Abstract
AIM: To assess attitudes and trends regarding the use of high-dose infliximab among pediatric gastroenterologists for treatment of pediatric ulcerative colitis (UC).
METHODS: A 19-item survey was distributed to subscribers of the pediatric gastroenterology (PEDSGI) listserv. Responses were submitted anonymously and results compiled in a secure website.
RESULTS: A total of 113 subscribers (88% based in the United States) responded (101 pediatric gastroenterology attendings and 12 pediatric gastroenterology fellows). There were 46% in academic medical institutions and 39% in hospital-based practices. The majority (91%) were treating >10 patients with UC; 13% were treating >100 patients with UC; 91% had prescribed infliximab (IFX) 5 mg/kg for UC; 72% had prescribed IFX 10 mg/kg for UC. Using a 5-point Likert scale, factors that influenced the decision not to increase IFX dosing in patients with UC included: “improvement on initial dose of IFX” (mean: 3.88) and “decision to move to colectomy” (3.69). Lowest mean Likert scores were: “lack of guidelines or literature regarding increased IFX dosing” (1.96) and “insurance authorization or other insurance issues” (2.34). “Insurance authorization or other insurance issues” was identified by 39% as at least somewhat of a factor (Likert score ≥ 3) in their decision not to increase the IFX dose. IFX 10 mg/kg was more commonly used for the treatment of pediatric UC among responders based in the United States (75/100) compared to non-United States responders (6/13, P = 0.047). Induction of remission was reported by 78% of all responders and 81% reported maintenance of remission with IFX 10 mg/kg. One responder reported one death with IFX 10 mg/kg.
CONCLUSION: IFX 10 mg/kg is more commonly used in the United States to treat pediatric UC. Efficacy and safety data are required to avoid insurance barriers for its use.
Keywords: Immunosuppression, Inflammatory bowel disease, Ulcerative colitis, Children, Pharmacology
INTRODUCTION
In the last decade, infliximab has become an alternative treatment for moderate to severe ulcerative colitis (UC). The landmark ACT1 and ACT2 trials in adults showed that infliximab (IFX) successfully induced remission in patients with corticosteroid sensitive and corticosteroid resistant UC[1]. More recently, the Pediatric Inflammatory Bowel Disease Collaborative Research Group showed that children with corticosteroid-dependent or corticosteroid-refractory UC treated with IFX have a significant decrease in corticosteroid dependency and avoidance of colectomy in 72% after 1 year and 61% after 2 years of treatment[2].
The Food and Drug Administration (FDA) has approved the use of IFX for treatment of moderate to severe UC for adult patients at a dose of 5 mg/kg[3]. However, in patients who lose responsiveness, the dose of IFX is commonly increased up to 10 mg/kg. The ACT1 and ACT2 trials demonstrated a response at this higher dose[1]. No studies have documented a benefit at this higher dose in pediatric patients.
Given the increased use of IFX for treatment of UC and the ongoing issues regarding efficacy, safety and insurance approval, we conducted a survey of pediatric gastroenterologists to collect information regarding dosing practices of IFX for the treatment of UC in the pediatric population. The results of this survey provide a glimpse into the attitudes of pediatric gastroenterologists regarding the use of high-dose IFX for pediatric UC.
MATERIALS AND METHODS
An anonymous survey was created using the web-based survey software SurveyMonkey.com® (Portland, OR). The survey was distributed to all United States based and international pediatric gastroenterologists participating in the pediatric gastroenterology (PEDSGI) listserv with the approval of the listserv administrator. This survey included an electronic consent form and was free of identifiers. In addition, the survey forms were secured using secure sockets layer encryption to maintain the Health Insurance Portability and Accountability Act compliance. An “Opt Out” link was available at any point during the survey. This survey was approved by the University of California, San Francisco Committee on Human Research.
