HIV infection is associated with decreased thrombin generation and an increased antithrombin level. These data suggest that HIV infection may not be associated with an increased propensity towards clotting.
Abstract
Background. Excess risk of cardiovascular disease occurs in effectively treated individuals with human immunodeficiency virus (HIV) infection. Although elevated plasma D-dimer levels are associated with increased morbidity and mortality, the impact of HIV infection on coagulation in vivo has not been well studied.
Methods. We measured D-dimers, antithrombin, endogenous thrombin potential (ETP; a functional measure of thrombin generation in vitro), thrombin/antithrombin complexes (TAT; a measure of thrombin generation in vivo), tissue factor, prothrombin fragment 1 + 2 (F1+2), and normalized APC sensitivity ratio (nAPCsr) in 199 HIV-positive men who were receiving antiretroviral therapy and had an undetectable HIV RNA level, in 79 HIV-positive untreated men, and in 39 uninfected controls.
Results. Median antithrombin levels were higher while the ETP was lower among HIV-infected adults (treated and untreated), compared with controls. There were few differences between coagulation markers in the 2 HIV groups. Compared with controls, the nAPCsr was lower in treated men and the TAT level was lower in untreated individuals. We observed little difference among measured levels of D-dimer, tissue factor, or F1+2 between HIV-infected individuals and controls. Antiretroviral therapy exposure was associated with a lower antithrombin level, a lower nAPCsr, and a lower ETP, while history of opportunistic infection was associated with a higher nAPCsr.
Conclusions. HIV infection is associated with decreased thrombin generation, as measured by the ETP, and an increased antithrombin level. These data suggest that HIV infection may not be associated with increased propensity toward clotting, as has been suggested on the basis of isolated measures of D-dimer levels.
The underlying mechanisms by which human immunodeficiency virus (HIV) infection and/or antiretroviral therapy are associated with cardiovascular events remain controversial but likely involve chronic inflammation [1]. Biomarker analysis in the SMART study revealed that higher levels of inflammation and coagulation biomarkers (high-sensitivity C-reactive protein [hsCRP], interleukin-6, and D-dimer) were associated with a higher risk of all-cause mortality [2]. Even among HIV-infected individuals with a HIV RNA load of ≤400 copies/mL, levels of these markers (and of cystatin C) were significantly higher than those in uninfected controls in the MESA study [3], suggesting that mortality among HIV-positive individuals with a treatment-suppressed viral load is linked to inflammatory and coagulation pathways. Data from the FRAM study also showed that hsCRP and fibrinogen were independent predictors of mortality [4]. The observations that D-dimer and fibrinogen levels are abnormally elevated and associated with disease progression among treated HIV-infected adults suggest that abnormalities in the coagulation system may be important in the pathogenesis of this disease. While acute inflammation is a potent prothrombotic stimulus [5], the mechanisms leading to thrombosis are complex and include a tightly regulated interaction between procoagulant and anticoagulant factors [6]. Initiation of antiretroviral therapy in severely immunocompromised HIV-infected individuals resulted in improvement of coagulation markers, although values remained abnormal compared with those for controls [7].
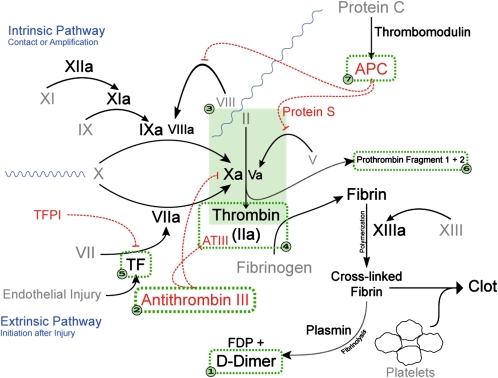
A comprehensive evaluation of the coagulation system in contemporary HIV-infected individuals with treatment-suppressed viral loads has not been described. Our objective was to conduct a detailed analysis of the coagulation system (Figure 1) in individuals with HIV infection, as compared with uninfected controls. Our assessment included the following markers: (1) D-dimers (a fibrin degradation product), (2) antithrombin (AT; an endogenous thrombin inhibitor), (3) endogenous thrombin potential (ETP; a functional measure of thrombin generation in vitro), (4) thrombin-antithrombin complexes (TAT; an integrated measure of thrombin generation over time in vivo), (5) tissue factor (TF; the integral membrane protein known to initiate thrombin formation), (6) prothrombin fragment 1 + 2 (F1+2; activation peptide generated during conversion of prothrombin to thrombin), and (7) normalized activated protein C (APC) sensitivity ratio (nAPCsr; a measure of the sensitivity to APC, the known inhibitor of thrombin generation).
Figure 1.
