Abstract
Purpose
To investigate the relationship between sperm apoptotic biomarkers and patient clinical characteristics, conventional sperm parameters and fertility potential.
Material and methods
Sperm analysis, phospholipid asymmetry, mitochondrial membrane potential (MMP) and DNA denaturation were assessed in 142 males of infertile couples. Seventy-three couples were allocated to the natural conception group, and 55 couples underwent IVF or ICSI.
Results
DNA denaturation correlated positively with age and negatively with testicular volume (TV). MMP correlated negatively with BMI and FSH and positively with TV. Normal viable sperm correlated positively with TV and negatively with age, BMI and FSH. DNA denaturation was associated with a significantly lower natural pregnancy rate (OR 5.4, 95% CI:1.3–22, p = 0.011).
Conclusion
Sperm apoptosis is related to male age, BMI, testicular volume and FSH. Among the apoptotic markers, only DNA denaturation has been found to predict natural pregnancy better than conventional sperm parameters.
Keywords: Patient clinical parameters, DNA denaturation, IUI, Conventional sperm parameters, Spontaneous pregnancy
Introduction
Diagnosis of male infertility has been mainly based on the analysis of conventional sperm parameters as recommended by the World Health Organization [43]. However, it has become apparent that none of these parameters alone or their combination are useful in the diagnosis of infertility [21].
To improve the prediction of fertility potential in clinical practice, the use of sperm function biomarkers, i.e. of the markers of apoptosis, has been proposed.
Apoptosis is increased in spermatozoa of infertile men affected by cryptorchidism, infection or varicocele [4]. Pathological conditions along the male genital tract impair sperm DNA [38]. Apoptotic markers measured by flow cytometry are less subjective than the sperm parameters determined by conventional sperm analysis.
However, there is still an unresolved question of whether sperm apoptotic markers have a prognostic power in clinical practice.
Among the apoptotic markers, DNA damage has been the most thoroughly investigated so far. It is considered a significant marker in the diagnosis of male infertility [3, 5] and in the prediction of natural conception [42]. According to some authors, it might provide additional information regarding the outcome of in vitro fertilization [6, 14, 26, 46]. However, there are authors denying this property to DNA damage [8, 12, 48].
In addition to DNA denaturation, potentially related to late apoptosis, we measured two other apoptosis signaling markers that appear earlier in apoptosis [16, 20], i.e. the changes of phosphatidylserine asymmetry in the plasma membrane and in the mitochondrial membrane potential (MMP). The assessment of MMP in sperm is important considering the functionality of mitochondria during apoptosis. The diagnostic value of these early apoptotic events has been proven [2, 17, 24]; nonetheless there is little [29] or no information on their predictive value of male fertility.
This retrospective study on male partners of infertile couples was designed to analyze the relationship between sperm apoptotic markers (changes in cytoplasmic membrane asymmetry, MMP and DNA integrity) in processed ejaculated sperm and patient clinical characteristics, conventional sperm parameters of neat sperm, and fertility potential. The latter was evaluated according to clinical pregnancy, achieved in cycles of natural conception and intrauterine insemination (IUI) or in classical in vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI) cycles.
Materials and methods
Patients and study design
A total of 142 consecutive couples attending the infertility outpatient clinic of the Reproductive Unit, Department of Obstetrics and Gynecology, University Medical Centre Ljubljana, were enrolled in the study. Experimental protocol was approved by the national medical ethics committee and written informed consent was obtained from all participants.
In male partners testicular volume was measured using Prader orchidometer. According to their body mass index (BMI), males were divided in two groups (< 30 and ≥30 kg/m2). Their serum FSH was measured by a solid-phase, two-site chemiluminescent immunometric assay (IMMULITE 1000 FSH, Diagnostic Products Corporation, Los Angeles, CA).
The couples in whom female infertility factor was ruled out and were able to conceive naturally or after IUI (n = 73), constituted the “Natural conception group”.
The IVF/ICSI group consisted of 55 couples: in 22 the cause of infertility was oligoasthenoteratozoospermia, in 21 unexplained male infertility, and in 12 tubal factor infertility. Fourteen couples did not continue infertility treatment and were not considered in the final analyses.
Sperm analysis and preparation
Semen samples were collected by masturbation after 2–5 days of sexual abstinence. After an hour, semen was assessed according to the WHO guidelines [43]. Sperm was considered to be normal when sperm count was ≥40.106 sperm, rapid progressive motility or motility “a” ≥25%, and normal morphology using strict criteria ≥14%. To prepare semen, an aliquot of semen was purified using a two-step (100%/40%) discontinuous Pure Sperm (Nidacon International AB, Mölndal, Sweden) gradient diluted in Sperm Preparation Medium (Origio, Måløv, Denmark). After centrifugation at 160 g for 30 min, motile sperm from the 100% layer were washed in Sperm Preparation Medium and centrifuged at 220 g for 10 min. Purified population was resuspended in 1 mL of the same medium. The concentration of sperm with motility “a” was estimated. This fresh semen was used for cytofluorometric analysis. Data were collected on a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA), and sperm phosphatidylserine exposure, MMP and DNA fragmentation were determined using CellQuest ™ (BD Biosciences) software at excitation wavelengths of 488 nm.
