In a longitudinal birth cohort conducted in Tanzania, where transmission of Plasmodium falciparum is intense, naturally occurring iron deficiency predicted reduced risk of malaria and mortality among infants and toddlers.
Abstract
(See the Editorial Commentary by Awah and Kaneko, on pages 1145–7.)
Background. Iron supplementation may increase malaria morbidity and mortality, but the effect of naturally occurring variation in iron status on malaria risk is not well studied.
Methods. A total of 785 Tanzanian children living in an area of intense malaria transmission were enrolled at birth, and intensively monitored for parasitemia and illness including malaria for up to 3 years, with an average of 47 blood smears. We assayed plasma samples collected at routine healthy-child visits, and evaluated the impact of iron deficiency (ID) on future malaria outcomes and mortality.
Results. ID at routine, well-child visits significantly decreased the odds of subsequent parasitemia (23% decrease, P < .001) and subsequent severe malaria (38% decrease, P = .04). ID was also associated with 60% lower all-cause mortality (P = .04) and 66% lower malaria-associated mortality (P = .11). When sick visits as well as routine healthy-child visits are included in analyses (average of 3 iron status assays/child), ID reduced the prevalence of parasitemia (6.6-fold), hyperparasitemia (24.0-fold), and severe malaria (4.0-fold) at the time of sample collection (all P < .001).
Conclusions. Malaria risk is influenced by physiologic iron status, and therefore iron supplementation may have adverse effects even among children with ID. Future interventional studies should assess whether treatment for ID coupled with effective malaria control can mitigate the risks of iron supplementation for children in areas of malaria transmission.
Understanding the influence of iron status on the risk of malaria is necessary for planning and implementing iron deficiency (ID) control programs in sub-Saharan Africa. Both ID and malaria are common in this region and are major causes of anemia. ID anemia is associated with impaired cognitive and motor development, reduced growth velocity, and anorexia in children [1, 2]. International guidelines recommend iron and folic acid supplementation in children under 2 years of age in areas with a high prevalence of anemia. Consequently, iron supplementation programs are being implemented in many countries as a primary strategy for preventing ID and anemia in pregnant women and in children.
Although the detrimental effects of ID argue for aggressive intervention, the safety of universal routine iron supplementation remains unclear. Children in a malaria-endemic region of Tanzania who were randomized to receive iron supplements suffered from a 15% increased all-cause mortality [3]. Whereas results from several earlier studies also identified increased malarial risk in individuals treated with iron [4, 5], other studies have not identified risk associated with iron supplementation programs [6–8].
We recently observed that maternal ID is associated with a 5.5-fold reduced prevalence of placental malaria [9], but few studies have examined the role of iron status in modifying malaria risk in unsupplemented children [10]. In the current study, we evaluated the hypothesis that naturally occurring ID confers protection from malaria outcomes in a birth cohort of Tanzanian children. We report that ID protects children from malaria infection, malaria morbidity, and mortality.
MATERIALS AND METHODS
Ethical Approval
Ethical clearance was obtained from the Institutional Review Board of Seattle Biomedical Research Institute and the Medical Research Coordinating Committee of the National Institute for Medical Research, Tanzania.
Study Population
Subjects participated in the Mother–Offspring Malaria Studies (MOMS) project, which is based at Muheza Designated District Hospital (DDH) in northeastern Tanzania. Mothers presenting at Muheza DDH for delivery were enrolled and provided signed, informed consent for themselves and for their newborns before participation in the study. Details of the MOMS study design, enrollment methods, and exclusion criteria have been published elsewhere [9, 11].
Inclusion Criteria and Clinical Monitoring
We monitored 785 children for Plasmodium falciparum infection from birth up to 3 years of age. Children were evaluated at routine, well-child visits by a clinician every 2 weeks from birth to 1 year of age, then monthly thereafter, including blood smear analysis. Routine blood samples were collected at 3, 6, and 12 months of age, then once every 6 months in the second and third years of life. Blood smears and blood samples were also collected any time the child became sick. Sick children were examined by a medical officer upon presentation to the hospital or mobile clinic. Treatment outside the study was minimized by active, weekly surveillance by our mobile clinics.
Clinical malaria was defined as asexual P. falciparum parasitemia by blood smear coupled with symptoms suggestive of malaria such as temperature >37.5°C, nausea or vomiting, irritability, and poor feeding. Prompt treatment was provided to sick children according to the guidelines of the Tanzanian Ministry of Health, and study participants were instructed to obtain all medications including antimalarials through the project staff.
Sample Collection and Processing
Venous blood was collected and stored at 4°C until processing. After centrifugation, plasma was stored at –80°C. P. falciparum parasitemia was determined by Giemsa-stained thick blood smears prepared from capillary or venous blood. Parasite density was expressed as the number of asexual stage parasites/200 white blood cells (WBCs) in the thick smear. Sickle cell trait was determined by electrophoresis (Helena Laboratories, Beaumont, TX). Ferritin, C-reactive protein (CRP), and soluble transferrin receptor (sTfR) were assayed in plasma as described previously [12]. Hemograms were obtained on an impedance-based analyzer (Abbott Cell Dyne 1200).