Coagulation pathway. We assessed the following 7 components of the coagulation cascade: (1) D-dimer, fibrin degradation product (FDP); (2) antithrombin III (antithrombin, AT), circulating inhibitor of thrombin; (3) endogenous thrombin potential (ETP), functional measure of thrombin generation in vitro; (4) thrombin-antithrombin complexes (TAT), antibodies to the thrombin-antithrombin complex, which is a measure of thrombin generation in vivo; (5) tissue factor (TF), protein needed for initiation of thrombin formation; (6) prothrombin fragment 1 + 2 (F1+2), activation peptide generated during conversion of prothrombin to thrombin; and (7) normalized activated protein C (APC) sensitivity ratio (nAPCsr), ETP with and without addition of APC, which is a measure of resistance to the enzymatic activity of APC.
MATERIALS AND METHODS
Study Participants
Study participants were recruited from clinical cohorts at San Francisco General Hospital between 2007 and 2009. For this analysis, we included the following individuals (1) HIV-seropositive men who were receiving combination antiretroviral therapy and had undetectable plasma HIV RNA levels, (2) HIV-seropositive men who were untreated and had a detectable HIV RNA load, and (3) HIV-seronegative men. Further details are provided in the supplementary materials.
Laboratory Assays
Venipuncture was performed on subjects who fasted for 12 hours, using a 21-gauge needle with a tourniquet. The first 5 mL of blood was discarded, and an additional 5 mL of blood was then drawn into Vacutainer tubes containing 3.8% sodium citrate. Whole blood was centrifuged at 1500× g for 10 minutes, and plasma was isolated and frozen at −80°C until use. Standard enzyme-linked immunosorbant assays for D-dimer (Asserachrom, DiagnosticaStago), TAT (Siemens), Prothrombin F1+2 (Siemens), and tissue factor (American Diagnostica) were performed on plasma samples according to the manufacturer’s protocol. Antithrombin activity was measured on plasma samples, using the 2-stage Actichrome antithrombin kit according to the manufacturer’s protocol (American Diagnostica). The ETP was measured using a fluorogenic thrombin substrate on a multiwell automated fluorescent plate reader (ThrombinoSCOPE, Netherlands), according to the manufacturer’s protocol. The nAPCsr was determined by dividing the ETP with and the ETP without 20 nM APC (Enzyme Research Laboratories).
Statistical Methods
Summary statistics of baseline demographic and clinical characteristics were compared using the Mann-Whitney U test for continuous parameters and the Fisher exact test for categorical parameters. Multivariable linear and relative risk regression were used to investigate the association of HIV status with coagulation markers. Candidate HIV-related factors were selected by means of Bayesian model averaging, using the BMA package for the R statistical computing language (R Development Core Team, Vienna, Austria). All other analyses were conducted using the SAS system, version 9.2 (SAS Institute, Cary, NC). Further details are provided in the supplementary materials.
RESULTS
Coagulation markers were measured in 199 HIV-infected antiretroviral-treated men, 79 HIV-infected untreated men, and 39 HIV-uninfected men, whose baseline characteristics are presented in Table 1. Compared with controls, treated HIV-infected men were older, had a greater prevalence of hypertension, and had a greater prevalence of dyslipidemia. Median current and nadir CD4+ T-cell counts were 459 and 100 cells/μL, respectively, among the treated individuals, and the median duration of highly active antiretroviral therapy was 7.8 years. Compared with the controls, the untreated HIV-infected men had lower low-density lipoprotein and high-density lipoprotein cholesterol values; 37% had HIV RNA levels >10 000 copies/mL.
Table 1.
Demographic and Clinical Characteristics of Men With and Men Without Human Immunodeficiency Virus (HIV) Infection
| HIV-Infected Men |
|||||
| Parameter | Receiving HAART, Undetectable HIV RNA Load (n = 199) | P (vs Uninfected Controls) | Untreated, Detectable HIV RNA Load (n = 79) | P (vs Uninfected Controls) | Uninfected Controls (n = 39) |
| Age (y) | 50 (45–58) | <.0001 | 45 (39–52) | .38 | 44 (34–52) |
| Race | |||||
| White | 141 (71) | .69 | 40 (51) | .037 | 27 (69) |
| African American | 26 (13) | 30 (38) | 6 (15) | ||
| Latino | 23 (12) | 7 (9) | 3 (8) | ||
| Other | 9 (5) | 2 (3) | 3 (8) | ||
| History of CAD | 15 (8) | .14 | 1 (1) | >.99 | 0 |
| Cigarette smoking | |||||
| Current | 45 (23) | .71 | 31 (39) | .35 | 11 (28) |
| Past | 60 (30) | 16 (20) | 12 (31) | ||
| Never | 93 (47) | 32 (41) | 16 (41) | ||
| Diabetes mellitus | 17 (9) | .083 | 6 (8) | .18 | 0 |
| Hypertension | 79 (40) | .0009 | 19 (24) | .22 | 5 (13) |
| Hypolipidemic agent use | 69 (35) | .023 | 5 (6) | .18 | 6 (15) |
| LDL-C level (mg/dL) | 107 (87–134) | .033 | 98 (80–115) | .0009 | 124 (96–151) |
| HDL-C level (mg/dL) | 44 (37–54) | .014 | 42 (35–51) | .0035 | 48 (45–59) |
| Triglyceride level (mg/dL) | 136 (92–237) | <.0001 | 89 (66–142) | .26 | 86 (58–122) |
| Total cholesterol level (mg/dL) | 185 (161–219) | .28 | 169 (139–193) | .0003 | 199 (171–220) |
| hsCRP level (mg/L) | 2.0 (0.9–4.8) | .13 | 1.9 (0.8–4.7) | .31 | 1.3 (0.5–3.8) |
| BMIa | 25 (23–28) | .21 | 26 (23–28) | .68 | 25 (24–28) |
| HCV infection | 29 (15) | 17 (22) | 2 (5) | ||
| Duration of HIV infection (y) | 16 (9–21) | 9 (3–17) | |||
| ART use (ever) | 199 (100) | 15 (19) | |||
| Current CD4+ T-cell count (cells/mm3) | 459 (248–674) | 499 (360–612) | |||
| Plasma HIV RNA load (copies/mL) | 100 (20–300) | 371 (286–500) | |||
| <75 | 199 (100) | 0 | |||
| 75–1999 | 0 | 30 (38) | |||
| 2000–9999 | 0 | 20 (25) | |||
| >10 000 | 0 | 29 (37) | |||
Data are median (interquartile range) or no. (%) of subjects.