Detection of phospholipid asymmetry in the sperm plasma membrane
The loss of plasma membrane is one of the earliest features of apoptosis. In apoptotic sperm, the membrane phospholipid phosphatidylserine is translocated from the inner to the outer leaflet of the plasma membrane, thereby exposing phosphatidylserine to the external cellular environment. Disruption of the membrane asymmetry was detected using fluorescein isothiocyanate-conjugated annexin V (Annexin V-FITC; Becton Dickinson Pharmingen, San Diego, CA, USA). Semen samples were diluted to 1 × 106 sperm/mL in 100 μL of binding buffer with 5 μg annexin V. The samples were simultaneously stained with 1 μg/mL propidium iodide (PI; Molecular Probes, Eugene, Oregon, USA). Unstained samples were used as negative fluorescence controls. Two replicate experiments were done for each sample, and the average values were used in further analyses. According to their reactivity to annexin V and PI, sperm were classified as viable (negative annexin V and negative PI), sperm in early apoptosis (annexin V positive and PI negative), and dead cells, permeable to PI (Fig. 1), Sperm membrane phospholipid asymmetry changes were expressed as percentages of viable, apoptotic and dead sperm.
Fig. 1.
Flow cytometry detection of phosphatidylserine exposure analyzed with annexin V and propidium iodide (PI). Sperm at the final concentration of 1 × 106/mL were incubated with annexin V and 1 μg/mL PI in 100 μL of binding buffer. In the left panel sperm cells were mostly annexin V and PI negative, indicating that they were viable and not undergoing apoptosis. The right panel represents a sample with three populations of cells: normal viable sperm were annexin V and PI negative, early apoptotic sperm were annexin V positive and PI negative, and dead sperm were annexin V ± and PI positive
Assessment of sperm mitochondrial membrane potential
Mitochondrial membrane potential (MMP) was measured by means of DiOC6(3) staining. PI was used as a supravital fluorescent stain. Because DiOC6(3) has a single wave-length emission, a normal MMP was attributed to cells with a high fluorescence signal; cells with lower DiOC6(3) emission (lower MMP) were defined as apoptotic. Sperm (1 × 106/mL) were incubated in 1 mL DiOC6(3) (0.5 nmol/L) in a 37°C water bath for 20 min. In the analysis we used only the percentage of viable sperm with normal MMP (Fig. 2).
Fig. 2.
Assessment of sperm mitochondrial membrane potential (MMP) measured by means of 3,3′-dihexyloxacarbocyanine iodide (DiOC6(3)). Sperm from each fresh sample were incubated with DiOC6(3). Ten thousand cells were analyzed by FACSCalibur flow cytometer. The left sample has a high percentage of viable sperm cells with normal MMP. The right sample has more cells with lower MMP
Sperm DNA denaturation
Sperm were treated with a pH 1.2 detergent solution containing 0.1% Triton X-100, 0.15 mol/L NaCl, and 0.08 N HCl for 30 s, and then stained with 0.02 mg/mL of purified acridine orange (AO; Molecular Probes) in a phosphate-citrate buffer, pH 6.0, at the final concentration of 1 × 106/mL. Cells were analyzed by FACSCalibur flow cytometer equipped with air-cooled Argon ion laser. Ten thousand events were accumulated for each measurement. Under these conditions, when excited with a 488 nm light source, AO intercalating with double-stranded DNA emits green fluorescence, and AO associated with single-stranded DNA emits red fluorescence. Thus, sperm chromatin damage can be quantified by flow cytometric measurements of the metachromatic shift from green (native, double-stranded DNA) to red (denatured, single-stranded DNA) fluorescence (Fig. 3). In the analysis we used the percentages of sperm with denaturated DNA only.
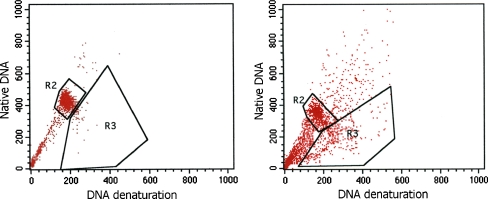
Fig. 3.
Flow cytometry analysis of sperm DNA denaturation. Sperm cells were stained with acridine orange. The left sample shows mostly native double-stranded DNA in the gate R2. In the right sample we can see two populations of cells: cells with native double-stranded DNA in the gate R2, and cells with denatured single-stranded DNA in the gate R3
IUI, IVF and ICSI cycles
Sperm were prepared using DGC. In IUI cycles, ovarian stimulation was performed using clomiphene citrate – 100 mg daily from day 5 to day 9 of the cycle – or recombinant human menopausal gonadotropin (Gonal F, Serono, Geneva, Switzerland) – 150 IU daily from day 2 of the cycle on. In IVF and ICSI cycles, ovarian stimulation was induced using a long protocol of gonadotropin-releasing hormone agonist buserelin acetate, (Suprefact; Hoechst AG, Frankfurt, Germany) and Gonal F 225 IU daily. Human chorionic gonadotropin (hCG, Primogonyl; Geneva, Serono) was given (10 000 IU i.m.) when follicles reached a mean diameter of 18 mm. Sperm insemination or oocyte retrieval was carried out 36 h after hCG injection. Embryos were cultured to the blastocyst stage. At most 2 best developed embryos were transferred.
