Abstract
AIM: To determine if there were any interactions between cardiac devices and small bowel capsules secondary to electromagnetic interference (EMI) in patients who have undergone small bowel capsule endoscopy (SBCE).
METHODS: Authors conducted a chart review of 20 patients with a cardiac pacemaker (CP) or implantable cardioverter defibrillator (ICD) who underwent continuous electrocardiographic monitoring during their SBCE from 2003-2008. authors searched for unexplained electrocardiogram (ECG) findings, changes in CP and ICD set parameters, any abnormality in transmitted capsule data, and adverse clinical events.
RESULTS: There were no adverse events or hemodynamically significant arrhythmias reported. CP and ICD set parameters were preserved. The majority of ECG abnormalities were also found in pre- or post- SBCE ECG tracings and the CP behavior during arrhythmias appeared appropriate. Two patients seemed to have episodes of undersensing by the CP. However, similar findings were documented in ECGs taken outside the time frame of the SBCE. One patient was observed to have a low signal encountered from the capsule resulting in lack of localization, but no images were lost.
CONCLUSION: Capsule-induced EMI remains a possibility but is unlikely to be clinically important. CP-induced interference of SBCE is also possible, but is infrequent and does not result in loss of images transmitted by the capsule.
Keywords: Small bowel capsule endoscopy, Cardiac pacemakers, Implantable cardioverter defibrillators, Electromagnetic interference, Telemetry review
INTRODUCTION
Small bowel capsule endoscopy (SBCE) is firmly established as a diagnostic modality in the evaluation of obscure gastrointestinal bleeding and Crohn’s disease. Based on concerns that radio signals that transmit images obtained from the capsule to an external sensory array could interfere with cardiac pacemaker functions, the US Food and Drug Administration required the manufacturer to insert language in the package insert that specifically contraindicates the use of capsule endoscopy in patients with these devices. When other diagnostic modalities fail to identify disease, many clinicians still perform capsule endoscopy in patients with pacemakers on the premise that the benefits of obtaining a diagnosis outweigh the proposed risks of the study. In addition, there is some skepticism over whether or not electromagnetic interference (EMI) by the capsule actually occurs and, if so, results in pacemaker malfunction[1]. There have been several preliminary studies suggesting that there are no verifiable clinically relevant malfunctions associated with the use of capsule endoscopy in patients with cardiac pacemakers[2-4]. Knowing the potential danger of EMI from prior studies, manufacturers of pacemakers and implantable cardioverter defibrillators (ICDs) have designed these devices to be shielded from small amounts of radiofrequency energy[5-7]. Transmissions to and from cardiac pacemakers to program the device occur in the 402 – 405 MHz band[8]. The radiofrequency energy from a small bowel capsule device may not be sufficient enough to cause clinically relevant malfunctions of the implanted cardiac pacemaker device as the capsule transmits images to the recorder in the 432-434.09 MHz band range (personal communication with Given Imaging, manufacturer of Pillcam SB™). The potential for adverse interactions between the cardiac device and ingested capsule has led us to perform capsule endoscopy under continuous electrocardiographic (ECG) telemetry monitoring. In this study, we review our experience with patients who had cardiac pacemakers or ICDs that underwent SBCE specifically looking for evidence implicating EMI between devices.
MATERIALS AND METHODS
We reviewed the charts of 20 patients (13 men, 7 women; mean age 71 years, range 57-80 years) with implanted pacing systems seen from September 2003 to June 2008. All of the patients presented with an indication for SBCE (either GI bleed or iron deficiency anemia) and gave written informed consent to the procedure after explaining risks, benefits, and alternatives. All SBCE investigations utilized the Pillcam SB™ capsule (Given Imaging Ltd., Isreal). The patients were advised to eat nothing after midnight, in compliance with an 8 hour fast prior to the procedure, but were allowed to take essential medications two hours before ingesting the capsule and at two hours post capsule ingestion. The sensor array was applied to the abdomen using adhesive pads, and was connected to the data recorder and battery pack. The battery pack was worn on a belt around the patient’s waist. Pacemaker nurse specialists performed interrogation of the cardiac device and adjusted settings according to a standardized protocol. All patients with ICDs had their sensing function turned off. Continuous ECG telemetry monitoring was performed during the study. The ECG data was transmitted to a central station which notifies nursing staff of abnormal rhythms. Concerning ECG waveforms such as premature ventricular contractions (PVCs), atrial fibrillation, brady-arrythmias, nonsustained ventricular tachycardia (NSVT) or sustained ventricular tachycardia (VT) were recorded and placed in the patient’s chart. Patients were instructed to avoid strenuous activity during the study period, and to call for help if they experienced concerning symptoms. When the patient had either passed the capsule or surpassed the life span of the battery, usually 8-9 h after the start of the examination, the capsule endoscopy data recorder was removed, and the ECG leads disconnected for those without an indication for continued telemetry monitoring. The cardiac device was then re-interrogated by the pacemaker nurse specialist to ensure proper function.
