenstein R, Buttemer WA. Life and death: metabolic rate, membrane composition, and life span of animals. Physiol Rev. 2007;87(4):1175–1213. doi: 10.1152/physrev.00047.2006. [DOI] [PubMed] [Google Scholar]
- 7.Edrey YH, Park TJ, Kang H, Biney A, Buffenstein R. Endocrine function and neurobiology of the longest-living rodent, the naked mole-rat. Exp Gerontol. 2011;46(2–3):116–123. doi: 10.1016/j.exger.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Bennett NC, Faulkes CG. African Mole-Rats: Ecology and Eusociality. Cambridge, UK: Cambridge University Press; 2000. [Google Scholar]
- 9.Blanga-Kanfi S, Miranda H, Penn O, Pupko T, DeBry RW, Huchon D. Rodent phylogeny revised: analysis of six nuclear genes from all major rodent clades. BMC Evol Biol. 2009;9:71. doi: 10.1186/1471-2148-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deuve JL, Bennett NC, Britton-Davidian J, Robinson TJ. Chromosomal phylogeny and evolution of the African mole-rats (Bathyergidae) Chromosome Res. 2008;16(1):57–74. doi: 10.1007/s10577-007-1200-8. [DOI] [PubMed] [Google Scholar]
- 11.Michener CD. Comparative social behavior of bees. Annu Rev Entomol. 1969;14:299. [Google Scholar]
- 12.Jarvis JU. Eusociality in a mammal: cooperative breeding in naked mole-rat colonies. Science. 1981;212(4494):571–573. doi: 10.1126/science.7209555. [DOI] [PubMed] [Google Scholar]
- 13.Holmes MM, Goldman BD, Goldman SL, Seney ML, Forger NG. Neuroendocrinology and sexual differentiation in eusocial mammals. Front Neuroendocrinol. 2009;30(4):519–533. doi: 10.1016/j.yfrne.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pinto M, Jepsen KJ, Terranova CJ, Buffenstein R. Lack of sexual dimorphism in femora of the eusocial and hypogonadic naked mole-rat: a novel animal model for the study of delayed puberty on the skeletal system. Bone. 2010;46(1):112–120. doi: 10.1016/j.bone.2009.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faulkes CG, Abbott DH, Jarvis JU. Social suppression of ovarian cyclicity in captive and wild colonies of naked mole-rats, Heterocephalus glaber. J Reprod Fertil. 1990;88(2):559–568. doi: 10.1530/jrf.0.0880559. [DOI] [PubMed] [Google Scholar]
- 16.Clarke FM, Faulkes CG. Hormonal and behavioural correlates of male dominance and reproductive status in captive colonies of the naked mole-rat, Heterocephalus glaber. Proc Biol Sci. 1998;265(1404):1391–1399. doi: 10.1098/rspb.1998.0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Westlin LM, Bennett NC, Jarvis JUM. Relaxation of reproductive suppression in non-breeding female naked mole-rats, Heterocephalus glaber. J Zool. 1994;234:177–188. [Google Scholar]
- 18.Buffenstein R. Negligible senescence in the longest living rodent, the naked mole-rat: insights from a successfully aging species. J Comp Physiol B. 2008;178(4):439–445. doi: 10.1007/s00360-007-0237-5. [DOI] [PubMed] [Google Scholar]
- 19.Buffenstein R, Pinto M. Endocrine function in naturally long-living small mammals. Mol Cell Endocrinol. 2009;299(1):101–111. doi: 10.1016/j.mce.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Honeycutt R, Nelson K, Schlitter D, Sherman P. Genetic variation within and among populations of the naked mole-rat: evidence from mitochondrial and nuclear genomes. In: Sherman P, Jarvis J, Alexander R, editors. The Biology of the Naked Mole-Rat. Princeton, NJ: Princeton University Press; 1991. pp. 195–208. [Google Scholar]
- 21.Reeve HK, Westneat DF, Noon WA, Sherman PW, Aquadro CF. DNA “fingerprinting” reveals high levels of inbreeding in colonies of the eusocial naked mole-rat. Proc Natl Acad Sci U S A. 1990;87(7):2496–2500. doi: 10.1073/pnas.87.7.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bennett NC, Jarvis JUM, Davies KC. Daily and seasonal temperatures in the burrows of African rodent moles. S Afr J Zool. 1988;23(3):189–195. [Google Scholar]