Demographic information was obtained by asking responders to provide data regarding their specialty (pediatric gastroenterology attending, pediatric gastroenterology trainee, or other specialty), practice type (academic, private, hospital based or other), practice size (< 10, 10-50, 51-100, 101-200 or > 200 patients) and location (Western United States, Midwest United States, Northeast United States, South United States, Europe, Middle East, Asia or “Other”).
To determine the extent of IFX use among pediatric gastroenterologists, responders were asked, “Have you ever prescribed IFX at 5 mg/kg?” Those pediatric gastroenterologists that responded positively were asked, “What were the diagnoses for those patients receiving IFX 5 mg/kg (Crohn’s, UC, inflammatory bowel disease-unspecified (IBD-U) or other)?” Responders were then asked, “Have you ever prescribed IFX at 10 mg/kg?” Those that responded positively were again asked, “What were the diagnoses for those patients receiving IFX 10 mg/kg (Crohn’s, UC, IBD-U or other)?” Responders who listed UC as a diagnosis were subsequently asked to specify all indications for which they prescribed IFX 10 mg/kg (steroid refractory UC, mild-to-moderate UC for induction or maintenance of remission, moderate-to-severe UC for induction or maintenance of remission, severe UC for induction or maintenance of remission).
Difficulty in achieving dose escalation was evaluated by asking those with experience in prescribing IFX 5 mg/kg: “… was an increase in IFX dosing considered at any point?” Responders who answered positively were subsequently asked: “…was an increase in IFX dose achieved?”
To further evaluate barriers to IFX dose escalation, a 5-point Likert scale was created whereby responders were asked to rank (scale of 1-5) factors that may have influenced their decision not to increase IFX to 10 mg/kg. The factors listed included: (1) patient improvement on the initial dose of IFX; (2) patient improvement on alternate medical therapy; (3) decision to move to colectomy; (4) lack of guidelines or literature regarding dose escalation; and (5) insurance authorization or other insurance issues. Responders were also asked to list other reasons that may have influenced their decision not to increase IFX dosing.
To evaluate the variation in administration of concomitant immunosuppressive medications, responders were asked to specify all medications instituted prior to IFX 10 mg/kg. In a separate question, responders were asked to specify all medications instituted concomitant with IFX 10 mg/kg for the treatment of UC. Answer choices included: corticosteroids, aminosalicylates, azathioprine (AZA), 6-mercaptopurine (6-MP), and methotrexate. Responders were also asked to specify any other medications that were not listed.
Methods for administration of IFX 10 mg/kg such as dosing interval and continuous or episodic treatment were not surveyed.
Responders were also asked to select any adverse events their patients experienced as a result of IFX 10 mg/kg. Answer choices included: worsening UC, headache, arthralgia, profound anemia, infection, oncologic process, neurologic event, antibodies against IFX or other autoimmune antibodies and infusion reactions. Responders were asked to specify any other adverse event that was not listed.
Finally, responders were asked to list all outcomes their patients experienced as a result of IFX 10 mg/kg (clinical remission, maintenance of remission, colectomy, continued or worsening colitis, death). Again, responders were asked to specify any other unlisted outcome.
Response frequencies were tabulated and expressed as percentages of total responses. Differences among groups with regard to frequency and Likert scale variables were assessed for significance using a Wilcoxon Rank Sum test for comparing two groups, or a Kruskal-Wallis test for more than two groups as the data were not normally distributed. Differences between categorical variables were tested using χ2 and Fisher exact tests. Differences were considered significant for P ≤ 0.05.
RESULTS
Demographics
Our survey yielded 113 responders (5.7%) out of a total of 1993 subscribers to the PEDGI listserv. Of those responders, 101 were pediatric gastroenterology attendings and 12 were trainees in pediatric gastroenterology programs. Most responders identified themselves as based in an academic medical institution (46.0%) or a hospital-based practice (38.9%). A minority of respondents identified themselves as working in a private practice (12.4%). Most responders also identified themselves as practicing within the United States (88.5%). Ninety-one percent of responders reported practices that treat at least 10 patients with UC, 23.0% reported 50 to 100 patients, and 13.3% reported over 100 patients with UC.