Several different models of pacemaker and ICDs produced by three manufacturers were studied see Table 1. The different types of pacemakers are categorized according to the NASPE/BPEG (North American Society of Pacing and Electrophysiology/ British Pacing and Electrophysiology Group) generic pacemaker code. The first letter identifies the chamber paced, the second letter identifies the chamber sensed (V, ventricular; A, atrial; D, dual ventricular or atrial), the third letter identifies the response to sensing (I, inhibited; T, triggered; D, dual), and the fourth letter identifies the response rate (R). The following parameters were assessed: adverse events occurring during and immediately after the capsule study; abnormal rhythms detected during telemetry monitoring; if available, ECG tracings taken prior to ingestion of the capsule and following the completion of the study for comparison; changes in set parameters that are documented in pacemaker interrogation reports; and findings concerning for oversensing or undersensing were interpreted by a staff electrophysiologist.
Table 1.
Pacemakers and implantable cardioverter defibrillators studied
| Patient | Manufacturer | Device | Model | Implanted | Mode | Polarity |
| No. 1 | Medtronic | ICD | Virtuoso DR | Oct. 2006 | AAI↔DDD | Bipolar |
| No. 2 | St Jude | CP | Integrity AF pacesetter | Aug. 2001 | DDDR | Bipolar |
| No. 3 | Medtronic | ICD | Concerto DWK | Apr. 2007 | DDD→VOO | Bipolar |
| No. 4 | Medtronic | CP | Enrhythm DR | Feb. 2006 | AAI↔DDD →VOO | Bipolar |
| No. 5 | St Jude | CP | Integrity DR | Aug. 2003 | DDDR | Bipolar |
| No. 6 | Medtronic | ICD | Concerto DWK | Oct. 2007 | DDD→VOO | Bipolar |
| No. 7 | Medtronic | CP | Kappa KDR901 | Oct. 2004 | DDDR | Bipolar |
| No. 8 | St Jude | CP | Affinity SR pacesetter | Sept.2000 | VVIR | Unipolar |
| No. 9 | St Jude | CP | Integrity DR | Mar. 2003 | DDDR | Bipolar |
| No. 10 | Medtronic | ICD | Virtuoso VR | Jun. 2007 | VVIR→ OO | Bipolar |
| No. 11 | Medtronic | CP | Sigma SSR | VVIR→ OO | Bipolar | |
| No. 12 | St Jude | ICD | Atlas HF | Feb. 2004 | DDDR→ OO | Bipolar |
| No. 13 | Medtronic | CP | Enrhythm DR | Aug. 1997 | DDDR | Bipolar |
| No. 14 | Medtronic | CP | EnpulseE2DR01 | Oct. 2004 | DDD→ OO | Bipolar |
| No. 15 | CP | |||||
| No. 16 | CP | |||||
| No. 17 | ||||||
| No. 18 | ||||||
| No. 19 | ELA | CP | Brio | Sept. 2003 | DDD | Bipolar |
| No. 20 | Medtronic | CP | Enrhythm DR | Aug. 2006 | DDDR↔AAIR→ DOO | Bipolar |
Blank spaces represent information that was unavailable. PPM: Permanent pacemaker; ICD: Implantable cardioverter defibrillator; CP: Permanent pacemaker.
Each patient’s final capsule endoscopy report was reviewed to evaluate for pacemaker induced interference of images transmitted by the capsule. We considered any alteration in the appearance of the images transmitted by the capsule, inability to localize the capsule, or any change in the strength of the transmitted signal to be positive markers for interference.