Bold numbers indicate a P value of < .05.
Abbreviations: ART, antiretroviral therapy; CAD, coronary artery disease; HAART, highly active antiretroviral therapy; HCV, hepatitis C virus; HDL, high-density lipoprotein cholesterol; hsCRP, high-sensitivity C-reactive protein; LDL-C, low-density lipoprotein cholesterol.
Body mass index (BMI) is defined as the weight in kilograms divided by the square of the height in meters.
Levels of Coagulation Markers
Baseline levels of coagulation markers are presented in Table 2. Antithrombin activity was higher in both treated and untreated HIV-infected men, compared with controls. The ETP was lower in treated and untreated HIV-infected men than in controls. The nAPCsr was lower in treated men, compared with controls (median 0.67 vs. 0.96, P = .017), while TAT was lower in untreated individuals, compared with controls (3.3 μg/L vs. 4.7, P < .01). Values for antithrombin, TAT, and D-dimers were mostly within the normal reference ranges, with the exception of F1+2 (Supplementary Table 1). Levels of F1+2, tissue factor, and D-dimers were similar in the 3 groups.
Table 2.
Descriptive Summary of Coagulation Markers at Baseline in Men With and Men Without Human Immunodeficiency Virus (HIV) Infection
| HIV-Infected Men |
|||
| Parameter | Receiving HAART, Undetectable HIV RNA Load (n = 199) | Untreated, Detectable HIV RNA Load (n = 79) | Uninfected Controls (n = 39) |
| Antithrombin level (%) | |||
| Median (IQR) | 108 (82–126)a | 109 (84–127)a | 85 (67–102) |
| Mean ± SD | 104 ± 33 | 106 ± 32 | 87 ± 29 |
| TAT level (μg/L) | |||
| Median (IQR) | 3.7 (2.7–6.9) | 3.3 (2.5–4.9)a | 4.7 (3.0–11.0) |
| Mean ± SD | 8.3 ± 11.6 | 7.2 ± 11.9 | 9.6 ± 12.6 |
| F1+2 level (pM) | |||
| Median (IQR) | 318 (218–653) | 381 (235–836) | 396 (240–905) |
| Mean ± SD | 618 ± 688 | 760 ± 805 | 811 ± 837 |
| D-dimer level (FEU) | |||
| Median (IQR) | 308 (127–585) | 361 (181–591) | 315 (115–624) |
| Mean ± SD | 382 ± 303 | 431 ± 318 | 382 ± 292 |
| nAPCsr | |||
| Median (IQR) | 0.67 (0.31–1.17)b | 0.89 (0.47–1.3) | 0.96 (0.52–1.49) |
| Mean ± SD | 0.81 ± 0.66 | 0.98 ± 0.72 | 1.17 ± 0.91 |
| Tissue factor level (pg/mL, interpolated) | |||
| Median (IQR) | 85.1 (67.7–106.1) | 80.8 (65.8–107.8) | 90.0 (74.3–115.1) |
| Mean ± SD | 91.3 ± 33.4 | 90.7 ± 35.8 | 95.1 ± 29.4 |
| ETP (nM thrombin) | |||
| Median (IQR) | 1247 (1022–1462)c | 1223 (981–1419)c | 1465 (1272–1614) |
| Mean ± SD | 1240 ± 392 | 1217 ± 417 | 1468 ± 297 |
Abbreviations: ETP, endogenous thrombin potential; FEU, fibrinogen equivalent units; F1+2, prothrombin fragment 1 + 2; HAART, highly active antiretroviral therapy; HIV, human immunodeficiency virus; IQR, interquartile range; nAPCsr, normalized activated protein C sensitivity ratio; SD, standard deviation; TAT, thrombin-antithrombin complexes.