Statistical analysis
The Statistical Program for Social Sciences (SPSS, version 17; SPSS Inc., Chicago, IL) was used for statistical analysis. The Spearman correlation test, Mann-Whitney test, and analysis of variance were used to evaluate relationships between sperm apoptotic markers and male age, total testicular volume, BMI, FSH and conventional sperm parameters.
Mann-Whitney test, analysis of variance, logistic regression and chi-squared test were used to detect whether conventional sperm parameters and apoptotic markers differed with regard to the achievement of natural pregnancy or to the outcome of the first IVF/ICSI attempt (embryo development to the blastocyst stage and occurrence of a pregnancy).
Results
The characteristics of the enrolled male partners of infertile couples are presented in Table 1.
Table 1.
Clinical characteristics of 142 infertile couples included in the study
| Characteristic | Value |
|---|---|
| Male age (years) | 33.7 ± 5.4 (24.0–55.0) |
| Female age (years) | 31.6 ± 4.5 (21.0–44.0) |
| Duration of infertility (years) | 2.6 ± 2.4 (0.5–15.0) |
| Range of infertility (primary/secondary) | 106/36 |
| BMI (kg/m2) | 25.9 ± 3.3 (19.4–39.2) |
| Total testicular volume (mL) | 33.1 ± 9.1 (12.0–67.0) |
| FSH (IU/L) | 4.8 ± 2.9 (1.4–17.7) |
| Neat semen | |
| Sperm count (x106) | 197.3 ± 167.9 (0.4–781.0) |
| Sperm motility “a” (%) | 27.9 ± 11.7 (0.0–50.0) |
| Normal sperm morphology (%) | 24.3 ± 14.2 (0.0–59.0) |
| Sperm processed by density–gradient centrifugation | |
| Dead sperm (%) | 28.7 ± 16.8 (4.0–76.0) |
| Apoptotic sperm (%) | 11.4 ± 11.9 (0.5–69.0) |
| Normal viable sperm (annexin V negative and PI negative) (%) | 59.0 ± 22.4 (2.0–91.0) |
| Viable sperm with normal MMP (%) | 65.4 ± 23.9 (2.0–97.0) |
| Sperm with DNA denaturation (%) | 15.0 ± 14.2 (0.1–95.0) |
Values are either mean ± SD (range) or percentage (%)
BMI body mass index; MMP mitochondrial membrane potential
Correlations between sperm apoptotic markers and clinical and hormonal characteristics are presented in Table 2.
Table 2.
Correlations between apoptotic markers in the processed sperm and clinical and hormonal characteristics of male partners of infertile couples
| Male age | BMI* | Total testicular volume | FSH | |
|---|---|---|---|---|
| Dead sperm (%) | NS | NS | NS | NS |
| Apoptotic sperm (%) | NS | p = 0.005 | NS | r = 0.227, p = 0.008 |
| Normal viable sperm (%) | r = −0.244, p = 0.004 | p = 0.012 | r = 0.259, p = 0.002 | r = −0.253, p = 0.003 |
| Viable sperm with normal MMP (%) | NS | p = 0.002 | r = 0.295, p < 0.001 | r = −0.262, p = 0.002 |
| Sperm with DNA denaturation (%) | r = 0.206, p = 0.014 | NS | r = −0.168, p = 0.045 | NS |
r Spearman (rank) coefficient; NS not significant (p > 0.05)
*Mann-Whitney test and analysis of variance were used to evaluate relationships between sperm apoptotic markers and body mass index (BMI). According to BMI, males were divided in two groups (<30 and ≥30 kg/m2). Men with BMI ≥30 kg/m2 (n = 17) had more apoptotic, fewer normal viable sperm and lower percentage of sperm with normal MMP than men with BMI < 30 kg/m2 (n = 125)
The percentage of apoptotic sperm was significantly correlated with FSH (r = 0.227, p = 0.008) and BMI (p = 0.005).
The percentage of normal viable sperm (annexin V negative and PI negative) was significantly correlated with male age (r = −0.244, p = 0.004), testicular volume (r = 0.259, p = 0.002), BMI (p = 0.012), and FSH (r = −0.253, p = 0.003).
Viable sperm with normal mitochondrial membrane potential was determined using DiOC6(3) staining (Fig. 4). The percentage of viable sperm with normal MMP was significantly correlated with testicular volume (r = 0.295, p < 0.001), BMI (p = 0.002), and FSH (r = −0.262, p = 0.002).
Fig. 4.