Case Definitions
Severe malaria was defined according to the World Health Organization criteria [13] as a positive blood smear and one or more of the following: (1) respiratory distress defined by respiratory rate of >40/minute for children older than 2 months of age or a respiratory rate of >50/minute for children less than 2 months of age, in conjunction with clinical signs of respiratory distress; (2) a history of 2 or more convulsions in the 24 hours before or during hospitalization; (3) prostration defined by inability to sit unaided; (4) hypoglycemia defined by glucose <2.2 mmol/L; or (5) severe anemia defined by Hgb <5 g/dL. Hyperparasitemia was defined as parasitemia >2500/200 WBC.
Malaria-associated mortality was defined as death with a positive blood film obtained during the terminal illness. One child who died of bacterial meningitis but had a positive blood film was adjudicated as a nonmalarial death.
ID was defined as ferritin concentration <30 ng/mL when CRP was <8.2 μg/mL (ID in the absence of inflammation) or ferritin concentration <70 ng/mL when CRP was >8.2 μg/mL (ID in the presence of inflammation) [9, 14]. Secondary definitions of ID included the following: ferritin <12 ng/mL [15], ferritin <30 ng/mL [16], ferritin <12 ng/mL and CRP <6 μg/mL or ferritin ≤50 ng/mL and CRP ≥6 μg/mL [17], and sTfR (μg/mL)/log10 ferritin (ng/mL) > 1.5 [18] or 5.6 [19].
Statistical Analysis
Association between parasite density and concurrent body iron status was evaluated using generalized estimating equation (GEE)-based linear regression models to account for the correlation between measurements at multiple time points from the same child. Likewise, associations between prevalence data (ie, parasitemia, hyperparasitemia, or severe malaria) and concurrent iron status were estimated with GEE-based logistic regression models. Cross-sectional analyses considered outcomes concurrent with iron status measures. Longitudinal analyses examined the relationship between iron status measured at aparasitemic routine visits and subsequent risk of a malarial event. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated as estimates of risk. Cox proportional hazards analysis was used to evaluate the impact of iron status on mortality. In Cox models, iron status was coded as an unlagged time-varying covariate [20–22]. All GEE models were adjusted for Hemoglobin S, village, bed net use, low birth weight, and hemoglobin levels. In Cox models, these covariates were assessed and retained if P < .1. Age was considered as both a confounding covariate and as an effect modifier (interaction term) in our models.
RESULTS
Baseline Characteristics of the Study Population
A total of 785 children were included in this study with an average of 113 weeks of follow-up. Table 1 describes the baseline characteristics of the study population. Malaria parasitemia was frequent and intense: 16% (5082 of 32 641) of routine blood smears were positive with a mean parasite density of 829 parasites per 200 WBCs.
Table 1.
Muheza Birth Cohort Characteristics, n = 785
| Low Birth Weighta n (%) | 65 (8.3%) |
| Sex n (%) | |
| Male | 401 (51.1%) |
| Female | 384 (48.9%) |
| Hemoglobin ASb n (%) | 124 (16.1%) |
| Village of residence n (%) | |
| Magila- Mkumba | 130 (16.6%) |
| Muheza Township | 354 (45.1%) |
| Mkanyageni, Mkuzi, Mtindiro | 172 (21.9%) |
| Bwembwera, Potwe | 129 (16.4%) |
| Bed Net Use n (%) | |
| Treated | 111 (14.1%) |
| Untreated | 294 (37.5%) |
| None | 247 (31.5%) |
| Unknown | 133 (16.9%) |
| Hemoglobin g/dL LS mean (SE) | 10.8 (2.8) |
Abbreviations: LS, mean adjusted for repeated measures on an individual over time; SE, standard error.
n = 2 missing birth weight.
n = 13 missing Hemoglobin S type.
Prevalence of ID in the Study Area
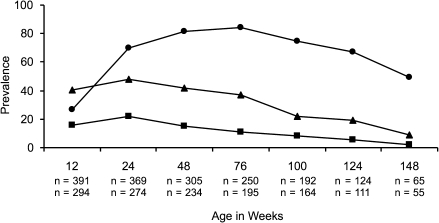
Because parasitemia can increase ferritin levels (resulting in underestimation of the prevalence of ID), we examined the prevalence of ID in samples obtained from aparasitemic children during routine visits (n = 1696). The prevalence of ID was low in neonates but increased rapidly during infancy (Figure 1). At routine, aparasitemic visits, children with ID had significant increases in the odds of moderate anemia (Hgb <8 G/dL, OR [95% CI] = 1.75 [1.13, 2.70], P = .01) and severe anemia (Hgb <5 G/dL, OR [95% CI] = 4.45 [1.94, 10.22], P < .001) compared with children who were iron-replete.
Figure 1.