P < .01, compared with uninfected control men.
P < .05, compared with uninfected control men.
P < .001, compared with uninfected control men.
Multivariable Analysis of HIV Infection and Coagulation Markers
We next examined the association of HIV infection with antithrombin activity, nAPCsr, ETP, and TAT in multivariable analysis. As noted above, in unadjusted analysis, antithrombin activity was higher in both treated and untreated HIV-infected persons, compared with controls. After controlling for age, race, and hsCRP level, HIV infection remained associated with higher levels of antithrombin activity (Table 3). Both treated and untreated HIV infection were associated with lower ETP, even after multivariable adjustment. Results were similar in a sensitivity analysis in which we used all available coagulation markers in a repeated measures analysis (Supplementary Table 2). Treated HIV infection was associated with lower nAPCsr (−0.25; P = .044), compared with controls, but the association weakened after controlling for age, race, and hsCRP level (−0.22; P = .12).
Table 3.
Association of Human Immunodeficiency Virus (HIV) Infection With Baseline Coagulation Markers
| HAART Use/Undetectable HIV RNA Load (n = 199) vs Uninfected Controls (n = 39) |
Untreated/Detectable HIV RNA Load (n = 79) vs Uninfected Controls (n = 39) |
|||
| Outcome | Estimate (95% CI) | P | Estimate (95% CI) | P |
| Antithrombin level (%) | ||||
| Unadjusted | 17.9 (7.1–28.0) | <.0001 | 17.3 (5.7–30.0) | .0049 |
| Adjusted for age and race | 16.8 (5.2–28.3) | .0024 | 17.7 (5.3–31.7) | .0039 |
| Adjusted for age, race, and hsCRP level | 16.1 (4.2–27.9) | .0068 | 18.0 (4.6–31.4) | .0070 |
| nAPCsr | ||||
| Unadjusted | −0.25 (−.50 to −.011) | .044 | −0.022 (−.33 to .25) | .84 |
| Adjusted for age and race | −0.22 (−.50 to .026) | .088 | −0.055 (−.40 to .23) | .67 |
| Adjusted for age, race, and hsCRP level | −0.22 (−.51 to .042) | .12 | −0.002 (−.34 to .28) | .99 |
| ETP (nM thrombin) | ||||
| Unadjusted | −186.2 (−285.5 to −91.6) | <.0001 | −191.3 (−313.0 to −58.0) | .0025 |
| Adjusted for age and race | −170.2 (−292.4 to −61.9) | .0019 | −171.8 (−312.4 to −33.3) | .016 |
| Adjusted for age, race, and hsCRP level | −169.5 (−292.3 to −69.6) | .0005 | −202.3 (−348.5 to −54.3) | .0034 |
Estimates of HIV infection vs control effects were determined by means of robust linear regression models.
Bold numbers indicate a P value of < .05.
Abbreviations: CI, confidence interval; ETP, endogenous thrombin potential; HAART, highly active antiretroviral therapy; HIV, human immunodeficiency virus; hsCRP, high-sensitivity C-reactive protein; nAPCsr, normalized activated protein C sensitivity ratio.
The prevalence of elevated TAT level (defined as >11 μg/L) was less frequent in treated HIV-infected (18.2%) and untreated HIV-infected (11.4%), compared with controls (25.6%), although the difference in relative risk (RR) did not reach statistical significance (treated vs control: RR, 0.71 [P = .27]; untreated vs control: RR, 0.44 [P = .051]). Little change in risk was seen after adjustment for demographic characteristics and hsCRP level (data not shown).
HIV-infected individuals with an elevated hsCRP level (ie, >3 mg/L) had levels of antithrombin, TAT, and F1+2 and a nAPCsr that were similar to those with an hsCRP level of <1 mg/L (Supplementary Table 3). By contrast, levels of D-dimer and ETP were elevated and the level of tissue factor was lower in HIV-infected individuals with hsCRP level of >3 mg/L, compared with those with hsCRP level of <1. However, levels of ETP and D-dimer in HIV-infected individuals with a hsCRP level of >3 mg/dL were not significantly higher than those in controls.
Sensitivity analyses were performed that excluded HCV infected persons, because most pro- and anti-coagulant proteins are made or modified in the liver, and HCV infection is associated with lower hsCRP [8]. The association of HIV infection with coagulation markers showed little change in sensitivity analyses which excluded hepatitis C virus (HCV)–infected persons (data not shown). We found no statistically significant interactions between HCV infection and hsCRP level with respect to the coagulation markers.
Association of HIV-Related Factors With Coagulation Markers
Because HIV infection was associated with different levels of several markers, even after adjustment for demographic characteristics and hsCRP level, we next examined models in HIV-infected participants alone, to investigate associations with HIV-specific characteristics. There appeared to be little association between age, race, or HIV-related factors and antithrombin level, with the exception of tenofovir exposure, which was associated with lower levels of this biomarker (–2.6% per year of exposure; P = .020) (Table 4).
