Abstract
We analyze the relationship between age of survival, morbidity, and disability among centenarians (age 100–104 years), semisupercentenarians (age 105–109 years), and supercentenarians (age 110–119 years). One hundred and four supercentenarians, 430 semisupercentenarians, 884 centenarians, 343 nonagenarians, and 436 controls were prospectively followed for an average of 3 years (range 0–13 years). The older the age group, generally, the later the onset of diseases, such as cancer, cardiovascular disease, dementia, and stroke, as well as of cognitive and functional decline. The hazard ratios for these individual diseases became progressively less with older and older age, and the relative period of time spent with disease was lower with increasing age group. We observed a progressive delay in the age of onset of physical and cognitive function impairment, age-related diseases, and overall morbidity with increasing age. As the limit of human life span was effectively approached with supercentenarians, compression of morbidity was generally observed.
Keywords: Centenarian, Supercentenarian, Compression of morbidity, Oldest old, Health span
Supercentenarians are exceedingly rare, occurring at a rate of about 1 per 5 million in industrialized nations and far less frequently in less developed countries (1). Kestenbaum and Ferguson (2) reported that in 2000, there were 32,920 centenarians in the United States, and of these, 105 or 0.3% were aged 110 years and older. According to the Social Security Administration’s 1900 cohort life table, of 100,000 people born in 1900, for men and women, respectively, 0.51% and 3.2% lived to age 100–104 years, 0.03% and 0.25% were predicted to live to age 105–109 years, and 0% and 0.004% were predicted to live to age 110+ years (3). Bourbeau (4) estimated that 7 in 1,000 people (0.7%) born at the turn of the last century lived to become a centenarian and 1 in 100,000 (0.001%) live to be 110 years or older. Though we often read about age claims greater than 115 years old, 99% of these are false (1). The oldest validated case is Jeanne Calment, who lived for 122 years and 165 days and she died in 1997 (5). Only a few people have approached this age (119 was the next oldest) over the past 14 years, indicating how extremely rare it is to even come close to the current life-span record. Notably, these two oldest humans were women, and approximately 10 of 11 supercentenarians are women (6).
A few studies have provided insight into the medical conditions and functional abilities of supercentenarians. The New England Centenarian Study (NECS) published a descriptive case series of 32 supercentenarians revealing that 41% required minimal or no assistance in activities of daily living and fewer than 15% had a history of a vascular-related disease (7). A study of 12 age-validated supercentenarians in Okinawa found that 83% did not have a major clinically evident disease up to age 105 (8), and the earlier case series of 32 participants also found that diseases that were common in younger centenarians (eg, 100–104 years), such as heart disease and stroke, were rare among supercentenarians.
Previously, we reported that although centenarians in the NECS (average age 103 years) generally do not experience disability until their early to mid-nineties, a substantial percentage of them experience age-related diseases for a longer period of time (9,10), an observation that would not be consistent with the compression of morbidity hypothesis if one assumed human life span to approximate 100 years of age (11,12). We showed that approximately 43% of centenarians in the NECS had onset of age-related diseases (heart disease, diabetes, cancer, skin cancer, osteoporosis, thyroid condition, hypertension, stroke, dementia, and chronic obstructive pulmonary disease [COPD]) prior to age 80, another 42% after the age of 80, and 15% did not have such diseases at age 100. However, given our and others’ observations that numerous age-related diseases are both rare and delayed among supercentenarians, we suspected that participants around the age of 100 might not be old enough to assess the interrelationships between morbidity, disability, and survival at the approximate limit of human life span. We present here an analysis of both medical disease history and physical and cognitive functional status data for 104 participants, aged 110 years and older and compare these findings with younger age groups of the NECS sample. We hypothesize Fries is indeed correct that as the practical limit of human life span is approached (eg, supercentenarians), morbidity is compressed toward the end of life and thus health span (morbidity- and disability-free period of life) approximates life span. Furthermore, with such compression, there is a rapid terminal decline in functional status and organ reserve thus supporting the assertion of a fixed life span.
METHODS
Participants
The NECS began in 1994 as a population-based study of all centenarians living within eight towns in the Boston area (13) and expanded enrollment to include centenarians from throughout the United States (www.bumc.bu.edu/centenarian) in 2000 (13). Since 1997, the NECS has made a concerted effort to locate and recruit supercentenarians, aged 110 years and older in addition to recruiting younger participants. Potential participants were identified from state voter registries, responses to nursing home and senior center mailings, news items appearing in print and on the World Wide Web, and enrollment inquiries made directly to the NECS. The only exclusion criterion for the NECS has been the inability to validate age. Birth certificates were available for age validation in 30% of participants. This relative low availability rate of birth certificates is not surprising given that birth certificates or what is more formally called the U.S. birth registration area did not become established until 1915, and all the states were not included in this effort until 1933 (14). Therefore, most of the birth certificates came from foreign-born participants or those generated by local municipalities (rather than at the state level). In the remaining 70% of participants, we relied upon U.S. census data from the early 1900s, which noted the participant’s birth year and month as well as similar information for their parents and siblings. We noted the documented birth date and checked that the birth date made sense relative to the ages of the parents and siblings who were also noted in the census record (1,15,16).
The NECS also enrolled referent participants who were participants without a familial predisposition for exceptional longevity. This referent group was composed of spouses of offspring of centenarians and participants of the same birth cohort as the offspring but where at least one parent died at age 73 years, which is the average life expectancy for the 1900 birth cohort.
Participants underwent informed consent, and the study was overseen up until 2001 by Beth Israel Deaconess Medical Center's Institutional Review Board (Boston, MA) and thereafter by the Boston University Medical Campus Institutional Review Board.
We included in the analysis 1,761 long-lived participants from 1,594 families that fell into the following age groups: supercentenarians (aged 110–119 years, n = 104, 13% men, 87% women), semisupercentenarians (aged 105–109 years, n = 430, 20% men, 80% women), younger centenarians (aged 100–104 years, n = 884, 25% men, 75% women), and nonagenarians (aged 87–99, n = 343, 38% men, 62% women). The NECS sample includes nonagenarian participants for the most part because 87% are siblings of enrolled or deceased centenarians. The younger referent group (described earlier) consisted of 436 subjects, aged 47–96 years.
Data
Data were collected via questionnaires administered to the participant or proxy over the telephone or via the mail and included medical history, current medications, and family pedigree information. The medical history questionnaire was introduced in early 1999, and hence, for about 20% of participants, the medical history was not recorded. These 20% were not included in the portion of the analysis that required medical data. Measures of physical (Barthel Index [17,18]) and cognitive function (Blessed Information-Memory-Concentration [BIMC] Test [19,20]) were obtained annually. The Barthel Index measures the ability to perform activities of daily living. Scores of 80–100 indicate independent functioning, 60–79 require minimal assistance, 40–59 indicate partial dependence, 20–39 indicate very dependent, and 0–19 indicate total dependence for performing activities of daily living. The BIMC Test, a brief test of global cognition, can have a maximum total score of 37 points. Scores of 34 or greater represent no clinically significant impairment, 27–33 indicate mild impairment, 21–26 signify moderate impairment, and less than 20 are associated with severe impairment (20).