The prevalence of iron deficiency (ID) (circles), hemoglobin <10 g/dL (triangles), and hemoglobin <8 g/dL (squares) in children by age group. ID and hemoglobin were determined on samples obtained at routine, aparasitemic visits. The sample size was n = 1696 for ID measures and n = 1327 for hemoglobin measures. Age-specific sample sizes reflect ID measures on top row and hemoglobin measures on bottom row.
ID Is Associated With Decreased Prevalence and Intensity of Parasitemia
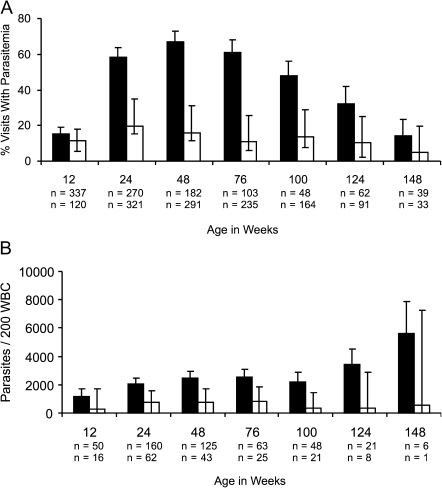
At routine visits (n = 2345), children with ID had 6.6-fold lower odds of concurrent malaria parasitemia compared with children who were iron-replete after adjusting for potential confounders (OR [95% CI] = 0.15 [0.12, 0.19], P < .001; Figure 2A). This effect was significantly modified by age (P < .001).
Figure 2.
Iron deficiency (ID) is associated with lower proportion of visits with parasitemia (A) and lower parasite density (B) in all age groups. Iron status measures were obtained at routine visits. (A) Percentage of visits with parasitemia in children at different age groups stratified by iron status. (B) Mean parasite count/200 white blood cells in parasitemic children at different age groups stratified by iron status. Children with ID had 6.6-fold lower odds of malaria parasitemia (odds ratio [95% confidence interval; CI] = 0.15 [0.12, 0.19], P < .001) and 3.9-fold lower parasite count (P < .001) compared with children with normal iron stores, even after accounting for potential confounders. Visits with normal iron stores (black bars) and ID (white bars) are shown. Samples sizes for visits with normal iron stores are given above samples sizes with ID. Error bars represent 95% CIs. WBC, white blood cells.
At routine, parasitemic visits (n = 649), children with ID had 3.9-fold lower parasite density compared with children who were iron-replete after adjusting for potential confounders (adjusted mean parasite density [standard error] 390 per 200 WBC [59] versus 1526 per 200 WBC [127], P < .001; Figure 2B). This effect was also significantly modified by age (P < .001).
ID Is Associated With Reduced Clinical Malaria
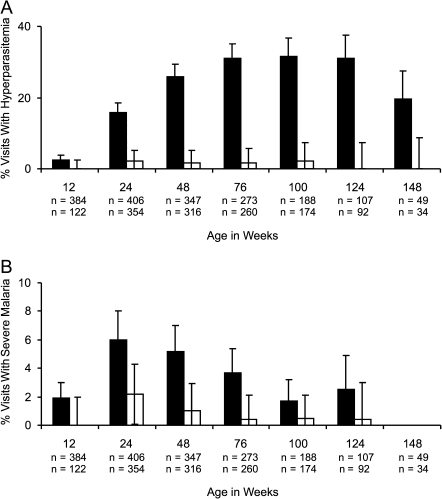
At routine and sick visits (n = 3106), children with ID had a 24.0-fold lower odds of concurrent hyperparasitemia than children who were iron-replete (OR [95% CI] = 0.04 [0.02, 0.07], P < .001) after adjusting for potential confounders. The effect of ID to decrease hyperparasitemia risk became stronger with age (P = .005; Figure 3A). In addition, children with ID had a 4.0-fold lower odds of concurrent severe malaria compared with children who were iron-replete after adjusting for potential confounders (OR [95% CI] 0.25 [0.14, 0.46], P < .001; Figure 3B).
Figure 3.
Iron deficiency (ID) is associated with lower proportion of visits with hyperparasitemia (A) and severe malaria (B). Iron status measures were obtained at both routine and hospital visits. (A) Percentage of visits with hyperparasitemia in different age groups stratified by iron status. (B) Percentage of visits with severe malaria in different age groups stratified by iron status. Children with ID had a 24.0-fold lower odds of hyperparasitemia (odds ratio [OR; 95% confidence interval; CI] = 0.04 [0.02, 0.07], P < .001) and a 4.0-fold lower odds of severe malaria (OR [95% CI] 0.25 [0.14, 0.46], P < .001) than children with normal iron status, even after adjusting for potential confounders. Visits with normal iron stores (black bars) and ID (white bars) are shown. Samples sizes for visits with normal iron stores are given above samples sizes with ID. Error bars represent 95% CIs.