Table 4.
Multivariable Analysis of Factors Associated With Levels of Coagulation Markers Among 278 Men With Human Immunodeficiency Virus Infection
| Outcome |
||||||
| AT Level |
nAPCsr |
ETP |
||||
| Parameter | Estimate (95% CI) | P | Estimate (95% CI) | P | Estimate (95% CI) | P |
| Treated/undetectable vs untreated/detectable | 8.5 (−2.1 to 19.2) | .12 | −0.12 (−.31 to .08) | .23 | 118.6 (−18.3 to 255.6) | .089 |
| Age (per decade) | −0.8 (−5.1 to 3.4) | .69 | 0.02 (−.07 to .10) | .69 | −4.2 (−56.8 to 48.4) | .88 |
| African American vs white | 2.2 (−7.8 to 12.2) | .66 | 0.02 (−.18 to .21) | .86 | −41.7 (−169.8 to 86.4) | .52 |
| Latino/other vs white | 3.1 (−7.6 to 13.9) | .57 | −0.02 (−.23 to .19) | .87 | 30.1 (−106.7 to 167.0) | .66 |
| Prior opportunistic infection | −2.7 (−11.8 to 6.3) | .55 | 0.39 (.21 to .56) | <.0001 | 115.5 (.6 to 230.5) | .049 |
| NNRTI (per year) | −0.7 (−2.0 to .6) | .28 | −0.03 (−.06 to −.01) | .013 | −19.5 (−35.6 to −3.4) | .019 |
| Didanosine (ever) | 0.5 (−9.5 to 10.6) | .92 | −0.32 (−.51 to −.12) | .0014 | … | |
| HCV infection | 2.8 (−8.5 to 14.2) | .62 | … | −307.8 (−452.3 to −163.3) | <.0001 | |
| BMIa | −0.4 (−1.3 to .4) | .32 | … | 18.7 (7.7 to 29.8) | .0012 | |
| Tenofovir (per year) | −2.6 (−4.7 to −.4) | .020 | … | −42.3 (−68.5 to −16.1) | .0020 | |
| hsCRP (per doubling) | 1.2 (−1.1 to 3.5) | .31 | … | 31.3 (2.1–60.6) | .036 | |
Estimates are calculated from linear mixed models with random intercepts and slopes to account for repeated measures.
Bold numbers indicate a P value of < .05.
Abbreviations: AT, antithrombin III; CI, confidence interval; ETP, endogenous thrombin potential; HCV, hepatitis C virus; hsCRP, high-sensitivity C-reactive protein; nAPCsr, normalized activated protein C sensitivity ratio; NNRTI, nonnucleoside reverse-transcriptase inhibitor.
Body mass index (BMI) is defined as the weight in kilograms divided by the square of the height in meters.
History of opportunistic infection was associated with a higher nAPCsr, while cumulative exposure to nonnucleoside reverse-transcriptase inhibitors (NNRTIs) and any exposure to didanosine were independently associated with a lower nAPCsr. We found little association between nadir CD4+ T-cell count and nAPCsr (data not shown). An elevated nAPCsr suggests either genetic or acquired resistance to APC and is a known risk factor for venous thrombotic events [9]. Factors independently associated with a lower ETP included HCV infection, cumulative exposure to tenofovir, and cumulative exposure to NNRTI, while hsCRP level, body mass index, and history of opportunistic infection were associated with a higher ETP.
In both unadjusted and adjusted analyses, a higher hsCRP level was associated with a decreased risk of having an elevated TAT level, while exposure to tenofovir was associated with an increased risk of having an elevated TAT level (Table 5). Abacavir use had little association with antithrombin level, nAPCsr, ETP, or TAT level (data not shown).
Table 5.
Factors Associated With Elevated Thrombin-Antithrombin III (TAT) Levels Among 278 Men With Human Immunodeficiency Virus Infection
| Unadjusted |
Adjusted |
|||
| Parameter | Relative Risk (95% CI) | P | Relative Risk (95% CI) | P |
| Treated/undetectable vs untreated/detectable | 1.30 (.78–2.16) | .32 | 0.64 (.28–1.47) | .30 |
| Age (per decade) | 1.09 (.87–1.38) | .46 | 1.08 (.84–1.39) | .54 |
| African American vs white | 0.94 (.56–1.58) | .81 | 1.10 (.59–2.05) | .76 |
| Latino/other vs white | 0.76 (.42–1.38) | .37 | 0.84 (.44–1.63) | .61 |
| hsCRP (per doubling) | 0.84 (.74–.96) | .0078 | 0.84 (.74–0.96) | .0089 |
| Tenofovir (ever) | 1.61 (1.06–2.45) | .024 | 2.87 (1.34–6.16) | .0069 |
A TAT level in the highest quartile (ie, >11 μg/L) is considered to be elevated. Estimates are calculated from a relative risk regression model, using generalized estimating equations to account for repeated measures.
Bold numbers indicate a P value of < .05.