- 23.Larson J, Park TJ. Extreme hypoxia tolerance of naked mole-rat brain. Neuroreport. 2009;20(18):1634–1637. doi: 10.1097/WNR.0b013e32833370cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carnes BA, Olshansky SJ. A biologically motivated partitioning of mortality. Exp Gerontol. 1997;32(6):615–631. doi: 10.1016/s0531-5565(97)00056-9. [DOI] [PubMed] [Google Scholar]
- 25.Brett R. The population structure of naked mole-rat colonies. In: Sherman P, Jarvis J, Alexander R, editors. The Biology of the Naked Mole-Rat. Princeton, NJ: Princeton University Press; 1991. pp. 97–136. [Google Scholar]
- 26.Lacey E, Sherman P. Social organization of naked mole-rat colonies: evidence for division of labor. In: Sherman P, Jarvis J, Alexander R, editors. The Biology of the Naked Mole-Rat. Princeton, NJ: Princeton University Press; 1991. pp. 275–336. [Google Scholar]
- 27.Edrey YH, Hanes M, Pinto M, Mele J, Buffenstein R. Successful aging and sustained good health in the naked mole rat: a long-lived mammalian model for biogerontology and biomedical research. ILAR J. 2011;52(1):41–53. doi: 10.1093/ilar.52.1.41. [DOI] [PubMed] [Google Scholar]
- 28.O’Connor TP, Lee A, Jarvis JU, Buffenstein R. Prolonged longevity in naked mole-rats: age-related changes in metabolism, body composition and gastrointestinal function. Comp Biochem Physiol A Mol Integr Physiol. 2002;133(3):835–842. doi: 10.1016/s1095-6433(02)00198-8. [DOI] [PubMed] [Google Scholar]
- 29.Fries JF. Frailty, heart disease, and stroke: the compression of morbidity paradigm. Am J Prev Med. 2005;29(5 suppl 1):164–168. doi: 10.1016/j.amepre.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 30.Ikeno Y, Hubbard GB, Lee S, et al. Housing density does not influence the longevity effect of calorie restriction. J Gerontol A Biol Sci Med Sci. 2005;60(12):1510–1517. doi: 10.1093/gerona/60.12.1510. [DOI] [PubMed] [Google Scholar]
- 31.Liang S, Mele J, Wu Y, Buffenstein R, Hornsby PJ. Resistance to experimental tumorigenesis in cells of a long-lived mammal, the naked mole-rat (Heterocephalus glaber) Aging Cell. 2010;9(4):626–635. doi: 10.1111/j.1474-9726.2010.00588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123(8):933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 33.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 34.Lakatta EG. Age-associated cardiovascular changes in health: impact on cardiovascular disease in older persons. Heart Fail Rev. 2002;7(1):29–49. doi: 10.1023/a:1013797722156. [DOI] [PubMed] [Google Scholar]
- 35.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285(26):1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 36.Miller V, Best P. Implications for reproductive medicine: sex differences in cardiovascular disease. Sex Reprod Menopause. 2011;9(3):21–28. [PMC free article] [PubMed] [Google Scholar]
- 37.Furman RH. Are gonadal hormones (estrogens and androgens) of significance in the development of ischemic heart disease. Ann N Y Acad Sci. 1968;149(2):822–833. doi: 10.1111/j.1749-6632.1968.tb53838.x. [DOI] [PubMed] [Google Scholar]
- 38.Kuhl H. Mechanisms of sex steroids. Future developments. Maturitas. 2004;47(4):285–291. doi: 10.1016/j.maturitas.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 39.Pal L, Kallen AN. Cardiovascular disease and ovarian function. Curr Opin Obstet Gyn. 2011;23(4):258–267. doi: 10.1097/GCO.0b013e3283488a21. [DOI] [PubMed] [Google Scholar]
- 40.Huhtaniemi I, Forti G. Male late-onset hypogonadism: pathogenesis, diagnosis and treatment. Nat Rev Urol. 2011;8(6):335–344. doi: 10.1038/nrurol.2011.47. [DOI] [PubMed] [Google Scholar]
- 41.Evert J, Lawler E, Bogan H, Perls T. Morbidity profiles of centenarians: survivors, delayers, and escapers. J Gerontol A Biol Sci Med Sci. 2003;58(3):232–237. doi: 10.1093/gerona/58.3.m232. [DOI] [PubMed] [Google Scholar]