ID Predicts Resistance to Malaria Infection and Disease
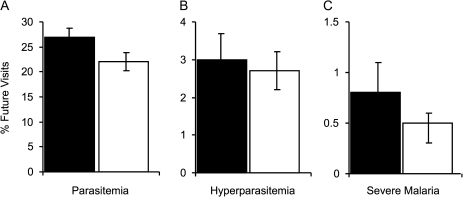
In cross-sectional analyses, inflammation accompanying malaria can lead to misclassification of children with ID as iron-replete (because ferritin is an acute phase reactant), and thus the conclusion that ID protects from concurrent malaria. To avoid this design concern, we prospectively examined the relationship between ID at each iron status determination and risk of future malaria outcomes. We adjusted for potential confounders, the number of iron status measures per child, the average age of the child at their iron status measurements, and the follow-up time interval after each iron status determination (ie, the time interval until next iron status determination or until completion of study). In GEE-based logistic regression models, children who were ID at routine aparasitemic visits had a 1.3-fold decreased odds of parasitemia (OR [95% CI] = 0.77 [0.66, 0.89], P < .001) and a 1.6-fold decreased odds of severe malaria (OR [95% CI] = 0.62 (0.39, 0.98), P = .04) during subsequent months compared with children with normal iron stores, whereas the risk of hyperparasitemia was not different in these groups (P = not significant [NS]). Concordant results were obtained when the proportion of future visits with these outcomes was analyzed in GEE-based linear regression models (Figure 4).
Figure 4.
Iron deficiency (ID) predicts decreased incidence of subsequent malaria events. Iron status, measures were obtained at routine, aparasitemic visits. For each iron status determination, we examined the relationship between iron status and the proportion of future visits with (A) parasitemia, (B) hyperparasitemia, or (C) severe malaria after adjusting for potential confounders and the number of iron status measures per child, the average age of the child at their iron status measurements, and the follow-up time interval. The time interval examined extended until the child had a subsequent iron status determination or completed the study. Children who were ID at routine aparasitemic visits had an 18% decreased mean incidence of parasitemia (P < .001), a 10% decreased mean incidence of hyperparasitemia (P = not significant), and a 38% decreased mean incidence of severe malaria (P = .07) during the subsequent months compared with children without ID. Error bars represent 95% confidence intervals.
ID Predicts Decreased All-Cause and Malaria-Associated Mortality
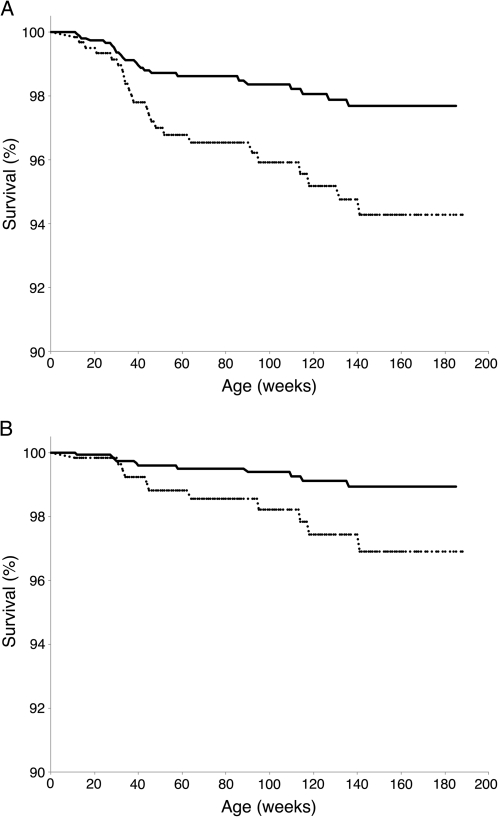
In Cox proportional hazards models, during periods of ID, children had 60% reduced all-cause mortality (HR [95% CI] = 0.40 [0.16, 0.96], P = .04; Figure 5A) and 66% reduced malaria-associated mortality (HR [95% CI] = 0.34 [0.09, 1.3], P = .11; Figure 5B) for the 3 years of follow-up compared with iron-replete periods.
Figure 5.
Iron deficiency (ID) predicts decreased risk of subsequent (A) all-cause mortality and (B) malaria-associated mortality. We evaluated the relationship between iron status measured at well-child, aparasitemic visits and mortality in Cox proportional hazards models. ID status was coded as a time-varying covariate. We assessed Hemoglobin S, village, bed net use, low birth weight, and hemoglobin level as potential confounders; however, none of these variables was associated with mortality at P < .1 and were therefore not included in the model. In each graph, dashed lines indicate normal iron status (totaling 42 135 person weeks) and solid lines indicate iron-deficient status (totaling 45 483 person weeks). During periods of ID, children had 60% reduced all-cause mortality (hazards ratio [HR; 95% confidence interval; CI] = 0.40 [0.16, 0.96], P = .04) and 66% reduced malaria-associated mortality (HR [95% CI] = 0.34 [0.09, 1.3], P = .11) over the 3 years of follow-up compared with iron-replete periods.