RESULTS
Twenty patients with cardiac pacemakers or ICDs implanted subcutaneously over the chest in the infraclavicular region were studied (13 men, seven women; mean age of 71 years, range of 57-80 years old). The indication for SBCE was either anemia or gastrointestinal bleeding of unknown origin. The indications for pacemaker or ICD included symptomatic tachy- or bradyarrhythmia, primary prevention for cardiomyopathy, or was not documented. Of these 20, four charts lacked an interrogation report, so baseline characteristics and changes that took place for the capsule study could not be assessed. Telemetry reports were available for all of these patients except two, and hence, 18 patients were included in the analysis of data from telemetry monitoring. Of the 16 patients whose interrogation reports were available to us, 15 devices were programmed to the bipolar output and sensing configuration and one pacemaker was committed to unipolar settings. Eleven of the 16 devices were pacemakers and five were ICDs. There were 10 different models placed between 1997 and 2007 that were manufactured by three brands—10 by Medtronic, Inc. (Minneapolis, MN), five by St Jude Medical, Inc. (St. Paul, MN), and one by ELA Medical (Arvada, CO). The pacing configuration in eight of 16 devices was changed for purposes of the capsule study, while the other eight retained their pre-SBCE set parameters. The devices were programmed to the following pacing modes: three were set to DDD, six to DDDR, one to DOO, four to VOO, one to VVIR, and one to AAI→DDD (Table 1). A pacemaker nurse specialist checked pacemaker function pre- and post procedure for each patient. Following the capsule study, none of the pre-SBCE configurations were found to be altered per review of the chart and interrogation reports.
Runs of PVCs were reported in six of 18 patients. A few patients’ alarms went off for what the telemetry system called PVCs, but on further review, these were thought to be artifact or insignificant findings. The clinically relevant PVCs were found in other ECG tracings within these patients’ charts before, after, or before and after the capsule endoscopy took place, suggesting that these abnormalities were part of the patient’s underlying cardiac rhythm derangement and had not been induced by the capsule. There was uncertainty over the exact number of PVCs in one patient because of the proximity of a PVC with the end of data recording. Similarly, NSVT- defined as three or more consecutive ventricular beats with a duration of less than 30 s, was seen in three patients, and all of these were documented before, after, or before and after the procedure. The number of beats of NSVT varied for each patient, but none of these episodes developed into sustained VT (> 30 consecutive beats or greater than 30 s in length) or ventricular fibrillation. Underlying arrhythmias such as atrial fibrillation, atrial flutter with variable AV block, and bradycardia were seen in several patients. All episodes of atrial fibrillation – five total, and the single incident of atrial flutter were documented in prior EKGs and/or in telemetry readings before or following the completion of the capsule endoscopy. The one patient with bradycardia (heart rate < 60) was found to have this in other ECGs. Six out of 18 patients were free of any irregularities during telemetry monitoring (Table 2). One patient had a shorter time on telemetry monitoring than capsule study duration, presumably from early defecation of the capsule or late application of ECG monitoring; regardless, no significant events were noted per chart review. A hemodynamically significant arrhythmia was not recorded for any patient, nor was there documentation of symptoms during the aforementioned arrhythmias.
Table 2.