The medical history questionnaire collected information about the presence and ages of onset for numerous age-related illnesses including the following that were used in the later described analysis: cancer type, cardiovascular disease (CVD, defined as angina pectoris, cardiac arrhythmia, congestive heart failure, and/or myocardial infarction), COPD (defined as emphysema or chronic bronchitis), dementia, diabetes mellitus, hypertension (HTN, defined as being on an antihypertensive medication or being told they have hypertension), osteoporosis or hip, wrist or spine fracture, and stroke. Annual follow-up was instituted in August, 2003 and included administration of the Barthel Index questionnaire and the BIMC test and updates on illnesses, hospitalizations, and medications. We collected medical history data in approximately 83% of participants and more than 90% of supercentenarians. We reviewed medical records on 98 participants to assess concordance with the medical histories reported by the participants and/or their proxies via the medical history questionnaire, and concordance for the illnesses (CVD, cancer, COPD, dementia, diabetes, and stroke) used in the analysis was 100%.
Statistics
Participants were grouped into four age groups, based upon age at death or, for those still alive, their ages on February 28, 2011. The only participants who were still alive were some of the supercentenarians (who had recently been enrolled).
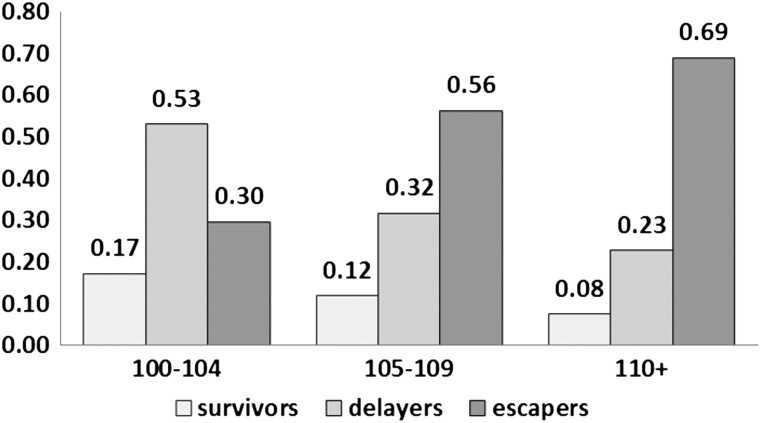
According to the Centers for Disease Control, in 2009, the top 10 leading causes of death among people aged 65+ years were heart disease, malignant neoplasms, chronic lower respiratory diseases, Alzheimer’s disease, diabetes mellitus, influenza and pneumonia, kidney diseases, accidents, stroke, and septicemia (21). For most analyses, we compared centenarians in the different age groups according to the ages of onset for the diseases on the leading cause list that were included in our medical history questionnaire (CVD, cancer, COPD, dementia, diabetes, and stroke), and we also included hypertension and osteoporosis. Prevalence of survivors, delayers, and escapers was estimated by the frequencies of participants with onset of at least one age-related disease (cancer, CVD, COPD, dementia, diabetes, and stroke) prior to age 80 (survivors), between 80 and 100 years (delayers), and 100+ years (escapers; Figure 1) as previously described (9). For the prevalence calculations, hypertension was not included because if present, it was being treated with medication, thus markedly decreasing its effect upon morbidity and osteoporosis was not included because it was not among the leading causes of death, noted earlier.
Figure 1.
Frequency of survivors (onset of at least one disease prior to age 80 years), delayers (onset of at least one disease between ages of 80 and 99), and escapers (onset of at least one disease after age 100 years) among three age groups: centenarians (100–104 years), semisupercentenarians (105–109 years), and supercentenarians (110+ years). Diseases included in this analysis were cancer, cardiovascular disease, chronic obstructive pulmonary disease, dementia, diabetes, and stroke.
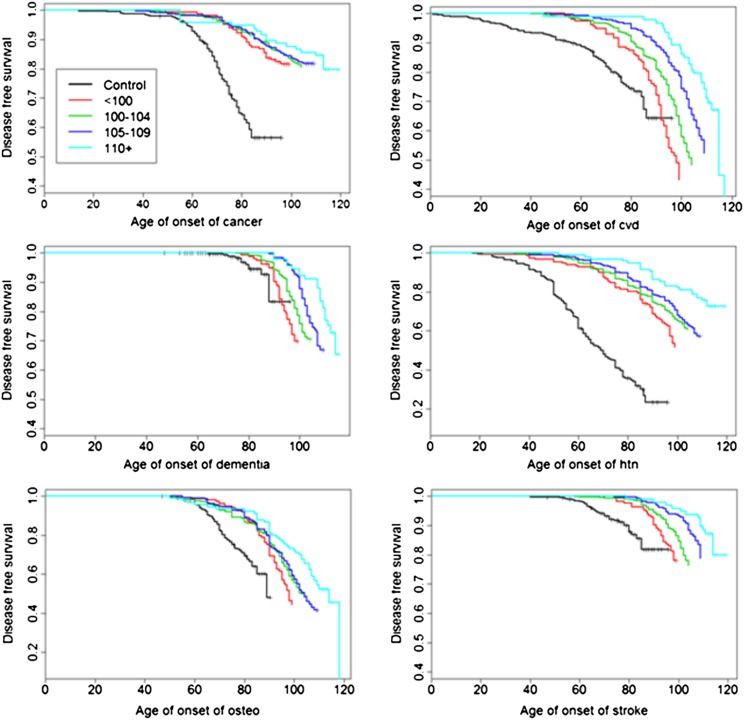
Ages of onset of age-related diseases are displayed with Kaplan–Meyer curves (Figure 2), and significant differences in hazard rates among groups were tested using Bayesian survival analysis with Weibull regression. This approach tailors the Cox proportional hazard regression to model an accelerated hazard for increasing ages and offers a simple multivariate parametric approach for the estimation of risk and quantiles of specific survival (22). The hypothesis of proportional hazards was assessed by plotting the log(−log[survival]) against the log(age) to show linearity. Some examples are provided in the Supplementary Material. Within family correlation was modeled using normally distributed random effects (23,24). Comparisons between the age groups of centenarians and controls were summarized by hazard ratios (HRs; Table 1) and by the age estimated to reach specific percentiles of survival (Figure 3). The estimates were computed using Markov Chain Monte Carlo, and uninformative priors were assumed for all parameters (essentially, all parameter values were assumed to be, a priori, equally likely). At least 20,000 simulated values were used to estimate parameters and produced summaries of model fit. Note that the grouping of centenarians into strata defined by the ages at death may not be exogenous variables (26) because the causal relation between age of onset of disease and age at death is not clear (27). However, the goal of this analysis was not to show the predictive effect of age of onset of disease on mortality but rather to study whether, consistent with the hypothesis of compression of morbidity, the ages of onset of disease change in the different age groups.
Figure 2.
Distribution of disease-free survival of common age-related diseases in controls and centenarians stratified by age at death. The survival curves were generated using Kaplan–Meyer estimators. Vertical ticks represent censored events (dead or alive participants without events). “Osteo” is osteoporosis, “htn” is hypertension, and “cvd” is cardiovascular disease.
Table 1.