DISCUSSION
ID-associated morbidities support international guidelines for iron supplementation in children under 2 years of age in areas with a high prevalence of anemia [1, 2]. Unfortunately, iron plus folate supplementation given during a recent randomized, placebo-controlled trial involving N = 42, 546 children living in a high P. falciparum transmission area resulted in a 15% increase in all-cause mortality [3], and substudy analyses suggested that enhanced malarial morbidity was concentrated in children who were iron-replete at baseline. Although malaria morbidity and mortality were not increased in some other iron supplementation trials [6–8], this result has raised concern over routine iron supplementation programs in malarious areas.
Because iron supplementation may exacerbate malaria outcomes, we examined whether naturally occurring ID confers protection in children residing in a high transmission area of Tanzania. Specifically, we examined the influence of iron status on malaria risk in both cross-sectional and longitudinal analyses in a birth cohort of children who were receiving intensive, active health surveillance and prompt antimalarial treatment. We found that ID was highly prevalent and, despite intensive blood smear monitoring and prompt antimalarial treatment, children who were iron-replete had significantly more malaria infection, morbidity, and mortality.
We identified a high prevalence of ID in our birth cohort which far exceeds that reported from healthy European children [23] but is consistent with results from another study in rural Tanzania [24]. The proportion of mothers with ID at delivery in our cohort was 78.4% [9], which mirrors the ID frequency in their offspring after 1 year (Figure 1). This concordance may reflect a shared iron-poor diet, or inherited, potentially beneficial, mutations at any of several iron transport, storage, regulatory, or heme synthesis genes [25].
Children living in malaria-endemic areas acquire antidisease and antiparasite immunity as they age, and this process is accompanied by the acquisition of antiparasite antibodies [26]. We demonstrate for the first time that ID also provides strong protection against parasitemia and malaria-associated morbidity and mortality during childhood. Because ID modifies malaria risk, iron status should be assessed in studies that evaluate the impact of other factors, such as parasite exposure, genetic polymorphisms, or therapeutic interventions, on malaria outcomes.
Despite many iron supplementation trials in malarious areas, remarkably few studies have examined the relationship between iron status and malaria in unsupplemented populations. In a longitudinal study of children (n = 240) between 8 months and 8 years of age who were living in a low transmission area on the coast of Kenya, those with ID had a 30% lower incidence of mild clinical malaria (any parasitemia accompanied by axillary temperature >37.5°C) during 12 months of follow-up compared with children who were iron-replete [10]. In our study from Tanzania, ID reduced parasitemia (23%) and severe malaria (38%) to a roughly similar degree that it reduced mild clinical malaria risk in Kenya.
Several trials have demonstrated that iron supplementation exacerbates malaria. Iron supplementation increased the risk of malaria among children from Papua New Guinea [4] and the risk of fever associated with malarial parasitemia in Gambian children [27]. Recently, a community-based, randomized, controlled study in Zanzibar found that children who received iron plus folic acid supplementation had significantly increased mortality or hospital admission and had a trend toward increased mortality compared with children who received placebos [3]. In contrast, a recent meta-analysis of iron supplementation trials in malarious areas did not find increased mortality in individuals who were given iron; however, the average length of follow-up in the pooled studies was less than 4 months, the analysis included children up to the age of 18 years, an age at which malaria-associated mortality is limited, and baseline iron status was rarely assessed [28].
The assessment of iron status in the context of infection and inflammation is daunting, and no ideal methods are available [9, 15–19, 29]. We defined iron status primarily based on serum ferritin levels [30]. Because serum ferritin is an acute phase protein and thus may increase during inflammation, some inflamed individuals with ID will be misclassified as iron-replete. We addressed this potential misclassification in several ways. First, we measured CRP concurrently as a marker of inflammation, and we adjusted the threshold ferritin level used to define ID cutoffs based on CRP levels [9, 14, 31]. We recognize that this approach remains susceptible to residual misclassification if the CRP elevation resolves before the ferritin response [32]. Second, we restricted our cross-sectional analyses of concurrent iron status and parasitemia to samples obtained during routine, nonsick visits (Figure 2). Third, we repeated our cross-sectional analyses of the impact of concurrent iron status on parasitemia, hyperparasitemia, and severe malaria (Figure 3) by using several secondary definitions of ID (see Supplementary Table 1). These alternative definitions of ID are reportedly less influenced by concurrent inflammation [15–19, 31], and all demonstrated similar relationships with malaria risk. Fourth, we examined the relationship between ID and future malaria risk, and we restricted our analyses to children who provided samples during routine, aparasitemic visits (Figures 4 and 5). This restriction significantly attenuates the possibility of misclassification of inflamed individuals with ID as iron-replete, and the longitudinal nature of the analysis allows directional inference.
Strikingly, ID measured at aparasitemic routine visits was a strong predictor of decreased mortality (Figure 5). During periods of ID, children had 60% reduced all-cause mortality and 66% reduced malaria-associated mortality over the 3 years of follow-up compared with iron-replete periods. These results may underestimate the decreased mortality attributable to ID because other macronutrient and micronutrient deficiencies that are frequently coincident with ID are causally linked to increased morbidity and mortality [33].