Initiation of IFX
Ninety-one percent (103/113) of respondents had prescribed IFX 5 mg/kg for treatment of UC, while 71.7% (81/113) had prescribed IFX 10 mg/kg for treatment of UC. The indications for prescribing IFX 10 mg/kg are listed in Table 1. A large majority of respondents selected steroid refractory UC as an indication for IFX 10 mg/kg (70.5%). In addition, a majority of respondents listed severe UC for induction and maintenance of remission as an indication for prescribing IFX 10 mg/kg (55.1% and 64.1%, respectively). A large number of respondents had also used IFX 10 mg/kg for treatment of moderate-to-severe UC, both for induction and maintenance of remission (38.5% and 62.8%, respectively).
Table 1.
Indication for initiation of infliximab 10 mg/kg
| Answer options | Percentage (n) |
| Steroid refractory UC | 70.5 (55) |
| Severe UC for maintenance of remission | 64.1 (50) |
| Moderate-to-severe UC for maintenance of remission | 62.8 (49) |
| Severe UC for induction of remission | 55.1 (43) |
| Moderate-to-severe UC for induction of remission | 38.5 (30) |
| Mild-to-moderate UC for maintenance of remission | 9.0 (7) |
| Mild-to-moderate UC for induction of remission | 2.6 (2) |
| Other | 6.4 (5) |
78 respondents. UC: Ulcerative colitis.
The overall use of IFX 10 mg/kg to treat UC did not differ significantly when comparing responders in academic and non-academic practices. However, more responders working in non-academic practices had prescribed IFX 10 mg/kg for maintenance of remission for severe UC (29/39) compared with those in academic practices (19/39, P = 0.034).
Responders based in the United States were more likely to use IFX 10 mg/kg to treat UC (75/100) compared with non-United States responders (6/13, P = 0.047). However, the indication for initiation of IFX 10 mg/kg for UC did not differ between these two groups.
Increasing the IFX dose
Pediatric gastroenterologists will often increase IFX dosing in patients that respond poorly to IFX 5 mg/kg. Respondents who had prescribed IFX 5 mg/kg for the treatment of UC were subsequently asked, “Have you ever considered an increase in IFX dosing above 5 mg/kg?” Overwhelmingly, 87.4% (90/103) of those using IFX had at least considered an increase in IFX dosing.
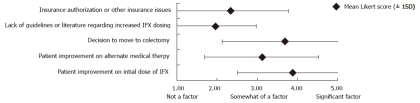
Using a 5-point Likert scale, respondents were asked to rank factors that may have influenced their decision not to increase IFX dosing in patients with UC (Figure 1). Among the 82 respondents, “improvement on initial dose of IFX” and “decision to move to colectomy” had the highest mean Likert scores (3.88 and 3.69, respectively). “Lack of guidelines or literature regarding increased IFX dosing” and “insurance authorization or other insurance issues” had the lowest mean Likert scores (1.96 and 2.34, respectively). Of note, however, 39.0% (32/82) identified “insurance authorization or other insurance issues” as at least somewhat of a factor (Likert score ≥ 3) in their decision not to increase the IFX dose in patients with UC.
Figure 1.
Factors that influenced pediatric gastroenterologists to not increase infliximab dosing depicted as a 5-point Likert scale. IFX: Infliximab.
Respondents were asked to list all medical therapies that may have been instituted prior to IFX 10 mg/kg (Table 2). Many of the respondents selected all the alternative medical therapies listed, and only one reported prescribing IFX 10 mg/kg without attempting any prior medical therapy. “Other” medications that were specified included tacrolimus, probiotics, metronidazole and other antibiotics.
Table 2.
Therapies introduced prior to infliximab 10 mg/kg
| Answer options | Percentage (n) |
| Corticosteroids | 96.2 (75) |
| Infliximab 5 mg/kg | 92.3 (72) |
| Aminosalicylates | 89.7 (70) |
| Azathioprine1 | 76.9 (60) |
| 6-mercaptopurine1 | 70.5 (55) |
| Methotrexate | 25.6 (20) |
| Other | 14.1 (11) |
| None | 1.3 (1) |
Reported use by physicians included either azathioprine or 6-mercaptopurine in different patients. 78 respondents.