Summary of important events observed on telemetry monitoring
| Patient | Device | Mode | Polarity | Induction of asynchronous mode | Undersensing | Oversensing | Threshold change | Symtoms | Holter findings |
| No. 1 | ICD | AAI↔DDD | Bipolar | No, occassional v-pacing on demand | No | No | No | No | PVC2 |
| No. 2 | CP | DDDR | Bipolar | No, occasional a-pacing and v-pacing on demand | Yes1 | No | No | No | A fib1, PVC1, Nonsustained VT1 |
| No. 3 | ICD | VOO | Bipolar | NA | NA | NA | No | No | Nonsustained VT1, PVC1, |
| No. 4 | CP | VOO | Bipolar | NA | NA | NA | No | No | A fib1, PVC |
| No. 5 | CP | DDDR | Bipolar | No, a-pacing and v-pacing on demand | Yes1 | No | No | No | A fib1, PVC1, |
| No. 6 | ICD | VOO | Bipolar | NA | NA | NA | No | No | PVC1 |
| No. 7 | CP | DDDR | Bipolar | No. Intrinsic rhythm without paced beats | No | No | No | No | No events |
| No. 8 | CP | VVIR | Unipolar | No, 100% v-paced on demand1 | No | No | No | No | No events |
| No. 9 | CP | DDDR | Bipolar | No, 100% a-paced on demand1 | No | No | No | No | No events |
| No. 10 | ICD | VOO | Bipolar | NA | NA | NA | No | No | No events |
| No. 11 | CP | VOO | Bipolar | NA | NA | NA | No | No | A fib1 |
| No. 12 | ICD | DOO | Bipolar | NA | NA | NA | No | No | No events |
| No. 13 | CP | DDDR | Bipolar | No, occasional v-pacing on demand | No | No | No | No | No events |
| No. 14 | CP | DOO | Bipolar | NA | NA | NA | No | No | No events |
| No. 15 | CP | 100% v-pacing | No | No events | |||||
| No. 16 | CP | 100% v-pacing | No | No events | |||||
| No. 17 | ICD | On demand pacing | No | Sinus brady1 | |||||
| No. 18 | CP | On demand pacing | No | A flutter1, nonsustained VT1 | |||||
| No. 19 | CP | DDD | Bipolar | No | No | ||||
| No. 20 | CP | DOO | Bipolar | No | No |
Blank spaces represent information that was unavailable.
Findings that were observed either before or after, or before and after the capsule study.
Findings that may have occurred before or early into the capsule study. NA: not applicable; CP: Permanent pacemaker; ICD: Implantable cardioverter defibrillator; a-pacing: Atrial pacing; v-pacing: ventricular pacing; A fib: Atrial fibrillation; Sinus brady: sinus bradycardia; VT: Nonsustained ventricular tachycardia; PVC: Premature ventricular contraction.
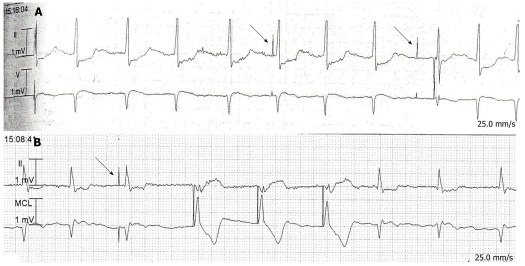
Eight of the 16 patients whose pacing interrogation reports were available were placed in an asynchronous mode (VOO or DOO) for the study and had their sensing capability turned off, and the other eight were placed in demand pacing mode (AAI→DDD, DDD, DDDR, or VVIR). Two patients being paced on demand were found to be in an asynchronous rhythm throughout the study. On further review, we noted that they had been receiving 100% of their beats from the pacemaker because of an underlying bradycardia that was found in old ECGs and those from after the completion of the study. Of the 8 patients in sensing mode, two had inappropriate pacer spikes due to undersensing of very subtle atrial fibrillation (Figure 1A and B). These patients had similar pacer spikes found in tracings that were recorded before and after the capsule endoscopy, respectively. The pacemaker responses to episodes of NSVT, PVCs, and atrial fibrillation in other tracings appeared appropriate.
Figure 1.
Electrocardiographic telemetry tracings from 2 patients with inappropriate pacer spikes (denoted by arrows). A: Undersensing of atrial fibrillatory waves is evident by the atrial pacer spikes preceding the 6th and 9th R waves; B: The atrial pacer spike preceding the 3rd R wave represents undersensing of a fibrillatory wave.
For details about the duration of the examination and location of the capsule at various time points, refer to Table 3. One out of 20 patients was found to have a low signal encountered from the capsule resulting in lack of localization. However, images were still able to be obtained from the exam. This patient had an ICD with sensing detections turned off. PVC’s were documented during their SBCE. The other 19 patients were free of image interference or other irregularities in data recording.
Table 3.