Sperm at the final concentration of 1 × 106/mL were incubated with DiOC6(3) and 1μg/mL propidium iodide (PI) and analyzed by confocal microscopy. Sperm in the upper left side of the photograph has normal mitochondrial membrane potential (MMP) and is defined as viable sperm with normal MMP. Sperm at the bottom right has low MMP and damaged plasma membrane which is permeable to PI
The percentage of sperm with DNA denaturation was correlated with male age (r = 0.206, p = 0.014) and testicular volume (r = −0.168, p = 0.045). DNA denaturation was significantly more frequent in men aged 36–55 years than in those aged 24–35 years (20.5% vs. 12.6%). The percentage of sperm with denaturated DNA was higher in men with total testicular volume of 12–29 mL than in those with testicular volume of 30–67 mL (19.2 mL vs. 12.9 mL).
A highly significant (p < 0.001) correlation was observed between dead sperm, apoptotic sperm, viable sperm, viable sperm with normal MMP, sperm with DNA denaturation and conventional sperm parameters.
In the Natural conception group, 28 (38.4%) men had abnormal sperm according to the WHO criteria. In 17 (37.8%) of the 45 men with normal sperm, sperm with DNA denaturation exceeded 10%.
There were no statistically significant differences between the Natural conception group and the IVF/ICSI group with regard to conventional sperm parameters and apoptotic markers.
In the Natural conception group, 16 female partners of the 73 couples conceived naturally (21.9% pregnancy rate per couple) or after IUI (5 couples in 10 cycles).
In the IVF/ICSI group, 55 couples underwent altogether 143 IVF or ICSI cycles, of which 28 were first IVF and 27 first ICSI cycles, considered in the analysis. Sixteen pregnancies (29.1% pregnancy rate per cycle) were obtained.
In the Natural conception group, the following parameters differed significantly according to whether women conceived or not: FSH, sperm motility “a” and normal morphology, viable sperm, viable sperm with normal MMP and DNA denaturation (Table 3).
Table 3.
Comparison of conventional sperm parameters and apoptotic markers in the Natural conception group according to whether the female partner conceived or not
| Natural conception group (n = 73) | |||
|---|---|---|---|
| Pregnancies (n/%) | 16/21.9 | ||
| Pregnancy | No pregnancy | p value* | |
| FSH (IU/L) | 3.2 ± 1.8 | 4.9 ± 3.1 | 0.011 |
| Male age (years) | 32.9 ± 4.1 | 33.9 ± 5.2 | 0.565 |
| Female age (years) | 32.5 ± 3.5 | 31.7 ± 4.4 | 0.293 |
| Sperm count (x106) | 295.8 ± 178.8 | 208.5 ± 192.5 | 0.053 |
| Sperm motility »a« (%) | 34.0 ± 9.2 | 26.2 ± 11.8 | 0.022 |
| Normal sperm morphology (%) | 31.5 ± 13.8 | 21.9 ± 14.1 | 0.028 |
| Dead sperm (%) | 22.9 ± 16.7 | 30.9 ± 17.6 | 0.082 |
| Apoptotic sperm (%) | 6.2 ± 4.9 | 14.2 ± 14.9 | 0.068 |
| Normal viable sperm (%) | 70.2 ± 17.2 | 54.3 ± 25.2 | 0.016 |
| Viable sperm with normal MMP (%) | 76.9 ± 16.0 | 60.9 ± 26.2 | 0.022 |
| Sperm with DNA denaturation (%) | 7.7 ± 7.6 | 18.8 ± 17.3 | 0.011 |
* as evaluated by Mann-Whitney U-test
The logistic regression model, in which we entered individual conventional sperm parameters or the information on normality or abnormality of sperm quality based on the combination of sperm parameters revealed that sperm parameters had a lower predictive value for the achievement of pregnancy than DNA denaturation. Nagelkerke’s R2 was 0.151 when sperm conventional parameters were used as predictors, and 0.291 when DNA denaturation and FSH were used as predictors. DNA denaturation and FSH were significant independent predictors.
Women whose male partners had ≥10% sperm with DNA denaturation had a five times lower chance to conceive naturally than women whose partners had <10% DNA denaturation (OR 5.4, 95% CI:1.3–22).
The pregnancy rate in women whose partners had normal sperm did not differ significantly from that in women whose partners had abnormal sperm (p = 0.06).
The pregnancy rate was higher in the couples whose sperm had less than 10% DNA denaturation than in those who had more than 10% denaturation. When dividing the couples with normal sperm according to the percentage of sperm with DNA denaturation, the pregnancy rate in women whose partners had <10% denaturation was higher (p = 0.005).
In the IVF/ICSI group, neither conventional sperm parameters in neat semen nor sperm apoptotic markers measured in semen prepared by DGC were predictive of embryo development to the blastocyst stage and achievement of pregnancy (Table 4).
Table 4.