Hazard Ratios of Common Age-Related Diseases for Centenarians and Their Siblings Stratified by Age Versus Controls (lines 1–4) and for Centenarians Versus Nonagenarians, Semisupercentenarians Versus Centenarians, and Supercentenarians Versus Semisupercentenarians (lines 5–7)
| Cancer |
CVD |
Dementia |
Hypertension |
Osteoporosis |
Stroke |
|||||||
| Est | 95% CI | Est | 95% CI | Est | 95% CI | Est | 95% CI | Est | 95% CI | Est | 95% CI | |
| HR (nonagenarians vs controls) | 0.12 | 0.09–0.17 | 0.55 | 0.40–0.76 | 0.65 | 0.35–1.31 | 0.17 | 0.13–0.22 | 0.41 | 0.30–0.56 | 0.22 | 0.14–0.36 |
| HR (centenarians vs controls) | 0.09 | 0.07–0.12 | 0.38 | 0.29–0.49 | 0.36 | 0.20–0.71 | 0.13 | 0.10–0.15 | 0.30 | 0.23–0.39 | 0.14 | 0.10–0.21 |
| HR (semisupercentenarians vs controls) | 0.08 | 0.06–0.11 | 0.26 | 0.19–0.34 | 0.21 | 0.11–0.44 | 0.11 | 0.09–0.14 | 0.27 | 0.21–0.36 | 0.07 | 0.05–0.11 |
| HR (supercentenarians vs controls) | 0.06 | 0.05–0.09 | 0.18 | 0.13–0.26 | 0.10 | 0.05–0.23 | 0.08 | 0.06–0.11 | 0.17 | 0.12–0.25 | 0.05 | 0.03–0.09 |
| HR (centenarians vs nonagenarians) | 0.79 | 0.57–1.07 | 0.68 | 0.53–0.89 | 0.56 | 0.39–0.80 | 0.74 | 0.58–0.95 | 0.73 | 0.56–0.95 | 0.63 | 0.43–0.93 |
| HR (semisupercentenarians vs centenarians) | 0.85 | 0.70–1.04 | 0.68 | 0.56–0.83 | 0.59 | 0.45–0.77 | 0.90 | 0.76–1.07 | 0.91 | 0.75–1.11 | 0.54 | 0.40–0.71 |
| HR (supercentenarians vs semisupercentenarians) | 0.81 | 0.59–1.09 | 0.71 | 0.53–0.95 | 0.49 | 0.31–0.76 | 0.70 | 0.53–0.91 | 0.62 | 0.45–0.85 | 0.73 | 0.49–1.07 |
Notes: Diseases and ages of onset are based on medical history and annual follow-up data. Cancer includes all cancers except skin cancers. Cardiovascular disease (CVD) is defined as angina pectoris, cardiac arrhythmia, congestive heart failure, and/or myocardial infarction. Osteoporosis is defined as diagnosis or fracture of wrist, femur, or spine. Estimates (Est) and 95% credible intervals (95% CI) were computed as the median, 2.5 and 97.5 percentiles from at least 20,000 samples generated from the posterior distribution of the parameters. The time of event was modeled using Weibull regression as described in the methods. HR = hazard ratio.
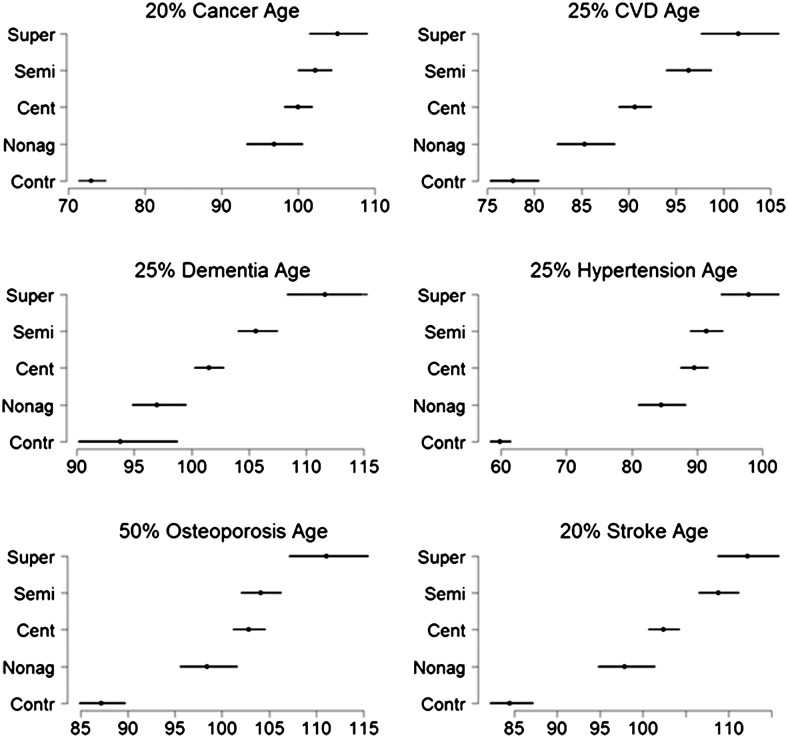
Figure 3.
Estimates of ages when specific percentiles of subjects first experienced specific age-related diseases. The percentiles are 20% for stroke and cancer; 25% for cardiovascular disease, dementia, and hypertension; and 50% for osteoporosis and were chosen based on reported prevalences of age-related diseases in (25). The estimates were computed using Weibull regression and the equation , where λ and v are the parameters of the hazard function .
The overall compression of morbidity was assessed by determining the survival rate after the onset of morbidity (Figure 4). The rates were analyzed using lognormal regression models, with mixed effects to adjust for familial relations. The referent group, described in the Participants section, was included in this analysis.
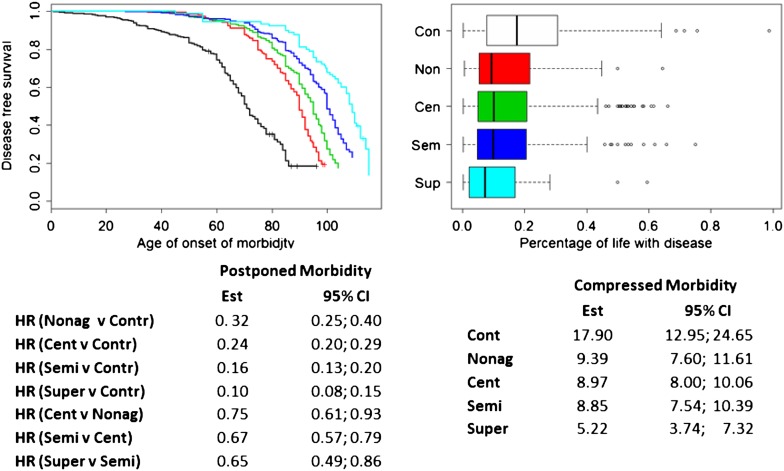
Figure 4.
Left: delay of morbidity. Morbidity-free survival in controls (black), nonagenarians (red), centenarians (green), semisupercentenarians (blue), and supercentenarians (pale blue). Morbidity was defined by either cancer, cardiovascular disease, dementia, diabetes, or stroke. The table shows the hazard ratios and 95% credible intervals that were estimated using Weilbull regression and Markov Chain Monte Carlo methods. Right: compression of morbidity. The boxplot displays the percentage of years spent with disease (as defined earlier) in controls and the different age groups of centenarians. Only participants with an age at death are included in this analysis. The table provides estimates of the median percentages of life spent with age-related disease and 95% CI that were estimated using a regression model with lognormal distributions.