- 42.Terry DF, Wilcox M, McCormick MA, Lawler E, Perls TT. Cardiovascular advantages among the offspring of centenarians. J Gerontol Med Sci. 2003;58(5):M425–M431. doi: 10.1093/gerona/58.5.m425. [DOI] [PubMed] [Google Scholar]
- 43.Barzilai N, Gabriely I, Gabriely M, Iankowitz N, Sorkin JD. Offspring of centenarians have a favorable lipid profile. J Am Geriatr Soc. 2001;49(1):76–79. doi: 10.1046/j.1532-5415.2001.49013.x. [DOI] [PubMed] [Google Scholar]
- 44.Gillebert TC, Van de Veire N, De Buyzere ML, De Sutter J. Time intervals and global cardiac function. Use and limitations. Eur Heart J. 2004;25(24):2185–2186. doi: 10.1016/j.ehj.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 45.Groban L, Kitzman DW. Diastolic function: a barometer for cardiovascular risk? Anesthesiology. 2010;112(6):1303–1306. doi: 10.1097/ALN.0b013e3181da89e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mandinov L, Eberli FR, Seiler C, Hess OM. Diastolic heart failure. Cardiovasc Res. 2000;45(4):813–825. doi: 10.1016/s0008-6363(99)00399-5. [DOI] [PubMed] [Google Scholar]
- 47.Basu R, Oudit GY, Wang X, et al. Type 1 diabetic cardiomyopathy in the Akita (Ins2WT/C96Y) mouse model is characterized by lipotoxicity and diastolic dysfunction with preserved systolic function. Am J Physiol Heart Circ Physiol. 2009;297(6):H2096–H2108. doi: 10.1152/ajpheart.00452.2009. [DOI] [PubMed] [Google Scholar]
- 48.Salmasi AM, Alimo A, Jepson E, Dancy M. Age-associated changes in left ventricular diastolic function are related to increasing left ventricular mass. Am J Hypertens. 2003;16(6):473–477. doi: 10.1016/s0895-7061(03)00846-x. [DOI] [PubMed] [Google Scholar]
- 49.Wilkenshoff UM, Hatle L, Sovany A, Wranne B, Sutherland GR. Age-dependent changes in regional diastolic function evaluated by color Doppler myocardial imaging: a comparison with pulsed Doppler indexes of global function. J Am Soc Echocardiogr. 2001;14(10):959–969. doi: 10.1067/mje.2001.116321. [DOI] [PubMed] [Google Scholar]
- 50.Park HS, Naik SD, Aronow WS, Ahn CW, McClung JA, Belkin RN. Age- and sex-related differences in the tissue Doppler imaging parameters of left ventricular diastolic dysfunction. Echocardiography. 2007;24(6):567–571. doi: 10.1111/j.1540-8175.2007.00434.x. [DOI] [PubMed] [Google Scholar]
- 51.Flu WJ, van Kuijk JP, Hoeks SE, et al. Prognostic implications of asymptomatic left ventricular dysfunction in patients undergoing vascular surgery. Anesthesiology. 2010;112(6):1316–1324. doi: 10.1097/ALN.0b013e3181da89ca. [DOI] [PubMed] [Google Scholar]
- 52.Halley CM, Houghtaling PL, Khalil MK, Thomas JD, Jaber WA. Mortality rate in patients with diastolic dysfunction and normal systolic function. Arch Intern Med. 2011;171(12):1082–1087. doi: 10.1001/archinternmed.2011.244. [DOI] [PubMed] [Google Scholar]
- 53.Hartley CJ, Taffet GE, Reddy AK, Entman ML, Michael LH. Noninvasive cardiovascular phenotyping in mice. ILAR J. 2002;43(3):147–158. doi: 10.1093/ilar.43.3.147. [DOI] [PubMed] [Google Scholar]
- 54.Tanaka N, Dalton N, Mao L, et al. Transthoracic echocardiography in models of cardiac disease in the mouse. Circulation. 1996;94(5):1109–1117. doi: 10.1161/01.cir.94.5.1109. [DOI] [PubMed] [Google Scholar]
- 55.Takimoto E, Kass DA. Role of oxidative stress in cardiac hypertrophy and remodeling. Hypertension. 2007;49(2):241–248. doi: 10.1161/01.HYP.0000254415.31362.a7. [DOI] [PubMed] [Google Scholar]
- 56.Andziak B, O’Connor TP, Buffenstein R. Antioxidants do not explain the disparate longevity between mice and the longest-living rodent, the naked mole-rat. Mech Ageing Dev. 2005;126(11):1206–1212. doi: 10.1016/j.mad.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 57.Andziak B, O’Connor TP, Qi W, et al. High oxidative damage levels in the longest-living rodent, the naked mole-rat. Aging Cell. 2006;5(6):463–471. doi: 10.1111/j.1474-9726.2006.00237.x. [DOI] [PubMed] [Google Scholar]