The mechanisms by which ID might limit parasite density are diverse and could involve both parasite and host-specific effects. Malaria parasites acquire iron in a transferrin-independent pathway [34], and chelation of intraerythrocytic iron reduces parasite growth [35]. In the host, chelation of iron increases cellular nitric oxide (NO) production and parasite killing in coculture systems [36] and similarly increases NO production in children being treated for cerebral malaria [37]. Iron inhibits the expression of inducible NO synthase (iNOS), and NO is a principal component of macrophage-mediated cytotoxicity towards P. falciparum [36]. Therefore, ID may amplify iNOS-mediated defenses against this pathogen, and iron chelation therapy has been associated with improved clinical course of cerebral malaria in some [38] but not all studies [39]. In addition, iNOS-mediated defenses control liver stage parasite development [40] and could contribute to the decreased frequency of parasitemia that we observed. Alternatively, ID may reflect concurrent hookworm infection; however, the relationship between hookworm infection and malaria outcomes remains unclear.
ID may pose a direct toxic insult to malarial parasites. Parasites detoxify heme by polymerizing it into crystals of hemozoin in a process that requires Fe+3/carboxylate bonds [41]. During ID erythropoiesis, ferrochelatase inserts zinc rather than iron into protoporphyrin IX [42]. Zinc protoporphyrin (ZPP) binds hemozoin crystals and inhibits crystal elongation, resulting in heme toxicity and possibly reduced parasite growth [43]. Measures of ZPP and the iron regulatory peptide hepcidin may identify the role of iron availability and iron sequestration in mediating protection from malaria in future studies.
Our results in a large birth cohort among children living in an area with intense P. falciparum transmission indicate that ID confers significant protection from malaria morbidity and mortality despite active, intense health surveillance. These findings are consistent with experimental evidence that links ID to reduced parasite fitness and enhanced host resistance. Our results suggest that the relationship between iron status and malaria is a continuum of risk that increases as individuals progress from ID to normal iron stores. For this reason, efforts to focus iron supplementation among individuals with ID may nevertheless increase the risk of malaria morbidity and mortality. These data warrant additional interventional studies to ascertain the benefits and risks of iron supplementation for children living in malaria-endemic regions representing a broad range of transmission pressures, but only if coupled with effective malaria control measures.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments.
We thank the Mother–Offspring Malaria Studies Project staff for their efforts in collecting clinical data, sample processing, and interpreting malaria blood smears. We also thank Sunthorn Pond-Tor for assistance with iron status measures.
Financial support.
This work was supported by grants from the US National Institutes of Health (grant AI52059) and the Bill & Melinda Gates Foundation (grant 1364; to P. E. D.). S. H. received grant support from Seattle BioMed.
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Oski FA, Honig AS, Helu B, Howanitz P. Effect of iron therapy on behavior performance in nonanemic, iron-deficient infants. Pediatrics. 1983;71:877–80. [PubMed] [Google Scholar]
- 2.Sheard NF. Iron deficiency and infant development. Nutr Rev. 1994;52:137–40. doi: 10.1111/j.1753-4887.1994.tb01406.x. [DOI] [PubMed] [Google Scholar]
- 3.Sazawal S, Black RE, Ramsan M, et al. Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community-based, randomised, placebo-controlled trial. Lancet. 2006;367:133–43. doi: 10.1016/S0140-6736(06)67962-2. [DOI] [PubMed] [Google Scholar]
- 4.Oppenheimer SJ, Gibson FD, Macfarlane SB, et al. Iron supplementation increases prevalence and effects of malaria: report on clinical studies in Papua New Guinea. Trans R Soc Trop Med Hyg. 1986;80:603–12. doi: 10.1016/0035-9203(86)90154-9. [DOI] [PubMed] [Google Scholar]
- 5.Smith AW, Hendrickse RG, Harrison C, Hayes RJ, Greenwood BM. The effects on malaria of treatment of iron-deficiency anaemia with oral iron in Gambian children. Ann Trop Paediatr. 1989;9:17–23. doi: 10.1080/02724936.1989.11748589. [DOI] [PubMed] [Google Scholar]