The scores of physical disability and cognitive impairment in the oldest old were analyzed using a Bayesian mixed model with random effects to account for repeated measurements and within-family correlations. Because both scores are defined within limited ranges, we used the logistic transformation of the normalized scores in the regression. The transformed scores were assumed to follow normal distributions, with expected values that were modeled as linear regression functions of gender, age at test taken, centenarian age group, and an interaction between age and centenarian age group that allows for varying rates of decline in different age groups. Gender and age groups were coded as dummy variables so that the regression coefficients (Table 2) could be interpreted as the difference from the reference group (female nonagenarians). The estimates were computed using Markov Chain Monte Carlo, uninformative priors were assumed for all parameters, and only statistically significant interactions (posterior 95% credible interval excluding 0) were retained in the final model. The predictive values from the final models were estimated on a grid of age values and used to infer trajectories of cognitive and functional declines (Figure 5) using the inverse logit transformation (28,29). Ages of onset of cognitive and functional declines were estimated as functions of the regression parameters. The compression of disability was described by the rates of decline of cognitive and physical functions in relation to age of death.
Table 2.
Estimates of the Regression Coefficients in the Models of Cognitive and Functional Decline
| Barthel Score |
Blessed Score |
|||
| Est | 95% CI | Est | 95% CI | |
| Age | −0.28 | −0.32 to −0.24 | −0.15 | −0.20 to −0.11 |
| Gender (male) | 0.96 | 0.76 to 1.16 | 0.51 | 0.33 to 0.68 |
| Cent | 0.76 | 0.40 to 1.12 | 0.49 | 0.16 to 0.83 |
| Semi | 2.1 | 1.67 to 2.48 | 1.28 | 0.91 to 1.66 |
| Super | 2.5 | 1.68 to 3.26 | 2.20 | 1.25 to 3.166 |
| Cent × Age | −0.12 | −0.17 to −0.06 | −0.07 | −0.12 to −0.01 |
| Semi × Age | −0.09 | −0.14 to −0.03 | −0.14 | −0.20 to −0.08 |
| Super × Age | Not significant | −0.14 | −0.24 to −0.04 | |
Notes: Estimate of the regression coefficients and 95% credible intervals for the logit transformation of Barthel score (columns 1 and 2) and Blessed score (columns 3 and 4). The regression coefficients labeled as “cent,” “semi,” and “super” are the effects of the centenarian groups and the regression coefficient labeled as “Cent × Age,” “Semi × Age,” and “Super × Age” are the interaction terms that change the rate of decline with age in the different centenarian age groups. For example, the rate of decline of the logit-transformed Barthel score with age in nonagenarians is −0.28; the rate of decline of the logit-transformed Barthel score with age in centenarians is −0.28 − 0.12 and is −0.28 − 0.09 in semisupercentenarians. Ages were centered at the mean value. The estimates of regression coefficients and 95% CI were computed as the median, 2.5 and 97.5 percentiles from 45,000 samples generated from the posterior distribution of the parameters, using Markov Chain Monte Carlo.
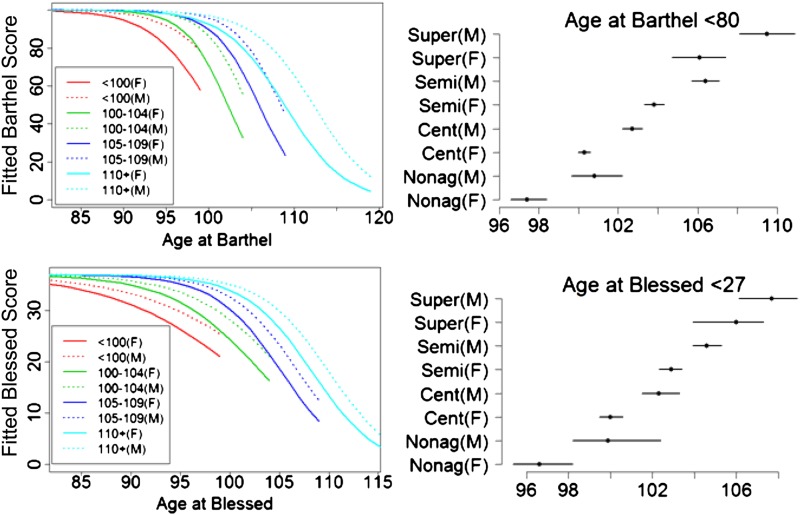
Figure 5.
Left panels: trajectories of physical (Barthel) and cognitive (Blessed Information-Memory-Concentration Test) functional declines fitted to the data. The right panels depict the age of onset of functional (Barthel score < 80) and cognitive declines (Blessed score < 27) in the different centenarian age groups by gender. The trajectories were computed using the inverse logistic transformation as explained in methods, and for each age group, they are truncated by the maximum age for their defined age ranges (eg, 99 years for nonagenarians, 104 for centenarians).
All analyses were conducted in OpenBugs (http://mathstat.helsinki.fi/openbugs/Home.html) and the statistical software R 2.11. Model specifications as well as details of simulations are available as Supplementary Material.
RESULTS
Delayed Onset of Age-Related Diseases
The frequency of “survivors” decreases and the frequency of “escapers” increases with age, such that 8%, 23%, and 69% of supercentenarians were survivors, delayers, and escapers, respectively, compared with 17%, 53%, and 30% in centenarians and 12%, 32%, and 56% of semisupercentenarians (Figure 1). This preliminary analysis shows that 17% of centenarians in the NECS survived at least 20 years (and hence at least 20% of their life span) with one or more age-related diseases. The rate of survivors decreased in supercentenarians, but the prevalence of delayers among supercentenarians shows that as many as 23% of these participants survived more than 30 years (eg, with an age-related disease after 80 years of age) with at least one age-related disease.
To better understand the relationship between delayed onset of morbidity and extended life span, we analyzed the age of onset of diseases using survival analysis. Figure 2 displays Kaplan–Meyer curves of disease-free survival, stratified by age, and demonstrates a consistent delay in the onset of major age-related diseases with increasing age of survival. Table 1 reports the Bayesian estimate of the HRs comparing participants stratified by age group that were obtained using Weibull regression. Diabetes and COPD were not included in this analysis because of the small numbers of events in supercentenarians (two cases of COPD and five cases of diabetes) that made the estimates unreliable. The analysis shows that centenarians and their younger nonagenarian siblings significantly delayed the onset of age-related diseases compared with the referent cohort, with a decreased HR as low as 0.09 for cancer (95% CI = 0.07–0.12) and 0.15 for hypertension (95% CI = 0.10–0.15). Less pronounced decreases in hazard were observed for dementia (HR = 0.36 comparing centenarians vs referent cohort, 95% CI = 0.20–0.71) and CVD (HR = 0.38 comparing centenarians vs referent cohort, 95% CI = 0.29–0.49).