- 58.Rodriguez KA, Wywial E, Perez VI, et al. Walking the oxidative stress tightrope: a perspective from the naked mole-rat, the longest living rodent. Curr Pharm Des. 2011;17(22):2290–2307. doi: 10.2174/138161211797052457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lambert AJ, Boysen HM, Buckingham JA, et al. Low rates of hydrogen peroxide production by isolated heart mitochondria associate with long maximum lifespan in vertebrate homeotherms. Aging Cell. 2007;6(5):607–618. doi: 10.1111/j.1474-9726.2007.00312.x. [DOI] [PubMed] [Google Scholar]
- 60.Willcox BJ, Curb JD, Rodriguez BL. Antioxidants in cardiovascular health and disease: key lessons from epidemiologic studies. Am J Cardiol. 2008;101(10A):75D–86D. doi: 10.1016/j.amjcard.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 61.Labinskyy N, Csiszar A, Orosz Z, et al. Comparison of endothelial function, O2-* and H2O2 production, and vascular oxidative stress resistance between the longest-living rodent, the naked mole rat, and mice. Am J Physiol Heart Circ Physiol. 2006;291(6):H2698–H2704. doi: 10.1152/ajpheart.00534.2006. [DOI] [PubMed] [Google Scholar]
- 62.Choy JC, Granville DJ, Hunt DW, McManus BM. Endothelial cell apoptosis: biochemical characteristics and potential implications for atherosclerosis. J Mol Cell Cardiol. 2001;33(9):1673–1690. doi: 10.1006/jmcc.2001.1419. [DOI] [PubMed] [Google Scholar]
- 63.Ungvari Z, Buffenstein R, Austad SN, Podlutsky A, Kaley G, Csiszar A. Oxidative stress in vascular senescence: lessons from successfully aging species. Front Biosci. 2008;13:5056–5070. doi: 10.2741/3064. [DOI] [PubMed] [Google Scholar]
- 64.Csiszar A, Pacher P, Kaley G, Ungvari Z. Role of oxidative and nitrosative stress, longevity genes and poly(ADP-ribose) polymerase in cardiovascular dysfunction associated with aging. Curr Vasc Pharmacol. 2005;3(3):285–291. doi: 10.2174/1570161054368616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Csiszar A, Labinskyy N, Orosz Z, Xiangmin Z, Buffenstein R, Ungvari Z. Vascular aging in the longest-living rodent, the naked mole rat. Am J Physiol Heart Circ Physiol. 2007;293(2):H919–H927. doi: 10.1152/ajpheart.01287.2006. [DOI] [PubMed] [Google Scholar]
- 66.Hill W, Porter A, Bloom R, Seago J, Southwick M. Field and laboratory studies on the naked mole rat, Heterocephalus glaber. Proc Zool Soc Lond. 1955;128(4):455–514. [Google Scholar]
- 67.Whitesall SE, Hoff JB, Vollmer AP, D’Alecy LG. Comparison of simultaneous measurement of mouse systolic arterial blood pressure by radiotelemetry and tail-cuff methods. Am J Physiol Heart Circ Physiol. 2004;286(6):H2408–H2415. doi: 10.1152/ajpheart.01089.2003. [DOI] [PubMed] [Google Scholar]
- 68.Savage VM, Gillooly JF, Woodruff WH, et al. The predominance of quarter-power scaling in biology. Funct Ecol. 2004;18(2):257–282. [Google Scholar]
- 69.McArdle W, Katch F, Katch V. Essentials of Exercise Physiology. New York: Lippincott Williams & Wilkins; 2010. [Google Scholar]
- 70.Zhang WG, Zhang GQ. Heart rate, lifespan, and mortality risk. Ageing Res Rev. 2009;8(1):52–60. doi: 10.1016/j.arr.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 71.Giannuzzi P, Imparato A, Temporelli PL, et al. Doppler-derived mitral deceleration time of early filling as a strong predictor of pulmonary capillary wedge pressure in postinfarction patients with left ventricular systolic dysfunction. J Am Coll Cardiol. 1994;23(7):1630–1637. doi: 10.1016/0735-1097(94)90667-x. [DOI] [PubMed] [Google Scholar]
- 72.Vasan RS, Larson MG, Benjamin EJ, Evans JC, Reiss CK, Levy D. Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a population-based cohort. J Am Coll Cardiol. 1999;33(7):1948–1955. doi: 10.1016/s0735-1097(99)00118-7. [DOI] [PubMed] [Google Scholar]