- 6.Iannotti LL, Tielsch JM, Black MM, Black RE. Iron supplementation in early childhood: health benefits and risks. Am J Clin Nutr. 2006;84:1261–76. doi: 10.1093/ajcn/84.6.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mebrahtu T, Stoltzfus RJ, Chwaya HM, et al. Low-dose daily iron supplementation for 12 months does not increase the prevalence of malarial infection or density of parasites in young Zanzibari children. J Nutr. 2004;134:3037–41. doi: 10.1093/jn/134.11.3037. [DOI] [PubMed] [Google Scholar]
- 8.Menendez C, Kahigwa E, Hirt R, et al. Randomised placebo-controlled trial of iron supplementation and malaria chemoprophylaxis for prevention of severe anaemia and malaria in Tanzanian infants. Lancet. 1997;350:844–50. doi: 10.1016/S0140-6736(97)04229-3. [DOI] [PubMed] [Google Scholar]
- 9.Kabyemela ER, Fried M, Kurtis JD, Mutabingwa TK, Duffy PE. Decreased susceptibility to Plasmodium falciparum infection in pregnant women with iron deficiency. J Infect Dis. 2008;198:163–6. doi: 10.1086/589512. [DOI] [PubMed] [Google Scholar]
- 10.Nyakeriga AM, Troye-Blomberg M, Dorfman JR, et al. Iron deficiency and malaria among children living on the coast of Kenya. J Infect Dis. 2004;190:439–47. doi: 10.1086/422331. [DOI] [PubMed] [Google Scholar]
- 11.Mutabingwa TK, Bolla MC, Li JL, et al. Maternal malaria and gravidity interact to modify infant susceptibility to malaria. PLoS Med. 2005;2:e407. doi: 10.1371/journal.pmed.0020407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coutinho HM, McGarvey ST, Acosta LP, et al. Nutritional status and serum cytokine profiles in children, adolescents, and young adults with Schistosoma japonicum-associated hepatic fibrosis, in Leyte, Philippines. J Infect Dis. 2005;192:528–36. doi: 10.1086/430929. [DOI] [PubMed] [Google Scholar]
- 13.WHO. Severe falciparum malaria. World Health Organization, Communicable Diseases Cluster. Trans R Soc Trop Med Hyg. 2000;94(Suppl 1):S1–90. [PubMed] [Google Scholar]
- 14.Leenstra T, Coutinho HM, Acosta LP, et al. Schistosoma japonicum reinfection after praziquantel treatment causes anemia associated with inflammation. Infect Immun. 2006;74:6398–407. doi: 10.1128/IAI.00757-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cook JD, Skikne BS. Iron deficiency: definition and diagnosis. J Intern Med. 1989;226:349–55. doi: 10.1111/j.1365-2796.1989.tb01408.x. [DOI] [PubMed] [Google Scholar]
- 16.Kuvibidila S, Yu LC, Ode DL, Warrier RP, Mbele V. Assessment of iron status of Zairean women of childbearing age by serum transferrin receptor. Am J Clin Nutr. 1994;60:603–9. doi: 10.1093/ajcn/60.4.603. [DOI] [PubMed] [Google Scholar]
- 17.van den Broek NR, Letsky EA, White SA, Shenkin A. Iron status in pregnant women: which measurements are valid? Br J Haematol. 1998;103:817–24. doi: 10.1046/j.1365-2141.1998.01035.x. [DOI] [PubMed] [Google Scholar]
- 18.Punnonen K, Irjala K, Rajamaki A. Serum transferrin receptor and its ratio to serum ferritin in the diagnosis of iron deficiency. Blood. 1997;89:1052–7. [PubMed] [Google Scholar]
- 19.Phiri KS, Calis JC, Siyasiya A, Bates I, Brabin B, van Hensbroek MB. New cut-off values for ferritin and soluble transferrin receptor for the assessment of iron deficiency in children in a high infection pressure area. J Clin Pathol. 2009;62:1103–6. doi: 10.1136/jcp.2009.066498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fleming TR, Harrington D. Counting processes, and survival analysis. New York, NY: John Wiley & Sons; 1991. [Google Scholar]
- 21.Therneau TM, Grambsch PM. Modeling survival data: extending the Cox model. New York, NY: Springer-Verlag; 2000. [Google Scholar]
- 22.Salvan A, Thomaseth K, Bortot P, Sartori N. Use of a toxicokinetic model in the analysis of cancer mortality in relation to the estimated absorbed dose of dioxin (2,3,7,8-tetrachlorodibenzo-p-dioxin, TCDD) Sci Total Environ. 2001;274:21–35. doi: 10.1016/s0048-9697(01)00729-x. [DOI] [PubMed] [Google Scholar]
- 23.Hay G, Sandstad B, Whitelaw A, Borch-Iohnsen B. Iron status in a group of Norwegian children aged 6–24 months. Acta Paediatr. 2004;93:592–8. [PubMed] [Google Scholar]
- 24.Mamiro PS, Kolsteren P, Roberfroid D, Tatala S, Opsomer AS, Van Camp JH. Feeding practices and factors contributing to wasting, stunting, and iron-deficiency anaemia among 3–23-month old children in Kilosa district, rural Tanzania. J Health Popul Nutr. 2005;23:222–30. [PubMed] [Google Scholar]