Respondents were also asked what therapies they had instituted concurrently with IFX 10 mg/kg (Table 3). Most used corticosteroids with IFX 10 mg/kg. In addition, aminosalicylates were commonly administered with IFX 10 mg/kg. Conversely, fewer prescribed AZA or 6-MP along with IFX 10 mg/kg. A small minority administered IFX 10 mg/kg alone. Methotrexate was also noted to be used concurrently with IFX 10 mg/kg.
Table 3.
Therapies instituted with infliximab 10 mg/kg
| Answer options | Percentage (n) |
| Corticosteroids | 70.5 (55) |
| Aminosalicylates | 61.5 (48) |
| Azathioprine1 | 39.7 (31) |
| 6-mercaptopurine1 | 34.6 (27) |
| Antibiotics | 44.9 (35) |
| None | 11.5 (9) |
| Methotrexate | 6.7 (5) |
| Other | 1.3 (1) |
Reported use by physicians included either azathioprine or 6-mercaptopurine for different patients. 78 respondents.
No significant differences in practice patterns with regards to medications initiated prior to and concurrent with IFX 10 mg/kg were observed between those in academic and non-academic practices.
A larger number of United States based responders had prescribed 6-MP prior to instituting IFX 10 mg/kg to treat UC (54/75, 72.0%) compared with non-United States based responders (1/6, 16.7%, P = 0.012). In addition, more United States based responders had prescribed 5-aminosalicylates concurrently with IFX 10 mg/kg (47/75, 62.7%) compared with non-United States responders (1/6, 16.7%, P = 0.039). Similarly, antibiotics was more commonly prescribed concurrently with IFX 10 mg/kg among United States responders (35/75, 46.7%) compared with non-United States responders (0/6, P = 0.034).
Outcomes with high-dose IFX
Table 4 lists the outcomes reported by respondents as a result of using IFX 10 mg/kg. A majority of pediatric gastroenterologists surveyed reported either clinical remission (61/78, 78.2%) or maintenance of remission with this dose (63/78, 80.8%). Over half of the surveyed pediatric gastroenterologists who used 10 mg/kg IFX had patients who continued to suffer worsening colitis (40/78, 51.3%) or who moved to colectomy (47/78, 60.3%).
Table 4.
Outcomes reported using infliximab 10 mg/kg
| Answer options | Percentage (n) |
| Maintenance of remission | 80.8 (63) |
| Clinical remission | 78.2 (61) |
| Colectomy | 60.3 (47) |
| Continued or worsening colitis | 51.3 (40) |
| Other | 2.6 (2) |
| Mortality | 1.3 (1) |
78 respondents.
Most pediatric gastroenterologists (50/78, 64.1%) reported no adverse effects of the higher dose (Table 5). The most common adverse events reported by prescribers were antibodies to IFX (14/78, 17.9%) and infusion reactions (13/78, 16.7%); 14.1% (11/78) respondents reported discontinuing IFX 10 mg/kg as a result of these adverse reactions. No oncologic or major neurologic events were reported as a consequence of administering IFX 10 mg/kg. However, a single respondent reported one death while using IFX 10 mg/kg.
Table 5.
Side effects and adverse reactions
| Answer options | Percentage (n) |
| None | 64.1 (50) |
| Antibodies to infliximab | 17.9 (14) |
| Infusion reaction or delayed hypersensitivity reaction | 16.7 (13) |
| Worsening ulcerative colitis | 14.1 (11) |
| Infection | 12.8 (10) |
| Headache | 9.0 (7) |
| Arthralgia | 9.0 (7) |
| Development of autoimmune antibodies (e.g., antinuclear antibodies, anti-dsDNA antibodies) | 5.1 (4) |
| Other | 2.6 (2) |
| Profound anemia | 1.3 (1) |
| Oncologic process | 0 (0) |
| Neurologic event (e.g., neuritis, neuropathy) | 0 (0) |
78 respondents.