Data from capsule study
| n | Minimum | Maximum | Mean | SD | |
| Duration of Holter monitoring | 20 | 05:54.4 | 08:30.0 | 07:48.0 | 01:48.0 |
| Time to pass pylorus | 20 | 00:03.1 | 02:38.6 | 00:41.1 | 00:45.0 |
| Time to pass cecum | 17 | 01:25.6 | End of recording |
DISCUSSION
Our experience with SBCE in patients with cardiac rhythm devices is consistent with other investigations done on capsule endoscopy in patients with cardiac pacemakers or ICDs, in that no clinically significant arrhythmias have been linked to electromagnetic interference from capsule transmission. However, it is well established that EMI between pacemakers, ICDs, and other medical devices is a real danger with potentially life-threatening consequences. For example, studies have shown that percutaneous catheter ablation of atrial arrhythmias can cause pacemaker malfunction and circuitry failure, necessitating replacement in some patients[9]. Devices which emit electromagnetic waves from a distance, such as digital cell phones, electronic surveillance systems, and electrocautery instruments may also interfere with pacemakers/ICDs[10,11]. Studies conducted on the C-net cellular phone system, which operates at 450 MHz, have shown a rate of interference up to 30%, clinically manifested as pacemaker mediated tachycardia and switch to interference mode, in some cases[12-14]. It is important to note that despite having a similar frequency, the radiated power of the video capsule is very low compared to that of the C-net mobile phone system, 50nW versus 2W, respectively[15]. Alternatively, equipment for electrocautery, defibrillation, catheter ablation, and lithotripsy are known to cause interference with cardiac devices, but operate at frequencies far below the 434.09 MHz used by the Pillcam SB™ capsule[3].
Studies on cell phones have demonstrated that interference is to some extent inversely proportional to the distance between the source of EMI and the cardiac device[13]. However, when the capsule travels past the heart, under most circumstances it will not come closer than fifteen centimeters from the implanted cardiac device, which is how far patients are told to keep mobile phones from their pacemaker[3]. The leads of the cardiac device may come much closer to the capsule as it traverses the esophagus, especially at the left atrium which is within centimeters of the esophagus. If proximity is indeed a factor, this is where a cardiac device is most vulnerable to EMI. However, exposure here is minimal as the capsule generally moves rapidly down the esophagus.
It is widely accepted that EMI is significantly reduced by a bipolar, as opposed to unipolar, configuration, and this is clinically relevant with regards to certain cellular phone and security systems[16,17]. However, Dubner et al performed a study using a SBCE simulation probe (Test Cap™, Given Diagnostic System) that emits electromagnetic waves at the same frequency as a real capsule and noted that EMI occurred in 4% of patients (four out of 100), and this occurred only in bipolar pacemakers[16]. Confounding this data is the fact that 95 of the 100 pacemakers in that study were programmed to bipolar mode. Similarly, all but one pacemaker in our study was configured in bipolar mode, so a direct comparison cannot be made.
Multiple studies have shown that body tissues serve as protection against EMI. Building on this concept, Bandorski et al (2008) and Payeras et al[3,18] built in vitro models, exposing pacemakers immersed in water or open to air to small bowel capsules. They found no interference in pacemaker function regardless of level of pacemaker sensitivity or bipolar versus unipolar settings. In the present study, we were not able to determine the exact time at which the capsules were swallowed, and thus cannot correlate the ECG abnormalities and episodes of undersensing with the location of the capsule.
In contrast to most observational studies, some prospective research points to the potential for clinically relevant EMI between capsules and cardiac devices. For example, the study utilizing the Test Cap™ by Dubner et al reported EMI when the capsule was hovering above the skin at a distance of less than 10 cm from the pacemaker. This effect was reproducible a week later in all four cases. The interference took the form of forcing the pacemaker into noise mode function, which is a safe-mode design that causes the pacemaker to change to an asynchronous pacing state when it cannot differentiate electromagnetic noise from a true signal. This change was reversible and there was no permanent damage to pacemaker. Pacemaker inhibition, which can have very serious consequences, was not observed and none of their patients developed symptoms[16]. It is thought that the site of entry for the noise signals was the unshielded part of the connector block which could occur as the swallowed capsule passes posterior to the heart while descending through the esophagus, consistent with studies on mobile phones[16,18]. This study very closely replicated a real capsule endoscopy, and involved a large number of patients. However, it was limited by the fact that several components of the capsule endoscopy system were not part of the simulation and the capsule was never actually swallowed. So, their findings may not be reliably translated to a real capsule study.