- 73.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289(2):194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 74.Kim EB, Fang X, Fushan AA, et al. Genome sequencing reveals insights into physiology and longevity of the naked mole rat. Nature. 2011;479(7372):223–227. doi: 10.1038/nature10533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Edrey Y, Buffenstein R. Animal models in aging and Alzheimer's disease. In: Hau, Schapiro S, editors. Handbook of Laboratory Animal Science, Volume II, Third Edition: Animal Models. New York: Taylor & Francis Group; In press. pp. 97–121. [Google Scholar]
- 76.de Magalhaes JP, Costa J, Church GM. An analysis of the relationship between metabolism, developmental schedules, and longevity using phylogenetic independent contrasts. J Gerontol A Biol Sci Med Sci. 2007;62(2):149–160. doi: 10.1093/gerona/62.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rabinovitch PS, Dai DF. Cardiac aging in mice and humans: the role of mitochondrial oxidative stress. Trends Cardiovas Med. 2009;19(7):213–220. doi: 10.1016/j.tcm.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Taffet GE, Pham TT, Hartley CJ. The age-associated alterations in late diastolic function in mice are improved by caloric restriction. J Gerontol Biol Sci. 1997;52(6):B285–B290. doi: 10.1093/gerona/52a.6.b285. [DOI] [PubMed] [Google Scholar]
- 79.Meyer TE, Kovacs SJ, Ehsani AA, Klein S, Holloszy JO, Fontana L. Long-term caloric restriction ameliorates the decline in diastolic function in humans. J Am Coll Cardiol. 2006;47(2):398–402. doi: 10.1016/j.jacc.2005.08.069. [DOI] [PubMed] [Google Scholar]
- 80.Bokov AF, Lindsey ML, Khodr C, Sabia MR, Richardson A. Long-lived ames dwarf mice are resistant to chemical stressors. J Gerontol A Biol Sci Med Sci. 2009;64(8):819–827. doi: 10.1093/gerona/glp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reddy AK, Amador-Noguez D, Darlington GJ, et al. Cardiac function in young and old Little mice. J Gerontol A Biol Sci Med Sci. 2007;62(12):1319–1325. doi: 10.1093/gerona/62.12.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Perez VI, Buffenstein R, Masamsetti V, et al. Protein stability and resistance to oxidative stress are determinants of longevity in the longest-living rodent, the naked mole-rat. Proc Natl Acad Sci U S A. 2009;106(9):3059–3064. doi: 10.1073/pnas.0809620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Salmon AB, Sadighi Akha AA, Buffenstein R, Miller RA. Fibroblasts from naked mole-rats are resistant to multiple forms of cell injury, but sensitive to peroxide, ultraviolet light, and endoplasmic reticulum stress. J Gerontol A Biol Sci Med Sci. 2008;63(3):232–241. doi: 10.1093/gerona/63.3.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lewis KN, Mele J, Hayes JD, Buffenstein R. Nrf2, a guardian of healthspan and gatekeeper of species longevity. Integr Comp Biol. 2010;50(5):829–843. doi: 10.1093/icb/icq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sykiotis GP, Habeos IG, Samuelson AV, Bohmann D. The role of the antioxidant and longevity-promoting Nrf2 pathway in metabolic regulation. Curr Opin Clin Nutr. 2011;14(1):41–48. doi: 10.1097/MCO.0b013e32834136f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Martin-Montalvo A, Villalba JM, Navas P, de Cabo R. NRF2, cancer and calorie restriction. Oncogene. 2011;30(5):505–520. doi: 10.1038/onc.2010.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tullet JMA, Hertweck M, An JH, et al. Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans. Cell. 2008;132(6):1025–1038. doi: 10.1016/j.cell.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yu C, Li Y, Holmes A, et al. RNA sequencing reveals differential expression of mitochondrial and oxidation reduction genes in the long-lived naked mole-rat when compared to mice. PLoS One. doi: 10.1371/journal.pone.0026729. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]