Comparison of conventional sperm parameters and apoptotic markers in the IVF/ICSI group according to the development of at least one blastocyst and occurrence of pregnancy. Only first IVF/ICSI cycles were considered in the analysis
| Pregnancies (n/%) | IVF/ICSI group (n = 55) | |||||
|---|---|---|---|---|---|---|
| 16 /29.1 | ||||||
| At least 1 blastocyst developed | No blastocyst | p value | Pregnancy | No pregnancy | p value | |
| Male age (years) | 36.0 ± 4.5 | 35.1 ± 5.0 | 0.618 | 36.0 ± 5.9 | 35.7 ± 4.4 | 0.822 |
| Female age (years) | 32.5 ± 4.0 | 33.5 ± 4.4 | 0.289 | 32.7 ± 4.1 | 32.0 ± 4.1 | 0.548 |
| FSH (IU/L) | 3.1 ± 1.7 | 4.9 ± 3.1 | 0.717 | 5.1 ± 3.6 | 4.6 ± 2.4 | 0.593 |
| Sperm count (x106) | 201.9 ± 176.5 | 191.5 ± 224.5 | 0.549 | 165.2 ± 166.3 | 213.2 ± 196.1 | 0.394 |
| Sperm motility »a« (%) | 25.9 ± 12.1 | 26.1 ± 10.2 | 0.731 | 24.0 ± 12.9 | 26.7 ± 11.1 | 0.454 |
| Normal sperm morphology (%) | 22.3 ± 14.5 | 18.6 ± 12.6 | 0.505 | 18.4 ± 12.7 | 22.6 ± 14.5 | 0.313 |
| Dead sperm (%) | 32.7 ± 18.5 | 25.3 ± 16.4 | 0.206 | 35.2 ± 16.7 | 28.9 ± 18.6 | 0.745 |
| Apoptotic sperm (%) | 13.4 ± 13.5 | 12.5 ± 17.0 | 0.961 | 14.2 ± 14.8 | 12.8 ± 14.3 | 0.248 |
| Normal viable sperm (%) | 53.6 ± 25.9 | 62.0 ± 21.4 | 0.379 | 50.5 ± 23.9 | 58.0 ± 25.3 | 0.317 |
| Viable sperm with normal MMP (%) | 62.2 ± 27.5 | 69.1 ± 22.6 | 0.401 | 56.9 ± 22.9 | 66.9 ± 27.3 | 0.203 |
| Sperm with DNA denaturation (%) | 16.1 ± 15.2 | 17.6 ± 16.2 | 0.832 | 19.2 ± 19.5 | 16.4 ± 19.2 | 0.745 |
Discussion
DNA denaturation correlated positively with age and negatively with testicular volume. MMP correlated negatively with BMI and FSH, and positively with testicular volume.
Sperm DNA denaturation is extensively reported to be increased with aging [33, 45]. We also found that the percentage of viable sperm (annexin-V negative and PI negative) declined with advancing male age. The association between age and increased plasma membrane translocation of phosphatidylserine has also been reported by Colin et al. [11].
We found that BMI was correlated positively with the percentage of apoptotic cells, and negatively with MMP. Previously, Chavarro et al. [10] found lower sperm count and ejaculate volume, and higher percentage of sperm with DNA fragmentation in men with BMI exceeding 30 kg/m2. On the other hand, sperm were not found to be impaired in healthy overweight male partners of subfertile couples [13], whereas Martini et al. [30] found a negative association between BMI and sperm motility; as this relation was concomitant with decreased levels of alpha-glucosidase, the authors of the latter study postulated that the impairment of sperm in men with increased BMI was due to a dysfunctional epididymis. The relationship between BMI and MMP has also been reported by La Vignera et al. [25].
We have found significant correlations between the testicular volume and sperm apoptotic markers; the percentages of normal viable sperm and viable sperm with normal MMP were correlated positively, and sperm with DNA denaturation were correlated negatively with the testicular volume. This is an important finding as testicular volume reflects the testicular function.
FSH plays an important role in germ cell survival and is a key regulator of testicular function, necessary for the maintenance of spermatogenesis in the adult. Our analysis revealed a positive correlation between FSH and the percentage of apoptotic sperm, and negative correlation between FSH and viable sperm. Furthermore, we have found a highly significant negative correlation between FSH and viable sperm with normal MMP, which confirms the findings by Ruwanpura et al. [36], obtained in experimental conditions. Similarly, the changes in MMP already observed in cases of inflammation [9, 35, 40, 47] were correlated with FSH, the main hormonal regulator of spermatogenesis; however, this relation did not exist with DNA denaturation, which is in agreement with the findings of Appasamy et al. [1].
We have observed a highly significant correlation between dead, apoptotic and viable sperm, viable sperm with normal MMP, sperm with DNA denaturation and conventional sperm parameters, which is in agreement with the findings in the literature [3, 6, 27, 41].
Increased sperm nuclear damage in infertile men with normozoospermia has already been reported [39]. This association is not clear. A disagreement between classical sperm analysis and DNA denaturation may be partly due to intra-laboratory variability. It should be taken into account when assessing male fertility potential. Indeed, the results obtained in this study indicate a powerful predictive value of DNA denaturation independently of the results of conventional sperm analysis.