DISCUSSION
This survey was designed to understand the attitudes and driving forces of pediatric gastroenterologists regarding the use of high-dose IFX for the treatment of UC. In our sample, a majority of the pediatric gastroenterologists surveyed had experience treating pediatric UC with IFX 10 mg/kg.
One of the strengths of this study is that the respondents were evenly split between academic institutions and non-academic practices, capturing the major practice settings of pediatric gastroenterologists.
One of the weaknesses of this study is the low response rate, which may represent a non-response bias. However, it should be noted that a large number of the 1993 PEDSGI listserv subscribers are not pediatric gastroenterologists. Many of the listserv subscribers are surgeons, pathologists, nurse practitioners and other healthcare professionals. Although the exact number of pediatric gastroenterologists that subscribe to the listserv is unknown, the response rate is likely much higher among this particular group. According to the American Board of Pediatrics, at the time this survey was conducted, 974 board certified pediatric gastroenterologists were registered in the United States[4]. Based on these numbers, our survey results represent the opinions of 9.3% (91/974) of the registered pediatric gastroenterologists practicing within the United States.
In addition, the results of this survey study are subject to recall bias. Respondents who have experienced more extreme outcomes with IFX or difficulty with insurance approval for higher doses of IFX are more likely to respond in an exaggerated manner.
While the results of this uncontrolled study are subject to various biases, they may also be indicative of the trends regarding the use of high-dose IFX among pediatric gastroenterologists.
IFX is effective for both induction and maintenance of remission for steroid-refractory and steroid-dependent UC[1,2,5]. Predictably, most of the pediatric gastroenterologists surveyed indicated steroid refractoriness as an indication for prescribing IFX 10 mg/kg. In addition, nearly all pediatric gastroenterologists surveyed indicated that both corticosteroids and IFX 5 mg/kg had been employed prior to increasing the IFX dose to 10 mg/kg. IFX 10 mg/kg may also be a corticosteroid-sparing agent as reflected in the response that fewer pediatric gastroenterologists reported using corticosteroids after the 10 mg/kg dose was instituted.
While nearly three-quarters of pediatric gastroenterologists surveyed have prescribed 6-MP or AZA therapy prior to instituting IFX, only about one-third of pediatric gastroenterologists surveyed have administered 6-MP or AZA concurrently with IFX 10 mg/kg. This practice may be in flux, as recent reports suggest improved disease responsiveness and lower risk of antibodies to IFX with concomitant immunomodulators, despite concerns of hepatosplenic T-cell lymphoma[6-10]. In our survey, no oncologic or serious neurologic adverse outcomes were reported. Future studies should further clarify the risk vs benefits of concomitant immunomodulator therapy.
Our results also suggest that pediatric gastroenterologists in the United States are more likely to prescribe IFX 10 mg/kg to treat UC compared with those working outside the United States. The only difference found for responders in academic compared with non-academic settings was an increased use of high-dose IFX for maintenance of remission for severe UC among non-academic pediatric gastroenterologists.
These differences highlight the lack of universal guidelines and need for further studies regarding the optimal use of IFX 10 mg/kg for the treatment of UC.
The most common reported side effects were antibodies to IFX and infusion reactions. Infections were also reported by 12.8% of responders. One respondent reported death as an adverse outcome. The events that lead to this death are not known. It is thought, however, that this fatality was not a result of oncologic, neurologic or infectious complications as the same respondent did not report experiencing any of these adverse effects while using IFX 10 mg/kg.
Most pediatric gastroenterologists surveyed reported improved outcome as a result of prescribing IFX 10 mg/kg, documenting a benefit of the higher dose in pediatric UC. However, over half of the respondents reported worsening disease and the need for colectomy, possibly due to loss of responsiveness, or in some cases a lack of any response to the increased dose.