Abbreviations: CI, confidence interval; hsCRP, high-sensitivity C-reactive protein.
DISCUSSION
In this cross-sectional analysis of coagulation-related markers in HIV-infected individuals, we found higher antithrombin activity and lower ETP in both untreated and effectively treated HIV adults, compared with uninfected controls. Treated individuals with undetectable HIV RNA levels had a lower nAPCsr, compared with controls, while untreated individuals had lower TAT levels. We did not find statistically significant differences in F1+2, tissue factor, or D-dimer levels in our study population. While the antithrombin level was higher in the HIV-infected group, we were not able to identify any HIV-related factors that were associated with this finding. In contrast, HIV-related factors, including a history of opportunistic infection, were associated with a higher nAPCsr, while ART exposure was associated with lower nAPCsr and ETP. Collectively, these comprehensive data suggest that the coagulation system—as defined by a series of well-validated biomarkers—is affected by HIV infection in a complex manner. The clinical significance of this effect is difficult to prove given the complicated changes observed in our study and other studies and the inability of any coagulation biomarker (including D-dimer level, which is commonly studied in HIV disease) to definitively characterize the degree to which coagulation is altered in vivo. Although these limitations preclude definitive conclusions, the most parsimonious interpretation of our data is that treated HIV infection is not associated with excess clotting (as suggested by prior studies, which relied entirely on measures of D-dimer levels) but may be associated with a lower risk of clotting (as suggested by the reduced ETP, lower nAPCsr, and elevated antithrombin level). This observation should be interpreted cautiously and will need to be replicated. Given the lack of consistency among biomarker changes in this and other studies, we believe there is strong rationale to perform well-controlled studies with hard clinical end points as outcomes to help resolve the role of coagulation system activation in HIV-associated morbidity and mortality.
Systemic inflammation is thought to shift the normal hemostatic balance to a net procoagulant state [5, 6, 10]. Thrombin is the main effector protease in the coagulation cascade. Its actions are varied and include anticoagulant/procoagulant, inflammatory, and proliferative effects [5]. Antithrombin functions as a circulating inhibitor of thrombin. We found that antithrombin activity was higher than expected in effectively treated and untreated HIV-infected individuals. While antithrombin levels have previously been shown to be significantly lower in patients with acute inflammatory conditions such as pneumonia [11] and sepsis [12], there are scant data on the effects of chronic inflammation on antithrombin levels. A single small study before the era of combination antiretroviral therapy showed normal levels of antithrombin and no association of antithrombin with degree of immunosuppression [13]. Further studies are needed to confirm our findings of high antithrombin activity among HIV-infected individuals.
ETP, a functional measure of thrombin generation in vitro, measures the total amount of thrombin generated in response to a standard triggering dose of tissue factor. ETP thus integrates all of the procoagulant and anticoagulant factors affecting thrombin generation. We found that HIV-infected individuals had a lower ETP, compared with controls. Among individuals without HIV infection, a higher ETP is predictive of an incident venous thrombosis event [14]. While a higher ETP did not predict recurrent events among individuals with a deep vein thrombosis [15], lower ETP was associated with a decreased risk of recurrence of deep vein thrombosis [16]. A study consisting mostly of South African women with advanced HIV infection also demonstrated that, while ETP increased with antiretroviral therapy, it remained significantly lower than that in uninfected controls [7]. In contrast to our study, a lower ETP has been reported among HIV-infected individuals receiving antiretroviral therapy, compared with untreated patients [17]. The clinical implications of the lower ETP in our study and other studies remain uncertain.
While it is tempting to attribute coagulation abnormalities to chronic inflammation in the setting of HIV infection, our results demonstrate little relationship between hsCRP level and coagulation factors; in fact (as shown in Table 3), adjustment for hsCRP level does not attenuate the effect of HIV infection. One possibility is that hsCRP level may not be the best assessment of inflammation among HIV-infected individuals, compared with other immunologic measures. For example, in the FRAM study, HIV RNA levels were not significantly associated with hsCRP level [18]. Also, antiretroviral therapy has been consistently shown to decrease levels of most markers of immune activation and inflammation [19, 20], but it has a limited effect on hsCRP level and in some controlled studies resulted in increased levels [8, 21]. Hence, it is not possible with our current data to investigate whether elevations in levels of inflammatory markers known to be affected by HIV infection predict alterations in the coagulation system.
In contrast to other studies [7, 15, 22], our study revealed a lower nAPCsr among individuals with effectively treated HIV infection. The differences between our findings and those of Jong et al may be attributable to differences in the study population, as the treatment response was highly variable [22]. Interestingly, 6% of HIV-infected adults in our study had a nAPCsr of 0, compared with none of the controls (P = .22). To our knowledge, there has never been a description of patients with abnormally low nAPCsr. Additional larger studies will be needed to explore this finding, which has not been previously reported among HIV-infected individuals.