Delays of onset and decreased hazards became more and more pronounced with older and older age groups, culminating in supercentenarians who had comparatively low HRs. Compared with controls, the HR for supercentenarians in the case of cancer was 0.06 (95% CI = 0.05–0.09); the HR for CVD was 0.18 (95% CI = 0.13–0.26); for dementia, it was 0.1 (95% CI = 0.05–0.23); for hypertension, it was 0.08 (95% CI = 0.06–0.11); for osteoporosis, it was 0.17 (95% CI = 0.12–0.25); and for stroke, the HR was 0.05 (95% CI = 0.03–0.09). Compared with semisupercentenarians, supercentenarians had a statistically significant reduced hazard for CVD (HR = 0.71; 95% CI = 0.53–0.95), dementia (HR = 0.49, 95% CI = 0.30–0.76), hypertension (HR = 0.70, 95% CI = 0.53–0.91), and osteoporosis (HR = 0.62, 95% CI = 0.45–0.85) and reduced but not statistically significant hazard for cancer (HR = 0.81, 95% CI = 0.59–1.09) and stroke (HR = 0.73, 95% CI = 0.49–1.07).
To further understand the delay in onset of these diseases with older and older ages, we estimated ages at which a specific percentile of participants reported a history of disease. The analysis confirms the substantial delay of onset of major diseases in the older participants and the increased delay among supercentenarians even compared with semisupercentenarians (Figure 3). For example, 25% of controls reported a history of CVD by age ∼75 years, whereas the age at which 25% of supercentenarians report a history of CVD was 102 years, a comparative delay in the onset of CVD of 27 years. Note that people born in 1900 who survived to at least age 40 had an average life expectancy of 68 years (30), and therefore, supercentenarians born in 1900 lived 47 years longer than other members of their birth cohort who survived the neonatal through young adulthood years. Among centenarians and semisupercentenarians, this 25th age percentile for CVD was, respectively, 91 and 96 years.
Our analysis shows that the onset of each individual disease is progressively postponed in the different age groups of centenarians with older and older age. To test whether the overall morbidity is postponed, we analyzed the morbidity-free survival in the controls, nonagenarians, and centenarian age groups. We defined onset of morbidity as the occurrence of at least one of the following diseases: CVD, cancer, COPD, dementia, diabetes, or stroke. Figure 4 (left panel) shows the extended morbidity-free survival for the various age groups relative to controls. The median age of onset of morbidity in controls was 71 years (95% CI = 70–74) years compared with 90 years in nonagenarians (95% CI = 89–92), 95 years in centenarians (95% CI = 94–96), 100 years in semisupercentenarians (95% CI = 100–102), and 109 years in supercentenarians (95% CI = 102–112). Note that because more than 85% of the nonagenarians were siblings of centenarians, they were likely healthier than average nonagenarians.
The parametric survival analysis shows the significantly reduced hazards of morbidity in nonagenarians and the different age groups of centenarians compared with controls. Supercentenarians had 0.10 times the hazard for morbidity compared with controls. The reduced hazard was also statistically significant compared with semisupercentenarians (HR = 0.65, 95% CI = 0.49–0.86). Note the decreasing trend in hazards comparing centenarians with nonagenarians (HR = 0.75, 95% CI = 0.61–0.93), semisupercentenarians with centenarians (HR = 0.67, 96% CI = 0.57–0.79), and supercentenarians with semisupercentenarians (HR = 0.65, 95% CI = 0.49–0.86). The decreasing trend of hazards should result in a substantial compression of morbidity, and consistent with this, the boxplots that display the rates of diseased years (Figure 4, right panel) show that older participants significantly compressed morbidity relative to controls: The median percentage of years with disease in nonagenarians was 9.39% (95% CI = 7.7–11.61), in centenarians (aged 100–104 years), it was 8.97% (95% CI = 8.00–10.06), and in semisupercentenarians, it was 8.85% (95% CI = 7.54–10.39) compared with a median percentage of years with disease of 17.9% (95% CI = 12.95–24.65) in controls. Supercentenarians compressed morbidity more than the other age groups of centenarians: The regression analysis of the percentages of diseased years showed that supercentenarians had a median percentage of 5.22% diseased years (95% CI = 3.74–7.32). Note that the nonoverlapping intervals indicated that the differences reach statistical significance. The reduced percentage of years with disease in the supercentenarians was reflected by the larger percentage of participants who escaped disease till the last 3 months of their lives or less (10%) compared with semisupercentenarians (4%) and centenarians (3%).
Decline of Cognitive Function
We obtained 2,075 BIMC scores in 1,374 participants (279 nonagenarians, 665 centenarians, 346 semisupercentenarians, and 81 supercentenarians) with two or more tests administered in more than 52% of participants and an average 3 years of follow-up. A small percentage of participants was not administered the test because of profound end-stage dementia (eg, noncommunicative, 7%). We analyzed the logistic transformation of BIMC scores using regression analysis, and Table 2 reports the estimates of the regression coefficients. The results show that gender, age at test, centenarian age group, and interaction between age and centenarian age group were all significantly associated with the BIMC scores. The positive coefficient of the male gender parameter shows that, assuming all other covariates were fixed, men had significantly better BIMC scores than women. The difference in scores increased with the magnitude of the regression coefficients so that supercentenarians at any given age had, on average, the highest BIMC score followed by semisupercentenarians, centenarians, and nonagenarians at the same age. The estimates of the regression coefficients for age represent the rates of cognitive decline for the transformed BIMC scores (−0.15 for nonagenarians, −0.15 − 0.07 = −0.22 for centenarians, and −0.15 − 0.14 = −0.29 for semisupercentenarians and supercentenarians). The negative sign of these estimates indicates that the BIMC scores decreased with age. The increasing magnitude of the age effects in older and older centenarians shows that the rate of cognitive decline accelerated at older ages.
To better understand the effect of centenarian age groups and the rates of cognitive decline, we generated the predicted trajectories as explained in the methods. The trajectories show the delay in declines of cognitive function with each older age group and that for each age group men have higher BIMC scores compared with women (Figure 5). Note the slightly accelerated decline in the semisupercentenarians and supercentenarians, which is consistent with a delay of onset of cognitive decline toward the end of life followed by a rapid decline. The bottom-right panel of Figure 5 shows the estimated age of onset of moderate impairment (BIMC < 27) in the different groups of centenarians segregated by gender. Male supercentenarians delayed the onset of moderate impairment to almost 108 years, with a gain of cognitive function (BIMC ≥ 27) of approximately 3 years compared with male semisupercentenarians and 5 years compared with male centenarians.
Decline of Physical Functions
A total of 2,681 Barthel Index scores were obtained for 1,605 participants: (313 nonagenarians, 794 centenarians, 394 semisupercentenarians, and 102 supercentenarians) with two or more tests administered in more than 80% of participants and an average of 3.75 years follow-up. All three groups had Barthel score ranges of 0–100. Gender, age at test, centenarian age group, and interaction between age and centenarian group were significantly associated with the Barthel scores (Table 2). Men had significantly higher Barthel scores than women, assuming all other covariates were fixed, as shown by the positive coefficient of the male gender parameter. The estimates of the regression coefficients for age represent the rate of decline for the transformed Barthel score. The coefficients were negative, consistent with the fact that physical functions declined with age. However, the rate of functional decline changed in the centenarian age groups: It was −0.28 for nonagenarians and supercentenarians, −0.28 − 0.12 = −0.4 for centenarians, and −0.28 − 0.09 = −0.37 for semisupercentenarians, suggesting that centenarians and semisupercentenarians had a more rapid decline than nonagenarians and supercentenarians. Figure 5 shows the trajectories of physical functional declines for older and older survival ages. Centenarians and semisupercentenarians have slightly accelerated declines compared with nonagenarians and supercentenarians. The right panel of Figure 5 shows the estimated age of onset of physical functional dependence (Barthel < 80) in the different groups of centenarians and gender. Male supercentenarians appeared to be functionally independent to almost 110 years, with a gain of approximately 4 years compared with male semisupercentenarians.