IFX is approved by the FDA for the treatment of moderate to severe UC at a dose of 5 mg/kg[3]. Insurance companies have often rejected appeals for reimbursement for increased doses of IFX due to lack of published data showing efficacy and safety for pediatric patients with UC. Despite this, however, our survey suggests that insurance approval or other insurance issues did not uniformly prevent pediatric gastroenterologists from prescribing IFX 10 mg/kg.
Insurance approval for higher doses of IFX is achieved via an exception process or appeal process. An exception process involves an initial request for a health plan to consider coverage for a prescribed medication therapy or to reevaluate the possible benefits. An appeal is a request for an insurance carrier to reconsider a coverage decision that was initially denied[11]. The exception and appeal processes require letters of support from the prescribing physician. The time-consuming processes to initiate higher doses of IFX provide some patients with potentially lifesaving therapy. On the other hand, this litigious process is often unsuccessful and may delay alternative intervention for some patients.
Modification of UC treatment often becomes necessary in patients who lose responsiveness to an initial dose or standard maintenance regimen of IFX. Modification may consist of an increase in dose, decrease in dosing interval (not queried here), or addition of alternative medical or surgical therapy[12]. Treatment strategies with IFX should optimize drug pharmacokinetics to maximize dose response and minimize the development of antibodies to IFX. This may delay the need to increase IFX dose and reduce the risk of complications. Among pediatric gastroenterologists who prescribe IFX, IFX 10 mg/kg has a recognized role and perceived benefit in the treatment of some pediatric UC patients. Future prospective controlled clinical trials using high-dose IFX and other anti-tumor necrosis factor-α antibody agents, and testing of specific dose increases may provide more standardized guidelines for the treatment of pediatric patients with UC.
COMMENTS
Background
In the last decade, infliximab (IFX) has become an alternative treatment of moderate to severe ulcerative colitis (UC) in both the adult and pediatric population. Based on current Food and Drug Administration recommendations the use of IFX for treatment of moderate to severe UC in adults is started at a dose of 5 mg/kg. However, in patients who lose responsiveness, the dose of IFX is commonly increased up to 10 mg/kg.
Research frontiers
No studies have documented benefit at this higher dose in pediatric patients. The efficacy, safety and insurance approval for high-dose IFX have also been of growing concern in the medical community. The authors conducted a survey of pediatric gastroenterologists to collect information regarding the use of IFX for the treatment of UC in the pediatric population.
Innovations and breakthroughs
Recently, the Pediatric Inflammatory Bowel Disease Collaborative Research Group (PIBDCRG) showed that children with corticosteroid-dependent or corticosteroid-refractory UC treated with IFX have a significant decrease in corticosteroid dependency and improved rates of colectomy. In the PIBDCRG study, dose escalation and decreased dose interval was at the discretion of prescribing physicians. The survey study conducted here is the first to report the extensive use of IFX 10 mg/kg by pediatric gastroenterologists for treatment of UC in the pediatric population.
Applications
This study offers a glimpse in the attitudes and practice patterns of pediatric gastroenterologists regarding the use of high-dose IFX for the treatment of pediatric UC. The results highlight the recognized role of IFX 10 mg/kg for the treatment of UC in the pediatric population and the need for more standardized guidelines regarding dose escalation.
Terminology
IFX is a biologic therapy used to treat autoimmune diseases such as UC. IFX is a monoclonal immunoglobulin G antibody that is genetically engineered to target tumor necrosis factor-alpha (TNF-α). By selectively targeting specific players in the inflammatory cascade such as TNF-α, biologic agents may spare the need for treatment with systemic corticosteroids and thereby reduce the number of side effects associated with such treatment.
Peer review
The authors examined the practice patterns of pediatric gastroenterologists regarding the use IFX for the treatment of UC. They found that a majority of those pediatric gastroenterologists surveyed have experience using IFX 10 mg/kg. In addition, significantly more pediatric gastroenterologists based in the United States have used IFX 10 mg/kg compared to those not based in the United States. Over one-third of pediatric gastroenterologists surveyed indicated insurance issues as a barrier to increasing IFX dosing.