Collectively, we found that patients with treatment-suppressed HIV infection have increased antithrombin activity and increased APC sensitivity. Both of these findings reflect a shift in the balance of procoagulant and anticoagulant factors toward more inhibition of thrombin rather than increased thrombin generation or activity. This is supported by the observation of a lower ETP, which is an ex vivo measurement of coagulation that incorporates many of the varied factors affecting thrombin generation. The clinical implications of these findings are unclear.
Our study has important limitations. Most notably, our study included only men, and we had a relatively small number of control subjects. This could explain the discrepancy between our study and the SMART study in terms of D-dimer levels. However, although our control group was small, we found D-dimer levels that were consistent with those reported in other large cohort studies (ie, ARIC, MESA, CHS) [23–25], suggesting that our trends were not influenced by unusually high levels of D-dimers in our control group. Of note, after unit conversion, the D-dimer levels in our HIV-infected individuals (308 fibrinogen equivalent units [FEU] in treated individuals, and 361 FEU in untreated individuals) are below the range of those reported at study entry for HIV-infected individuals in the SMART study (0.26 μg/mL, or 520 FEU), suggesting our HIV-infected group was relatively healthier [2]. However, the fact that we did not detect differences in D-dimer levels between HIV-infected patients and controls should offer caution in terms of overinterpreting the findings for the other markers in our study. In particular, the ETP results show that the potential for thrombin generation is decreased in plasma from HIV-infected individuals. This is seemingly at odds with the prior literature showing an increase in D-dimer levels in similar subjects. However, an elevation in D-dimer levels (as seen by others) and low ETP (our primary observation) could occur in at least 2 settings. First, high D-dimer levels might reflect an increase in fibrinolytic activity without a change in thrombin generation or fibrin formation. This is a testable hypothesis and one our group would like to address in the future. Second, it is also possible that low ETP occurs as the result of increased coagulation system activity in vivo, with consumption of procoagulant factors resulting in an artifactually lower ETP in the in vitro assay. However, if this were the case, one would expect to see an increase in levels of D-dimers, TAT, and F1+2, all of which are in vivo markers of coagulation system activity, and we did not observe increases in any of these markers.
In conclusion, among treated and untreated HIV-infected men, antithrombin activity (an inhibitor of thrombin) was high, compared with that in uninfected controls, while the ETP (an in vitro assessment of thrombin generation) was lower. The nAPCsr was lower among treated men, compared with controls, while the TAT level was lower in untreated individuals, compared with controls. Little difference was found in other coagulation markers, including D-dimers, tissue factor, and F1+2. Overall, coagulation markers were similar between treated and untreated HIV-infected individuals. These findings warrant further investigation, as the interplay between thrombosis and inflammation in the setting of HIV infection is clearly complex. Longitudinal studies with clinical end points are needed to determine the degree to which HIV disease alters the risk of clotting.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments.
P. Y. H, R. S, C. G, A. S, E. N, S. G. D, J. N. M, and E. J. W. contributed to the study design, data interpretation, and writing of the manuscript. R. S. performed all of the statistical analyses for the study. S. M. N, L. P. K, and E. W. performed the laboratory studies described in the article. A. S. and E. N. recruited all of the patients for the study and collected the clinical data for the study.
Financial support.
This work was supported by grants from the NIH (R01 HL095310). The SCOPE cohort was supported by the Centers for AIDS Research at UCSF (PO AI27763), CFAR Network of Integrated Systems (R24 AI067039), the UCSF CTSI (UL1 RR024131), and the NIAID (RO1 AI087145, K24AI069994, AI 76174).
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. 2011;62:141–55. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neuhaus J, Jacobs DR, Jr, Baker JV, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010;201:1788–95. doi: 10.1086/652749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tien PC, Choi AI, Zolopa AR, et al. Inflammation and mortality in HIV-infected adults: analysis of the FRAM study cohort. J Acquir Immune Defic Syndr. 2010;55:316–22. doi: 10.1097/QAI.0b013e3181e66216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Esmon CT. Inflammation and thrombosis. J Thromb Haemost. 2003;1:1343–8. doi: 10.1046/j.1538-7836.2003.00261.x. [DOI] [PubMed] [Google Scholar]
- 6.van der Poll T, Boer JD, Levi M. The effect of inflammation on coagulation and vice versa. Curr Opin Infect Dis. 2011;24:273–8. doi: 10.1097/QCO.0b013e328344c078. [DOI] [PubMed] [Google Scholar]