- 25.Iolascon A, De Falco L, Beaumont C. Molecular basis of inherited microcytic anemia due to defects in iron acquisition or heme synthesis. Haematologica. 2009;94:395–408. doi: 10.3324/haematol.13619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aponte JJ, Menendez C, Schellenberg D, et al. Age interactions in the development of naturally acquired immunity to Plasmodium falciparum and its clinical presentation. PLoS Med. 2007;4:e242. doi: 10.1371/journal.pmed.0040242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith AW, Hendrickse RG, Harrison C, Hayes RJ, Greenwood BM. Iron-deficiency anaemia and its response to oral iron: report of a study in rural Gambian children treated at home by their mothers. Ann Trop Paediatr. 1989;9:6–16. doi: 10.1080/02724936.1989.11748588. [DOI] [PubMed] [Google Scholar]
- 28.Ojukwu JU, Okebe JU, Yahav D, Paul M. Oral iron supplementation for preventing or treating anaemia among children in malaria-endemic areas. Cochrane Database Syst Rev. 2009;(3) doi: 10.1002/14651858.CD006589.pub2. CD006589. [DOI] [PubMed] [Google Scholar]
- 29.Northrop-Clewes CA. Interpreting indicators of iron status during an acute phase response—lessons from malaria and human immunodeficiency virus. Ann Clin Biochem. 2008;45:18–32. doi: 10.1258/acb.2007.007167. [DOI] [PubMed] [Google Scholar]
- 30.WHO. Assessing the iron status of populations: report of a Joint World Health Organization/Centers for Disease Control and Prevention technical consultation on the assessment of iron status at the population level. Geneva, Switzerland: WHO Press; 2004. pp. 6–8. [Google Scholar]
- 31.Kung'u JK, Wright VJ, Haji HJ, et al. Adjusting for the acute phase response is essential to interpret iron status indicators among young Zanzibari children prone to chronic malaria and helminth infections. J Nutr. 2009;139:2124–31. doi: 10.3945/jn.108.104026. [DOI] [PubMed] [Google Scholar]
- 32.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–54. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 33.Pelletier DL, Frongillo EA, Jr, Habicht JP. Epidemiologic evidence for a potentiating effect of malnutrition on child mortality. Am J Public Health. 1993;83:1130–3. doi: 10.2105/ajph.83.8.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanchez-Lopez R, Haldar K. A transferrin-independent iron uptake activity in Plasmodium falciparum-infected and uninfected erythrocytes. Mol Biochem Parasitol. 1992;55:9–20. doi: 10.1016/0166-6851(92)90122-z. [DOI] [PubMed] [Google Scholar]
- 35.Hershko C, Peto TE. Deferoxamine inhibition of malaria is independent of host iron status. J Exp Med. 1988;168:375–87. doi: 10.1084/jem.168.1.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fritsche G, Larcher C, Schennach H, Weiss G. Regulatory interactions between iron and nitric oxide metabolism for immune defense against Plasmodium falciparum infection. J Infect Dis. 2001;183:1388–94. doi: 10.1086/319860. [DOI] [PubMed] [Google Scholar]
- 37.Weiss G, Thuma PE, Mabeza G, Werner ER, Herold M, Gordeuk VR. Modulatory potential of iron chelation therapy on nitric oxide formation in cerebral malaria. J Infect Dis. 1997;175:226–30. doi: 10.1093/infdis/175.1.226. [DOI] [PubMed] [Google Scholar]
- 38.Gordeuk V, Thuma P, Brittenham G, et al. Effect of iron chelation therapy on recovery from deep coma in children with cerebral malaria. N Engl J Med. 1992;327:1473–7. doi: 10.1056/NEJM199211193272101. [DOI] [PubMed] [Google Scholar]
- 39.Thuma PE, Mabeza GF, Biemba G, et al. Effect of iron chelation therapy on mortality in Zambian children with cerebral malaria. Trans R Soc Trop Med Hyg. 1998;92:214–8. doi: 10.1016/s0035-9203(98)90753-2. [DOI] [PubMed] [Google Scholar]
- 40.Klotz FW, Scheller LF, Seguin MC, et al. Co-localization of inducible-nitric oxide synthase and Plasmodium berghei in hepatocytes from rats immunized with irradiated sporozoites. J Immunol. 1995;154:3391–5. [PubMed] [Google Scholar]
- 41.Pagola S, Stephens PW, Bohle DS, Kosar AD, Madsen SK. The structure of malaria pigment beta-haematin. Nature. 2000;404:307–10. doi: 10.1038/35005132. [DOI] [PubMed] [Google Scholar]
- 42.Rettmer RL, Carlson TH, Origenes ML, Jack RM, Labb RF. Zinc protoporphyrin/heme ratio for diagnosis of preanemic iron deficiency. Pediatrics. 1999;104:e37. doi: 10.1542/peds.104.3.e37. [DOI] [PubMed] [Google Scholar]
- 43.Iyer JK, Shi L, Shankar AH, Sullivan DJ., Jr Zinc protoporphyrin IX binds heme crystals to inhibit the process of crystallization in Plasmodium falciparum. Mol Med. 2003;9:175–82. doi: 10.2119/2003-00010.sullivan. [DOI] [PMC free article] [PubMed] [Google Scholar]