Reference values of conventional sperm parameters to diagnose male fertility as proposed by WHO [43, 44] have been largely criticized [21, 23]. Moreover, there is little consensus as to which sperm characteristic is the best predictor of fertility [28]. Consequently, different associations of sperm parameters have been proposed to predict pregnancy and miscarriage [7, 22, 32, 34]. We have found that conventional sperm parameters and apoptotic markers (membrane asymmetry loss, DNA denaturation and MMP) are related to natural conception and conception in IUI cycles, but not to IVF/ICSI conception. Logistic regression demonstrated that DNA denaturation and FSH combined are better predictors of natural pregnancy than conventional sperm parameters alone.
Like Giwercman et al. [18], we have found that in males whose partners conceived spontaneously, DNA denaturation was significantly lower than in males whose partners did not conceive naturally or after IUI. High quality non-apoptotic sperm, characterized by low levels of DNA damage, demonstrate high sperm capacitation [19], improved acrosome reaction [27] and oocyte penetration capacity [37].
FSH may be used as a surrogate for sperm quality [31]; its combination with DNA denaturation renders the effect of DNA denaturation to predict natural pregnancy increasingly potent. In contrast to couples who conceived spontaneously or after IUI, sperm apoptotic markers in men undergoing IVF or ICSI were not predictive of pregnancy. Assisted reproduction techniques enable the use of selected best quality sperm with low DNA denaturation [15] and less MMP disruption [20]. The negative effects of apoptosis on fertilization and pregnancy are less present or even absent. Another explanation for the absence of predictive power of DNA damage for IVF/ICSI outcomes might be that when using DGC, which reduces the percentage of sperm with DNA denaturation, there exist other types of DNA damage that are not detected by acridine orange [8], but additionally affecting IVF/ICSI outcomes. As a consequence, sperm DNA integrity may or may not have an impact on the clinical results of IVF and ICSI cycles [12].
Assessment of DNA damage in sperm has to be proposed to each infertile couple at the beginning of infertility workup to improve counselling and decision on the type of treatment.
Acknowledgments
Conflict of interest
There is no conflict of interest.
Footnotes
Capsule Sperm apoptotic markers as indicators and predictors of fertility
References
- 1.Appasamy M, Muttukrishna S, Pizzey AR, Ozturk O, Groome NP, Serhal P, et al. Relationship between male reproductive hormones, sperm DNA damage and markers of oxidative stress in infertility. Reprod Biomed Online. 2006;14:159–165. doi: 10.1016/S1472-6483(10)60783-3. [DOI] [PubMed] [Google Scholar]
- 2.Auger J, Ronot X, Dadoune JP. Human sperm mitochondrial function related to motility: a flow and image cytometric assessment. J Androl. 1989;10:439–448. doi: 10.1002/j.1939-4640.1989.tb00135.x. [DOI] [PubMed] [Google Scholar]
- 3.Aziz N, Said T, Paasch U, Agarwal A. The relationship between human sperm apoptosis, morphology and the sperm deformity index. Hum Reprod. 2007;22:1413–1419. doi: 10.1093/humrep/dem016. [DOI] [PubMed] [Google Scholar]
- 4.Baccetti B, Collodel G, Piomboni PJ. Apoptosis in human ejaculated sperm cells (notulae seminologicae 9) J Submicrosc Cytol Pathol. 1996;28:587–596. [PubMed] [Google Scholar]
- 5.Barroso G, Taylor S, Morshedi M, Manzur F, Gaviño F, Oehninger S. Mitochondrial membrane potential integrity and plasma membrane translocation of phosphatidylserine as early apoptotic markers: a comparison of two different subpopulations. Fertil Steril. 2006;85:149–154. doi: 10.1016/j.fertnstert.2005.06.046. [DOI] [PubMed] [Google Scholar]
- 6.Boe-Hansen GB, Fedder J, Ersbøll AK, Christensen P. The sperm chromatin structure assay as a diagnostic tool in the human fertility clinic. Hum Reprod. 2006;21:1576–1582. doi: 10.1093/humrep/del019. [DOI] [PubMed] [Google Scholar]
- 7.Bostofte E. Prognostic parameters in predicting pregnancy. A twenty-year follow-up study comprising semen analysis in 765 men of infertile couples evaluated by the Cox regression model. Acta Obstet Gynecol Scand. 1987;66:617–624. doi: 10.3109/00016348709022067. [DOI] [PubMed] [Google Scholar]