- 7.Jong E, Louw S, van Gorp EC, Meijers JC, ten Cate H, Jacobson BF. The effect of initiating combined antiretroviral therapy on endothelial cell activation and coagulation markers in South African HIV-infected individuals. Thromb Haemost. 2010;104:1228–34. doi: 10.1160/TH10-04-0233. [DOI] [PubMed] [Google Scholar]
- 8.Reingold J, Wanke C, Kotler D, et al. Association of HIV infection and HIV/HCV coinfection with C-reactive protein levels: the fat redistribution and metabolic change in HIV infection (FRAM) study. J Acquir Immune Defic Syndr. 2008;48:142–8. doi: 10.1097/QAI.0b013e3181685727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tans G, Van Hylckama Vlieg A, Thomassen MC, et al. Activated protein C resistance determined with a thrombin generation-based test predicts for venous thrombosis in men and women. Br J Haematol. 2003;122:465–70. doi: 10.1046/j.1365-2141.2003.04443.x. [DOI] [PubMed] [Google Scholar]
- 10.Esmon CT. Crosstalk between inflammation and thrombosis. Maturitas. 2004;47:305–14. doi: 10.1016/j.maturitas.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 11.Agapakis DI, Tsantilas D, Psarris P, et al. Coagulation and inflammation biomarkers may help predict the severity of community-acquired pneumonia. Respirology. 2010;15:796–803. doi: 10.1111/j.1440-1843.2010.01773.x. [DOI] [PubMed] [Google Scholar]
- 12.Kinasewitz GT, Yan SB, Basson B, et al. Universal changes in biomarkers of coagulation and inflammation occur in patients with severe sepsis, regardless of causative micro-organism [ISRCTN74215569] Crit Care. 2004;8:R82–90. doi: 10.1186/cc2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feffer SE, Fox RL, Orsen MM, Harjai KJ, Glatt AE. Thrombotic tendencies and correlation with clinical status in patients infected with HIV. South Med J. 1995;88:1126–30. doi: 10.1097/00007611-199511000-00008. [DOI] [PubMed] [Google Scholar]
- 14.ten Cate-Hoek AJ, Dielis AW, Spronk HM, et al. Thrombin generation in patients after acute deep-vein thrombosis. Thromb Haemost. 2008;100:240–5. [PubMed] [Google Scholar]
- 15.van Hylckama Vlieg A, Christiansen SC, Luddington R, Cannegieter SC, Rosendaal FR, Baglin TP. Elevated endogenous thrombin potential is associated with an increased risk of a first deep venous thrombosis but not with the risk of recurrence. Br J Haematol. 2007;138:769–74. doi: 10.1111/j.1365-2141.2007.06738.x. [DOI] [PubMed] [Google Scholar]
- 16.Hron G, Kollars M, Binder BR, Eichinger S, Kyrle PA. Identification of patients at low risk for recurrent venous thromboembolism by measuring thrombin generation. JAMA. 2006;296:397–402. doi: 10.1001/jama.296.4.397. [DOI] [PubMed] [Google Scholar]
- 17.Jong E, Louw S, Meijers JC, et al. The hemostatic balance in HIV-infected patients with and without antiretroviral therapy: partial restoration with antiretroviral therapy. AIDS Patient Care STDS. 2009;23:1001–7. doi: 10.1089/apc.2009.0173. [DOI] [PubMed] [Google Scholar]
- 18.Eastburn A, Scherzer R, Zolopa AR, et al. Association of low level viremia with inflammation and mortality in HIV-infected adults. PLoS One. 2011;6:e26320. doi: 10.1371/journal.pone.0026320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hunt PW, Martin JN, Sinclair E, et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003;187:1534–43. doi: 10.1086/374786. [DOI] [PubMed] [Google Scholar]
- 20.Baker JV, Neuhaus J, Duprez D, et al. Changes in inflammatory and coagulation biomarkers: a randomized comparison of immediate versus deferred antiretroviral therapy in patients with HIV infection. J Acquir Immune Defic Syndr. 2011;56:36–43. doi: 10.1097/QAI.0b013e3181f7f61a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shikuma CM, Ribaudo HJ, Zheng Y, et al. Change in high-sensitivity c-reactive protein levels following initiation of efavirenz-based antiretroviral regimens in HIV-infected individuals. AIDS Res Hum Retroviruses. 2011;27:461–8. doi: 10.1089/aid.2010.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jong E, Meijers JC, van Gorp EC, Spek CA, Mulder JW. Markers of inflammation and coagulation indicate a prothrombotic state in HIV-infected patients with long-term use of antiretroviral therapy with or without abacavir. AIDS Res Ther. 2010;7:9. doi: 10.1186/1742-6405-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cushman M, Folsom AR, Wang L, et al. Fibrin fragment D-dimer and the risk of future venous thrombosis. Blood. 2003;101:1243–8. doi: 10.1182/blood-2002-05-1416. [DOI] [PubMed] [Google Scholar]
- 24.Lutsey PL, Cushman M, Steffen LM, et al. Plasma hemostatic factors and endothelial markers in four racial/ethnic groups: the MESA study. J Thromb Haemost. 2006;4:2629–35. doi: 10.1111/j.1538-7836.2006.02237.x. [DOI] [PubMed] [Google Scholar]
- 25.Lange LA, Reiner AP, Carty CL, Jenny NS, Cushman M, Lange EM. Common genetic variants associated with plasma fibrin D-dimer concentration in older European- and African-American adults. J Thromb Haemost. 2008;6:654–9. doi: 10.1111/j.1538-7836.2008.02906.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.