There is the potential that capsule-induced EMI of the pacemaker may result in breakthrough arrhythmias secondary to undersensing or oversensing. We saw many arrhythmias, but virtually all of them were documented outside the timeframe of the SBCE study, and thus were unlikely to be the result of undersensing induced by the capsule. Most importantly, the pacemakers appeared to function appropriately during these arrhythmias, and each episode was brief, isolated, and was not associated with symptoms or changes in pacemaker set parameters. In addition, we cannot conclusively say why there was undersensing in two patients in our population, the most likely possibility is that the thresholds for atrial pacing were set too high, resulting in the pacemaker not sensing underlying low-amplitude fibrillatory atrial electrical activity. However, the possibility of capsule interference cannot be completely excluded. Knowing where in the abdomen the capsule happened to be at the time of interference would help since EMI seems to vary with distance from the source and position relative to the pacemaker.
It is important to determine whether or not ICDs, with their more complex program function and electronic circuitry, are susceptible to EMI. Both Bandorski et al and Leighton et al report no serious complications in respective papers involving SBCE in patients with ICDs[17,19,20]. We also studied patients with ICDs, but all of our patients had their ICD detection capability turned off prior to capsule ingestion, thus preventing the provision of shocks by the ICD in the setting of a dysrhythmia. In this regard, we are not able to say whether or not capsules may cause dysfunction of ICDs when sensing is activated.
Very few studies have reported problems with the capsule system caused by cardiac pacemakers or other sources of EMI. A 2011 retrospective study by Bandorski found gaps the capsule video processing in two of 13 patients on telemetry[20]. There was no capsule interference in 49 patients without telemetry monitoring, leading to their hypothesis that ECG-monitoring devices have the capacity to suppress processing of the capsule signal[20]. The incidence of interference of the capsule in this study was small at 5% (one out of 20 patients). Although interference prevented capsule localization, it was clinically insignificant as images were still obtained. However, because of the small patient population, we cannot reliably comment on the expected incidence of pacemaker or ECG-monitoring device induced EMI. In addition, it should be noted that obscuring or loss of images can occur in patients for many different reasons. In the population of patients at our medical center who have undergone capsule endoscopy that do not have pacemakers or ICDs, we have seen gaps in recording secondary to the sensor array not being plugged in, leads having fallen off or being improperly connected, discharged batteries, and many times, there is no clear explanation. Pacemakers, capsules, and ECG monitoring devices have different electromagnetic and radiofrequency characteristics depending on the type and brand, which may also play a role. To date, there been no large, prospective studies describing the incidence of capsule image loss.
In conclusion, in the largest study known to date with a complete review of continuous ECG-monitoring during capsule endoscopy, we observed no clinically significant interaction between the capsule endoscopy device and pacemakers or ICDs whose sensing capacity had been deactivated. Gaps in the recorded images were noted in one patient possibly due to EMI from the cardiac pacemaker, but EMI secondary to the ECG-monitoring device is another very plausible possibility. However, similar gaps occur in patients who do not have such devices. Undersensing and abnormal electrocardiographic findings were noted in a limited number of patients but were of no clinical importance. These unexpected findings likely represent underlying problems that were picked up incidentally and most likely were not due to EMI from the capsule. Based on our findings and review of previous studies on capsule endoscopy in patients with pacemakers, it appears safe to perform capsule endoscopy in these individuals without the use of telemetry monitoring. However, we cannot assume this with regards to our patients that had ICDs, as their sensing detections were turned off.
COMMENTS
Background
Electromagnetic interference (EMI) can cause pacemaker or implantable cardioverter defibrillator (ICD) malfunction in addition to loss of images or transmitted data from small bowel video capsules during capsule endoscopy. Although there is limited data on the clinical relevance of this interaction between the two devices, the US Food and Drug administration has mandated that manufacturers include language in the package insert contraindicating capsule endoscopy in patients with an implanted cardiac pacing device. Looking for electrocardiogram (ECG) abnormalities in patients with pacemakers during a capsule study could offer insight as to whether or not such an interaction actually takes place.
Research frontiers
The indications for capsule endoscopy are expanding and the technology continues to evolve. Normally an outpatient procedure, the package insert warning has led some providers to monitor their patients in an in-patient setting to mitigate the risks of EMI on pacemaker function. This adds cost to the procedure without a definite benefit and may limit the use of capsule endoscopy in select patients with a pacemaker or ICD.