- 8.Bungum M, Spanò M, Humaidan P, Eleuteri P, Rescia M, Giwercman A. Sperm chromatin structure assay parameters measured after density gradient centrifugation are not predictive for the outcome of ART. Hum Reprod. 2008;23:4–10. doi: 10.1093/humrep/dem353. [DOI] [PubMed] [Google Scholar]
- 9.Burrello N, Salmeri M, Perdichizzi A, Bellanca S, Pettinato G, D’Agata R, et al. Candida albicans experimental infection: effects on human sperm motility, mitochondrial membrane potential and apoptosis. Reprod Biomed Online. 2009;18:496–501. doi: 10.1016/S1472-6483(10)60125-3. [DOI] [PubMed] [Google Scholar]
- 10.Chavarro JE, Toth TL, Wright DL, Meeker JD, Hauser R. Body mass index in relation to semen quality, sperm DNA integrity, and serum reproductive hormone levels among men attending an infertility clinic. Fertil Steril. 2010;93:2222–2231. doi: 10.1016/j.fertnstert.2009.01.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colin A, Barroso G, Gómez-López N, Duran EH, Oehninger S. The effect of age on the expression of apoptosis biomarkers in human spermatozoa. Fertil Steril. 2010;94:2609–2614. doi: 10.1016/j.fertnstert.2010.04.043. [DOI] [PubMed] [Google Scholar]
- 12.Collins JA, Barnhart KT, Schlegel PN. Do sperm DNA integrity tests predict pregnancy with in vitro fertilization? Fertil Steril. 2008;89:823–831. doi: 10.1016/j.fertnstert.2007.04.055. [DOI] [PubMed] [Google Scholar]
- 13.Duits FH, Wely M, Veen F, Gianotten J. Healthy overweight male partners of subfertile couples should not worry about their semen quality. Fertil Steril. 2010;94:1356–1359. doi: 10.1016/j.fertnstert.2009.05.075. [DOI] [PubMed] [Google Scholar]
- 14.Duran EH, Gürgan T, Günalp S, Enginsu ME, Yarali H, Ayhan A. A logistic regression model including DNA status and morphology of spermatozoa for prediction of fertilization in vitro. Hum Reprod. 1998;13:1235–1239. doi: 10.1093/humrep/13.5.1235. [DOI] [PubMed] [Google Scholar]
- 15.Ebner T, Shebl O, Moser M, Mayer RB, Arzt W, Tews G. Easy sperm processing technique allowing exclusive accumulation and later usage of DNA-strandbreak-free spermatozoa. Reprod Biomed Online. 2011;22:37–43. doi: 10.1016/j.rbmo.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Espinoza JA, Paasch U, Villegas JV. Mitochondrial membrane potential disruption pattern in human sperm. Hum Reprod. 2009;24:2079–2085. doi: 10.1093/humrep/dep120. [DOI] [PubMed] [Google Scholar]
- 17.Gallon F, Marchetti C, Jouy N, Marchetti P. The functionality of mitochondria differentiates human spermatozoa with high and low fertilizing capability. Fertil Steril. 2006;86:1526–1530. doi: 10.1016/j.fertnstert.2006.03.055. [DOI] [PubMed] [Google Scholar]
- 18.Giwercman A, Lindstedt L, Larsson M, Bungum M, Spano M, Levine RJ, et al. Sperm chromatin structure assay as an independent predictor of fertility in vivo: a case-control study. Int J Androl. 2010;33:e221–e227. doi: 10.1111/j.1365-2605.2009.00995.x. [DOI] [PubMed] [Google Scholar]
- 19.Grunewald S, Kriegel C, Baumann T, Glander HJ, Paasch U. Interactions between apoptotic signal transduction and capacitation in human spermatozoa. Hum Reprod. 2009;24:2071–2078. doi: 10.1093/humrep/dep178. [DOI] [PubMed] [Google Scholar]
- 20.Grunewald S, Reinhardt M, Blumenauer V, Hmeidan AF, Glander HJ, Paasch U. Effects of post-density gradient swim-up on apoptosis signalling in human spermatozoa. Andrologia. 2010;42:127–131. doi: 10.1111/j.1439-0272.2009.00978.x. [DOI] [PubMed] [Google Scholar]
- 21.Guzick DS, Overstreet JW, Factor-Litvak P, Brazil CK, Nakajima ST, Coutifaris C, et al. Vogel DL; National Cooperative Reproductive Medicine Network: Sperm morphology, motility, and concentration in fertile and infertile men. N Engl J Med. 2001;345:1388–1393. doi: 10.1056/NEJMoa003005. [DOI] [PubMed] [Google Scholar]
- 22.Jedrzejczak P, Taszarek-Hauke G, Hauke J, Pawelczyk L, Duleba AJ. Prediction of spontaneous conception based on semen parameters. Int J Androl. 2008;31:499–507. doi: 10.1111/j.1365-2605.2007.00799.x. [DOI] [PubMed] [Google Scholar]
- 23.Joffe M. Semen quality analysis and the idea of normal fertility. Asian J Androl. 2010;12:79–82. doi: 10.1038/aja.2009.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kotwicka M, Jendraszak M, Jedrzejczak P. Phosphatidylserine membrane translocation in human spermatozoa: topography in membrane domains and relation to cell vitality. J Membr Biol. 2011;240:165–170. doi: 10.1007/s00232-011-9357-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.La Vignera S, Condorelli R, Vicari E, Calogero AE. Negative impact of increased body weight on sperm conventional and non-conventional flow cytometric sperm parameters. J Androl. 2012;33:53–58. [DOI] [PubMed]