Footnotes
Supported by DK060617 (MBH); DK080825 (JMW); DK077734 (NG); CCFA Career Development Award (NG); and NIH/NCRR UCSF-CTSI Grant, No. UL1 RR024131
Peer reviewer: Wojciech Blonski, MD, PhD, University of Pennsylvania, GI Research-Ground Centrex, 3400 Spruce St, Philadelphia, PA 19104, United States
S- Editor Sun H L- Editor Cant MR E- Editor Li JY
References
- 1.Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, Travers S, Rachmilewitz D, Hanauer SB, Lichtenstein GR, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–2476. doi: 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- 2.Hyams JS, Lerer T, Griffiths A, Pfefferkorn M, Stephens M, Evans J, Otley A, Carvalho R, Mack D, Bousvaros A, et al. Outcome following infliximab therapy in children with ulcerative colitis. Am J Gastroenterol. 2010;105:1430–1436. doi: 10.1038/ajg.2009.759. [DOI] [PubMed] [Google Scholar]
- 3.US Food and Drug Administration. Medication Guide for Remicade (Rem-eh-kaid) (Infliximab). Centocor Ortho Biotech Inc. February. 2011. Available from: http: //www.accessdata.fda.gov. Accessed June 22, 2011. [Google Scholar]
- 4.American Board of Pediatrics. The American Board of Pediatrics 2009-2010 Workforce Data. Available from: https: //www.abp.org. Accessed December 31, 2010.
- 5.Mamula P, Markowitz JE, Cohen LJ, von Allmen D, Baldassano RN. Infliximab in pediatric ulcerative colitis: two-year follow-up. J Pediatr Gastroenterol Nutr. 2004;38:298–301. doi: 10.1097/00005176-200403000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Mackey AC, Green L, Leptak C, Avigan M. Hepatosplenic T cell lymphoma associated with infliximab use in young patients treated for inflammatory bowel disease: update. J Pediatr Gastroenterol Nutr. 2009;48:386–388. doi: 10.1097/mpg.0b013e3181957a11. [DOI] [PubMed] [Google Scholar]
- 7.Baert F, Noman M, Vermeire S, Van Assche G, D’ Haens G, Carbonez A, Rutgeerts P. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn’s disease. N Engl J Med. 2003;348:601–608. doi: 10.1056/NEJMoa020888. [DOI] [PubMed] [Google Scholar]
- 8.Sokol H, Seksik P, Carrat F, Nion-Larmurier I, Vienne A, Beaugerie L, Cosnes J. Usefulness of co-treatment with immunomodulators in patients with inflammatory bowel disease treated with scheduled infliximab maintenance therapy. Gut. 2010;59:1363–1368. doi: 10.1136/gut.2010.212712. [DOI] [PubMed] [Google Scholar]
- 9.Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, Lichtiger S, D’Haens G, Diamond RH, Broussard DL, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383–1395. doi: 10.1056/NEJMoa0904492. [DOI] [PubMed] [Google Scholar]
- 10.Lichtenstein GR, Diamond RH, Wagner CL, Fasanmade AA, Olson AD, Marano CW, Johanns J, Lang Y, Sandborn WJ. Clinical trial: benefits and risks of immunomodulators and maintenance infliximab for IBD-subgroup analyses across four randomized trials. Aliment Pharmacol Ther. 2009;30:210–226. doi: 10.1111/j.1365-2036.2009.04027.x. [DOI] [PubMed] [Google Scholar]
- 11.Overview of the Exception and Appeals Process. Centocor Ortho Biotech Inc. April. 2009. Available from: http: //www.centocoraccessone.com. Accessed September 12, 2010. [Google Scholar]
- 12.Rutgeerts P, Vermeire S, Van Assche G. Biological therapies for inflammatory bowel diseases. Gastroenterology. 2009;136:1182–1197. doi: 10.1053/j.gastro.2009.02.001. [DOI] [PubMed] [Google Scholar]