Study Limitations
Several limitations to this study are evident. First, except for the supercentenarian sample, there may be a healthy volunteer bias given that participants who are healthier are more likely to participate in a study. However, we expect this effect to be approximately similar across all the age groups studied so that they do not affect the comparisons between centenarian age groups. In the case of supercentenarians, the healthy volunteer effect was less likely (but not absent) because the priority of the NECS for these participants was to obtain a blood sample for genetic studies, and both the study and families were less concerned about functional status of the participant in terms of their participation in the study. Additionally, 13% of the supercentenarian sample was male, but other resources suggest that the percentage of men should be higher (31). Because men tend to be healthier than women and because the healthy volunteer effect might be less in the supercentenarian group, these factors could result in an underestimate of better health and function among supercentenarians, thus biasing against our hypothesis. On the other hand, it is possible that the U.S. census estimate of the proportion of men at these most extreme ages is incorrect, in which case this bias is not an issue.
Our sample is also enriched for centenarians who have a familial history of exceptional longevity. Therefore, they are likely at even higher risk of achieving exceptional longevity than centenarians without such a familial history. The enrichment for familial longevity and the fact that this was not a population-based sample underscore that our findings are not reflective of all centenarians. Instead, we assert that many, but not all, supercentenarians exhibit compression of morbidity and disability, and our findings must be taken in the context of this select cohort.
DISCUSSION
Given our national enrollment efforts to date, we were able to avail ourselves of data from a substantial sample and age range of centenarians including 104 supercentenarians and 430 semisupercentenarians. Although there have been a substantial number of prevalence studies of age-related diseases and functional status in centenarians (7–9,25,32–42), limited for the most part to participants less than the age of 105 years, in this work we examined the age of onset of individual diseases, morbidity (age-related diseases combined), and cognitive and physical disability using survival analysis, and we were able to analyze these results across an age range of participants that encompasses 100-fold differences in survival probabilities among the oldest old.
Several statistically innovative methods were used in our analysis in order to deal with issues of censoring and varied periods of follow-up for this large sample of participants. We used Weibull regression for analysis of disease-free survival to be able to model an accelerated failure time. This is a simple specialization of Cox survival models that offers several advantages where statistical estimation may be challenging. Bayesian estimation is known to overcome limitations of parameter estimation, and it allows us to obtain accurate estimates of HRs as well as quantiles of specific survivals. Another innovative approach in this article was the use of Bayesian mixed models to study the trajectory of decline in centenarians stratified by age at death. The Bayesian method provides an elegant solution to the estimate of the trajectories and takes into account the overall uncertainty in the data.
The analysis reveals that comparing nonagenarians, centenarians (aged 100–104 years), semisupercentenarians, and supercentenarians, the older the age group, generally, the later the onset of major age-related diseases such as cancer, CVD, dementia, and stroke as well as of cognitive and functional decline. Along these lines, the HRs for these individual diseases became progressively less with older and older age. An indication of just how different the specific disease-free survival experiences of the centenarians are compared with the general population is the fact that the ages of onset for the diseases we looked at were substantially older than the median ages of death for the 1900 birth cohort.
For all the centenarian age groups, men fared better than women in terms of cognitive and physical functional status, and this phenomenon has been previously described (43). It may seem paradoxical that male centenarians are more fit than their female counterparts, whereas females have a much greater probability of surviving to extreme old age. A likely explanation, however, is that women are much better able to survive with age-related diseases and functional impairment compared with men, whereas the mortality associated with these conditions is higher for men. The result is a select survivor effect such that men who survive to extreme ages, relative to women, tend to not have the diseases and associated impairments that cause mortality at younger ages (44,45).
Some diseases were practically nonexistent among the supercentenarians, including Parkinson’s disease and diabetes. Perhaps, having these (and other) diseases is generally incompatible with survival to ages of 110 years and older, but because there was likely a healthy volunteer effect for all the age groups, it is possible that there are supercentenarians with these diseases, but we did not have the opportunity to include such participants in this study. Nonetheless, the marked delay in the age of onset and in some cases, the absence of age-related diseases suggest the utility of centenarians as controls for the study of such diseases. The hazards for these diseases become less with increasing age also suggesting that the power of centenarian participants for the discovery of factors that afford resistance to age-related diseases (or as controls for the discovery of predisposing factors) increases with age. The association between increasing age of the sample and the power to detect factors associated with exceptional longevity has previously been suggested by Tan and colleagues (46).
When we looked at overall morbidity (having one or more of the earlier age-related diseases) rather than individual diseases and at disease-free survival, the hazard for morbidity also declined with increasing age of the centenarian age groups. Simultaneously, the percentage of participants’ lives spent with one or more of the age-related diseases generally decreased with increasing age of the centenarian age groups. The median percentage of years with disease in controls was 17.9%, in nonagenarians, the median percentage was 9.4%; in centenarians (aged 100–104 years), it was 9.0%; in semisupercentenarians, it was 8.9%; and in supercentenarians, the regression analysis indicated a median percentage of 5.2% of life spent with disease. These findings are consistent with Fries’ (11) compression of morbidity hypothesis, predicting a decreased percentage of time spent with disease as the limit of life span is approached. The fact that we observe this compression also supports Fries’ contention that the human life span is fixed (rectangularization of the survival curve), but instead of a limit of life span being around 100 years as he suggested in 1980, survival to around 110–115 years is likely a more realistic practical limit. In fact, even in his 1980 article, Fries noted that the oldest age at the time was 114 years. Supporting this notion is that the prevalence of centenarians continues to grow (in 1990, the prevalence was about 1 per 10,000 and in 2010, the prevalence is about 1 per 5,000), but while there are many more supercentenarians alive today than there were in 1980, they still remain extremely rare at about 1 per 5 million in developed countries (1). Note that life span is defined by the oldest age ever achieved by a member of the species, and for humans, that age is 122 years and 164 days (born February 21, 1875 and died August 4, 1997 [5,47]). Despite a current population of nearly 7 billion people, no one with a validated age has come close to this record in the 14 years since Jeanne Calment’s death in 1997 except for Sarah Knauss, who died at the age of 119 years in 1999 (1,47).
Another consistency between our findings and Fries’s (11) predictions is his contention that exhaustion of organ reserve (the ability to restore homeostasis) is a key determinant of maximum life span. As cited in our results, we found that with each older age group, there was a larger percentage of participants who escaped disease (at least the diseases we included in our definition of morbidity) until the last 3 months or less of their lives (10% for supercentenarians, 4% for semisupercentenarians, and 3% for centenarians). These percentages would likely be greater if one uses a longer period of time prior to death for defining the period of time during which compression occurs. And, as noted earlier, the older the age group, the shorter the period of time spent with disease. In Figure 5, the trajectories of decline show that, on average, the supercentenarians in the study live to the limit of their functional and cognitive reserves. These findings support the hypothesis that underlying progressive diminution of functional reserve and adaptive capacity associated with aging, rather than prolonged chronic illness(es), become a key determinant of mortality as the limit of human life span is approached, just as Fries predicted.