Innovations and breakthroughs
Although all ICDs and pacemakers have a shield built into the device, it is still possible for high energy electromagnetic radiation to enter and cause sensing abnormalities or alterations in configured settings. Fortunately, small bowel capsules are designed in such a way that the radio energy emitted has a frequency and wattage that should not cause interference with a thoracic cardiac device when traveling through the abdomen under normal circumstances.
Applications
Given that clinically significant EMI with pacemaker function was not noted in this study, it may be safe to perform capsule endoscopy in out-patients without the use of continuous ECG telemetry monitoring as a precautionary measure. However, the incidence of interference with capsule function appears to be higher and we cannot comment on the impact of capsules on ICD function as these devices had their sensitivities turned off during the study. Large, prospective, randomized trials are needed before final recommendations can be made.
Terminology
EMI is a disturbance that affects an electrical circuit due to electromagnetic radiation emitted from an external source. Authors use this term interchangeably with radiofrequency interference and energy. Pacemaker sensing refers to the detection of heart rate and rhythm patterns by the cardiac device; under- and oversensing are electrocardiographic signs of pacemaker malfunction that may be due to EMI.
Peer review
This outcomes analysis study in which the authors review their experience performing capsule endoscopy on patients with pacemakers or ICDs while under continuous ECG monitoring is the largest experience of its kind reported in the literature. The results reiterate the concept that capsule endoscopy is a safe procedure to perform in patients with a pacemaker or an ICD with its detections turned off. EMI by one device on the other remains a possibility but appears to lack clinical significance.
Footnotes
Peer reviewer: Dr. Carlo M Girelli, Service of Gastroenterology and Digestive End, Hospital of Busto Arsizio, via A da Brescia, 1, Busto Arsizio 21052, Italy
S- Editor Yang XC L- Editor A E- Editor Yang XC
References
- 1.Guyomar Y, Vandeville L, Heuls S, Coviaux F, Graux P, Cornaert P, Filoche B. Interference between pacemaker and video capsule endoscopy. Pacing Clin Electrophysiol. 2004;27:1329–1330. doi: 10.1111/j.1540-8159.2004.00631.x. [DOI] [PubMed] [Google Scholar]
- 2.Leighton JA, Sharma VK, Srivathsan K, Heigh RI, McWane TL, Post JK, Robinson SR, Bazzell JL, Fleischer DE. Safety of capsule endoscopy in patients with pacemakers. Gastrointest Endosc. 2004;59:567–569. doi: 10.1016/s0016-5107(03)02879-7. [DOI] [PubMed] [Google Scholar]
- 3.Payeras G, Piqueras J, Moreno VJ, Cabrera A, Menéndez D, Jiménez R. Effects of capsule endoscopy on cardiac pacemakers. Endoscopy. 2005;37:1181–1185. doi: 10.1055/s-2005-870558. [DOI] [PubMed] [Google Scholar]
- 4.Dirks MH, Costea F, Seidman EG. Successful videocapsule endoscopy in patients with an abdominal cardiac pacemaker. Endoscopy. 2008;40:73–75. doi: 10.1055/s-2007-966785. [DOI] [PubMed] [Google Scholar]
- 5.Wilson BL, Broberg C, Baumgartner JC, Harris C, Kron J. Safety of electronic apex locators and pulp testers in patients with implanted cardiac pacemakers or cardioverter/defibrillators. J Endod. 2006;32:847–852. doi: 10.1016/j.joen.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Trigano A, Blandeau O, Dale C, Wong MF, Wiart J. Reliability of electromagnetic filters of cardiac pacemakers tested by cellular telephone ringing. Heart Rhythm. 2005;2:837–841. doi: 10.1016/j.hrthm.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 7.Lucas EH, Johnson D, McElroy BP. The effects of electronic article surveillance systems on permanent cardiac pacemakers: an in vitro study. Pacing Clin Electrophysiol. 1994;17:2021–2026. doi: 10.1111/j.1540-8159.1994.tb03793.x. [DOI] [PubMed] [Google Scholar]