Our results do not address whether or not the current increasing average life expectancy is accompanied by longer or shorter periods of morbidity and disability. Indeed, Crimmins and Beltrán-Sánchez (48) reviewed the relationship between morbidity and mortality among elderly Americans, and they concluded that as the population has been aging, morbidity and disability rates have been increasing. However, if rectangularization of the survival curve approximates human life span and life span is around 110 years, then it is possible that improvements in population morbidity and disability rates would not be observed with aging until median life expectancy is substantially higher than it is now. Studies suggest that this is possible when good health habits are the cause of increased life expectancy (49–52). Other studies suggest that even in the face of some poor health habits, people with a genetic predisposition to exceptional longevity can achieve very old age in good health and with good function (53).
With our recent success in enrolling a substantial number of participants whose survival approximates the limit of human life span, we address what happens to morbidity and disability with such survival. Our findings suggest that with older and older age beyond 100 years, many people have an increasing relative resistance to age-related diseases and disability and that survival to the supercentenarian years (eg, 110–122 years) approximates the limits of human functional or organ reserve to successfully contend with acute causes of death.
SUPPLEMENTARY MATERIAL
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/.
FUNDING
This work was supported by the National Institute on Aging (T.T.P.: K-24-AG025727) and the Glenn Medical Research Foundation.
Supplementary Material
References
- 1.Young RD, Desjardins B, McLaughlin K, Poulain M, Perls T. Typologies of extreme longevity myths. Curr Gerontol Geriatr Res. 2011:1–12. doi: 10.1155/2010/423087. http://www.hindawi.com/journals/cggr/2010/423087/Accessed December 1, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kestenbaum B, Ferguson RB. Number of centenarians in the United States Jan. 1, 1990, 2000 and 2010 based on improved medicare data. N Am Actuar J. 2005;10:1–6. http://ccp.ucr.ac.cr/creles/100m/m-rs05-1_xxvi.pdf. Accessed December 1, 2011. [Google Scholar]
- 3.Bell FC, Miller ML. Life Tables for the United States Social Security Area 1900–2100, Actuarial Study No. 120. 2005. http://www.ssa.gov/oact/NOTES/as120/LifeTables_Tbl_7_1900.html. Accessed September 5, 2011. [Google Scholar]
- 4.Bourbeau R. Demography. Tracking Down Supercentenarians. Universite de Montreal Research Bulletin; 2002. http://www.forum.umontreal.ca/forum_express/pages_a/demo.htmAccessed December 11, 2011. [Google Scholar]
- 5.Robine JM, Allard M. The oldest human. Science. 1998;279:1834–1835. doi: 10.1126/science.279.5358.1831h. [DOI] [PubMed] [Google Scholar]
- 6.Robine JM, Vaupel JW. Emergence of supercentenarians in low-mortality countries. N Am Actuar J. 2002;6:54–63. [Google Scholar]
- 7.Schoenhofen EA, Wyszynski DF, Andersen S, et al. Characteristics of 32 supercentenarians. J Am Geriatr Soc. 2006;54:1237–1240. doi: 10.1111/j.1532-5415.2006.00826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Willcox DC, Willcox BJ, Wang NC, He Q, Rosenbaum M, Suzuki M. Life at the extreme limit: phenotypic characteristics of supercentenarians in Okinawa. J Gerontol A Biol Sci Med Sci. 2008;63:1201–1208. doi: 10.1093/gerona/63.11.1201. [DOI] [PubMed] [Google Scholar]
- 9.Evert J, Lawler E, Bogan H, Perls T. Morbidity profiles of centenarians: survivors, delayers, and escapers. J Gerontol A Biol Sci Med Sci. 2003;58:232–237. doi: 10.1093/gerona/58.3.m232. [DOI] [PubMed] [Google Scholar]
- 10.Terry D, Sebastiani P, Andersen S, Perls T. Disentangling the roles of disability and morbidity in survival to exceptional old age. Arch Intern Med. 2008;168:277–283. doi: 10.1001/archinternmed.2007.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fries JF. Aging, natural death, and the compression of morbidity. N Engl J Med. 1980;303:130–135. doi: 10.1056/NEJM198007173030304. [DOI] [PubMed] [Google Scholar]
- 12.Fries JF. Frailty, heart disease, and stroke: the Compression of Morbidity paradigm. Am J Prev Med. 2005;29:164–168. doi: 10.1016/j.amepre.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Perls TT, Bochen K, Freeman M, Alpert L, Silver MH. Validity of reported age and centenarian prevalence in New England. Age Ageing. 1999;28:193–197. doi: 10.1093/ageing/28.2.193. [DOI] [PubMed] [Google Scholar]
- 14.Shapiro S. Development of birth registration and birth statistics in the United States. Popul Stud. 1950;4:86–111. [Google Scholar]
- 15.Desjardins B. Jeune B, Vaupel JW. Validation of extreme longevity cases in the past: the French-Canadian experience. Validation of Exceptional Longevity. Odense Monographs on Population Aging No. 6. Odense, Denmark: Odense University Press; 1999. http://www.demogr.mpg.de/books/odense/6/04.htmAccessed December 1, 2011. [Google Scholar]
- 16.Poulain M. On the age validation of supercentenarians. In: Maier H, Gampe J, Jeune B, Robine J-M, Vaupel JW, editors. Supercentenarians. Berlin, Germany: Springer; 2010. pp. 3–30. [Google Scholar]
- 17.Mahoney FI, Barthel DW. Functional evaluation: the Barthel index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 18.Sinoff G, Ore L. The Barthel activities of daily living index: self-reporting versus actual performance in the old-old (> or = 75 years) J Am Geriatr Soc. 1997;45:832–836. doi: 10.1111/j.1532-5415.1997.tb01510.x. [DOI] [PubMed] [Google Scholar]
- 19.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 20.Kawas C, Karagiozis H, Resau L, Corrada M, Brookmeyer R. Reliability of the Blessed Telephone Information-Memory-Concentration Test. J Geriatr Psychiatry Neurol. 1995;8:238–242. doi: 10.1177/089198879500800408. [DOI] [PubMed] [Google Scholar]
- 21.Kochanek KD, Xu J, Murphy SL, Minino AM, Kung H-C Division of Vital Statistics. Deaths: Preliminary Data for 2009. 4. Vol. 59. National vital statistics reports; 2011. www.cdc.gov/nchs/data/nvsr/nvsr59/nvsr59_04.pdfAccessed December 1, 2011. [PubMed] [Google Scholar]
- 22.Congdon P. Bayesian Statistical Modeling. 2nd ed. Chichester, UK: John Wiley & Sons; 2006. [Google Scholar]
- 23.Demidenko E. Mixed Models: Theories and Applications. Hoboken, NJ: John Wiley & Sons; 2004. [Google Scholar]