- 8.Establishment of a Medical Implant Communications Service in the 402-405 MHz band. Federal Communications Commission. Final rule. Fed Regist. 1999;64:69926–69934. [PubMed] [Google Scholar]
- 9.Vanerio G, Maloney J, Rashidi R, McCowan R, Castle L, Morant V, Wilkoff B, Simmons T. The effects of percutaneous catheter ablation on preexisting permanent pacemakers. Pacing Clin Electrophysiol. 1990;13:1637–1645. doi: 10.1111/j.1540-8159.1990.tb06866.x. [DOI] [PubMed] [Google Scholar]
- 10.Niehaus M, Tebbenjohanns J. Electromagnetic interference in patients with implanted pacemakers or cardioverter-defibrillators. Heart. 2001;86:246–248. doi: 10.1136/heart.86.3.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santucci PA, Haw J, Trohman RG, Pinski SL. Interference with an implantable defibrillator by an electronic antitheft-surveillance device. N Engl J Med. 1998;339:1371–1374. doi: 10.1056/NEJM199811053391905. [DOI] [PubMed] [Google Scholar]
- 12.Irnich W, Batz L, Müller R, Tobisch R. Electromagnetic interference of pacemakers by mobile phones. Pacing Clin Electrophysiol. 1996;19:1431–1446. doi: 10.1111/j.1540-8159.1996.tb03155.x. [DOI] [PubMed] [Google Scholar]
- 13.Hayes DL, Wang PJ, Reynolds DW, Estes M, Griffith JL, Steffens RA, Carlo GL, Findlay GK, Johnson CM. Interference with cardiac pacemakers by cellular telephones. N Engl J Med. 1997;336:1473–1479. doi: 10.1056/NEJM199705223362101. [DOI] [PubMed] [Google Scholar]
- 14.Hofgärtner F, Müller T, Sigel H. [Could C- and D-network mobile phones endanger patients with pacemakers?] Dtsch Med Wochenschr. 1996;121:646–652. doi: 10.1055/s-2008-1043051. [DOI] [PubMed] [Google Scholar]
- 15.Kolb C, Schmieder S, Lehmann G, Zrenner B, Karch MR, Plewan A, Schmitt C. Do airport metal detectors interfere with implantable pacemakers or cardioverter-defibrillators? J Am Coll Cardiol. 2003;41:2054–2059. doi: 10.1016/s0735-1097(03)00424-8. [DOI] [PubMed] [Google Scholar]
- 16.Dubner S, Dubner Y, Gallino S, Spallone L, Zagalsky D, Rubio H, Zimmerman J, Goldin E. Electromagnetic interference with implantable cardiac pacemakers by video capsule. Gastrointest Endosc. 2005;61:250–254. doi: 10.1016/s0016-5107(04)02834-2. [DOI] [PubMed] [Google Scholar]
- 17.Leighton JA, Srivathsan K, Carey EJ, Sharma VK, Heigh RI, Post JK, Erickson PJ, Robinson SR, Bazzell JL, Fleischer DE. Safety of wireless capsule endoscopy in patients with implantable cardiac defibrillators. Am J Gastroenterol. 2005;100:1728–1731. doi: 10.1111/j.1572-0241.2005.41391.x. [DOI] [PubMed] [Google Scholar]
- 18.Bandorski D, Irnich W, Brück M, Beyer N, Kramer W, Jakobs R. Capsule endoscopy and cardiac pacemakers: investigation for possible interference. Endoscopy. 2008;40:36–39. doi: 10.1055/s-2007-995353. [DOI] [PubMed] [Google Scholar]
- 19.Bandorski D, Diehl KL, Jaspersen D. [Capsule endoscopy in patients with cardiac pacemakers: current situation in Germany] Z Gastroenterol. 2005;43:715–718. doi: 10.1055/s-2005-858469. [DOI] [PubMed] [Google Scholar]
- 20.Bandorski D, Lotterer E, Hartmann D, Jakobs R, Brück M, Hoeltgen R, Wieczorek M, Brock A, de Rossi T, Keuchel M. Capsule endoscopy in patients with cardiac pacemakers and implantable cardioverter-defibrillators - a retrospective multicenter investigation. J Gastrointestin Liver Dis. 2011;20:33–37. [PubMed] [Google Scholar]