- 24.McArdle J, Prescott C. Mixed-effects variance components models for biometric family analyses. Behav Genet. 2005;35:631–652. doi: 10.1007/s10519-005-2868-1. [DOI] [PubMed] [Google Scholar]
- 25.Newman AB, Glynn NW, Taylor CA, et al. Health and function of participants in the Long Life Family Study: a comparison with other cohorts. Aging (Albany NY) 2011;3:63–76. doi: 10.18632/aging.100242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hogan JW, Roy J, Korkontzelou C. Handling drop-out in longitudinal studies. Stat Med. 2004;23:1455–1497. doi: 10.1002/sim.1728. [DOI] [PubMed] [Google Scholar]
- 27.Hekimi S. How genetic analysis tests theories of animal aging. Nat Genet. 2006;38:985–991. doi: 10.1038/ng1881. [DOI] [PubMed] [Google Scholar]
- 28.Neelon B, Swamy GK, Burgette LF, Miranda ML. A Bayesian growth mixture model to examine maternal hypertension and birth outcomes. Stat Med. 2011;30:2721–2735. doi: 10.1002/sim.4291. [DOI] [PubMed] [Google Scholar]
- 29.Elliott MR, Gallo JJ, Ten Have TR, Bogner HR, Katz IR. Using a Bayesian latent growth curve model to identify trajectories of positive affect and negative events following myocardial infarction. Biostatistics. 2005;6:119–143. doi: 10.1093/biostatistics/kxh022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arias E. United States Life Tables. 9. Vol. 56. National vital statistics reports: 2007. 2004. http://www.cdc.gov/nchs/data/nvsr/nvsr56/nvsr56_09.pdf. Accessed 1 December 2011. [PubMed] [Google Scholar]
- 31.U.S. Census Bureau. 2010 U.S. Census Factfinder. 2011. http://factfinder2.census.gov/faces/tableservices/jsf/pages/productview.xhtml?pid=DEC_10_SF1_QTP2&prodType=tableAccessed December 1, 2011. [Google Scholar]
- 32.Willcox BJ, Willcox DC, He Q, Curb JD, Suzuki M. Siblings of Okinawan centenarians share lifelong mortality advantages. J Gerontol A Biol Sci Med Sci. 2006;61:345–354. doi: 10.1093/gerona/61.4.345. [DOI] [PubMed] [Google Scholar]
- 33.Willcox BJ, Willcox DC, Ferrucci L. Secrets of healthy aging and longevity from exceptional survivors around the globe: lessons from octogenarians to supercentenarians. J Gerontol A Biol Sci Med Sci. 2008;63:1181–1185. doi: 10.1093/gerona/63.11.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andersen-Ranberg K, Vasegaard L, Jeune B. Dementia is not inevitable: a population-based study of Danish centenarians. J Gerontol B Psychol Sci. 2001;56:P152–P159. doi: 10.1093/geronb/56.3.p152. [DOI] [PubMed] [Google Scholar]
- 35.Atzmon G, Schechter C, Greiner W, Davidson D, Rennert G, Barzilai N. Clinical phenotype of families with longevity. J Am Geriatr Soc. 2004;52:274–277. doi: 10.1111/j.1532-5415.2004.52068.x. [DOI] [PubMed] [Google Scholar]
- 36.Capurso A, D’Amelio A, Resta F, et al. Epidemiological and socioeconomic aspects of Italian centenarians. Arch Gerontol Geriatr. 1997;25:149–157. [Google Scholar]
- 37.Darviri C, Demakakos P, Charizani F, et al. Assessment of the health status of Greek centenarians. Arch Gerontol Geriatr. 2008;46:67–78. doi: 10.1016/j.archger.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 38.Hagberg B, Bauer Alfredson B, Poon LW, Homma A. Cognitive functioning in centenarians: a coordinated analysis of results from three countries. J Gerontol B Psychol Sci. 2001;56:P141–P151. doi: 10.1093/geronb/56.3.p141. [DOI] [PubMed] [Google Scholar]
- 39.Luczywek E, Gabryelewicz T, Barczak A, et al. Neurocognition of centenarians: neuropsychological study of elite centenarians. Int J Geriatr Psychiatry. 2007;22:1004–1008. doi: 10.1002/gps.1780. [DOI] [PubMed] [Google Scholar]
- 40.Motta M, Ferlito L, Magnolfi SU, et al. Cognitive and functional status in the extreme longevity. Arch Gerontol Geriatr. 2008;46:245–252. doi: 10.1016/j.archger.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 41.Silver MH, Jilinskaia E, Perls TT. Cognitive functional status of age-confirmed centenarians in a population-based study. J Gerontol B Psychol Sci. 2001;56:P134–P140. doi: 10.1093/geronb/56.3.p134. [DOI] [PubMed] [Google Scholar]
- 42.Takayama M, Hirose N, Arai Y, et al. Morbidity of Tokyo-area centenarians and its relationship to functional status. J Gerontol A Biol Sci Med Sci. 2007;62:774–782. doi: 10.1093/gerona/62.7.774. [DOI] [PubMed] [Google Scholar]
- 43.Franceschi C, Motta L, Valensin S, et al. Do men and women follow different trajectories to reach extreme longevity? Italian Multicenter Study on Centenarians (IMUSCE) Aging (Milano) 2000;12:77–84. doi: 10.1007/BF03339894. [DOI] [PubMed] [Google Scholar]
- 44.Perls T, Fretts R. Why women live longer than men. Sci Am Press. 1998:100–107. [Google Scholar]
- 45.Perls TT. The oldest old. Sci Am. 1995;272:70–75. doi: 10.1038/scientificamerican0195-70. [DOI] [PubMed] [Google Scholar]
- 46.Tan Q, Zhao JH, Zhang D, Kruse TA, Christensen K. Power for genetic association study of human longevity using the case-control design. Am J Epidemiol. 2008;168:890–896. doi: 10.1093/aje/kwn205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glenday C, editor. Guinness Book of World Records. New York, NY: Bantam Books; 2010. [Google Scholar]
- 48.Crimmins EM, Beltrán-Sánchez H. Mortality and morbidity trends: is there compression of morbidity? J Gerontol B Psychol Sci Soc Sci. 2011;66:75–86. doi: 10.1093/geronb/gbq088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fraser GE, Shavlik DJ. Ten years of life: Is it a matter of choice? Arch Intern Med. 2001;161:1645–1652. doi: 10.1001/archinte.161.13.1645. [DOI] [PubMed] [Google Scholar]
- 50.Willcox BJ, He Q, Chen R, et al. Midlife risk factors and healthy survival in men. JAMA. 2006;296:2343–2350. doi: 10.1001/jama.296.19.2343. [DOI] [PubMed] [Google Scholar]
- 51.Chakravarty EF, Hubert HB, Lingala VB, Fries JF. Reduced disability and mortality among aging runners: a 21-year longitudinal study. Arch Intern Med. 2008;168:1638–1646. doi: 10.1001/archinte.168.15.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vita AJ, Terry RB, Hubert HB, Fries JF. Aging, health risks, and cumulative disability. N Engl J Med. 1998;338:1035–1041. doi: 10.1056/NEJM199804093381506. [DOI] [PubMed] [Google Scholar]
- 53.Rajpathak SN, Liu Y, Ben-David O, et al. Lifestyle factors of people with exceptional longevity. J Am Geriatr Soc. 2011;59:1509–1512. doi: 10.1111/j.1532-5415.2011.03498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.