- 26.Larson KL, DeJonge CJ, Barnes AM, Jost LK, Evenson DP. Sperm chromatin structure assay parameters as predictors of failed pregnancy following assisted reproductive techniques. Hum Reprod. 1999;15:1717–1722. doi: 10.1093/humrep/15.8.1717. [DOI] [PubMed] [Google Scholar]
- 27.Lee TH, Liu CH, Shih YT, Tsao HM, Huang CC, Chen HH, et al. Magnetic-activated cell sorting for sperm preparation reduces spermatozoa with apoptotic markers and improves the acrosome reaction in couples with unexplained infertility. Hum Reprod. 2010;25:839–846. doi: 10.1093/humrep/deq009. [DOI] [PubMed] [Google Scholar]
- 28.Lewis SEM. Is sperm evaluation useful in predicting human fertility? Reproduction. 2007;134:31–40. doi: 10.1530/REP-07-0152. [DOI] [PubMed] [Google Scholar]
- 29.Marchetti C, Obert G, Deffosez A, Formstecher P, Marchetti P. Study of mitochondrial membrane potential, reactive oxygen species, DNA fragmentation and cell viability by flow cytometry in human sperm. Hum Reprod. 2002;17:1257–1265. doi: 10.1093/humrep/17.5.1257. [DOI] [PubMed] [Google Scholar]
- 30.Martini AC, Tissera A, Estofán D, Molina RI, Mangeaud A, Cuneo MF, et al. Overweight and seminal quality: a study of 794 patients. Fertil Steril. 2010;94:1739–1743. doi: 10.1016/j.fertnstert.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 31.Meeker JD, Godfrey-Bailey L, Hauser R. Relationships between serum hormone levels and semen quality among men from an infertility clinic. J Androl. 2007;28:397–406. doi: 10.2164/jandrol.106.001545. [DOI] [PubMed] [Google Scholar]
- 32.Merwe FH, Kruger TF, Oehninger SC, Lombard CJ. The use of semen parameters to identify the subfertile male in the general population. Gynecol Obstet Invest. 2005;59:86–91. doi: 10.1159/000082368. [DOI] [PubMed] [Google Scholar]
- 33.Moskovtsev SI, Mullen JB, Lecker I, Jarvi K, White J, Roberts M, et al. Frequency and severity of sperm DNA damage in patients with confirmed cases of male infertility of different aetiologies. Reprod Biomed Online. 2010;20:759–763. doi: 10.1016/j.rbmo.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 34.Nallella KP, Sharma RK, Aziz N, Agarwal A. Significance of sperm characteristics in the evaluation of male infertility. Fertil Steril. 2006;85:629–634. doi: 10.1016/j.fertnstert.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 35.Rennemeier C, Frambach T, Hennicke F, Dietl J, Staib P. Microbial quorum-sensing molecules induce acrosome loss and cell death in human spermatozoa. Infect Immun. 2009;77:4990–4997. doi: 10.1128/IAI.00586-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruwanpura SM, McLachlan RI, Matthiesson KL, Meachem SJ. Gonadotrophins regulate germ cell survival, not proliferation, in normal adult men. Hum Reprod. 2008;23:403–411. doi: 10.1093/humrep/dem376. [DOI] [PubMed] [Google Scholar]
- 37.Said T, Agarwal A, Grunewald S, Rasch M, Baumann T, Kriegel C, et al. Selection of nonapoptotic spermatozoa as a new tool for enhancing assisted reproduction outcomes: an in vitro model. Biol Reprod. 2006;74:530–537. doi: 10.1095/biolreprod.105.046607. [DOI] [PubMed] [Google Scholar]
- 38.Sakkas D, Alvarez JG. Sperm DNA fragmentation. Mechanisms of origin, impact on reproductive outcome, and analysis. Fertil Steril. 2010;93:1027–1036. doi: 10.1016/j.fertnstert.2009.10.046. [DOI] [PubMed] [Google Scholar]
- 39.Saleh RA, Agarwal A, Nelson DR, Nada EA, El-Tonsy MH, Alvarez JG, et al. Increased sperm nuclear DNA damage in normozoospermic infertile men: a prospective study. Fertil Steril. 2002;78:313–318. doi: 10.1016/S0015-0282(02)03219-3. [DOI] [PubMed] [Google Scholar]
- 40.Schulz M, Sánchez R, Soto L, Risopatrón J, Villegas J. Effect of Escherichia coli and its soluble factors on mitochondrial membrane potential, phosphatidylserine translocation, viability, and motility of human spermatozoa. Fertil Steril. 2010;94:619–623. doi: 10.1016/j.fertnstert.2009.01.140. [DOI] [PubMed] [Google Scholar]
- 41.Shen HM, Dai J, Chia SE, Lim A, Ong CN. Detection of apoptotic alterations in sperm in subfertile patients and their correlations with sperm quality. Hum Reprod. 2002;17:1266–1273. doi: 10.1093/humrep/17.5.1266. [DOI] [PubMed] [Google Scholar]
- 42.Spanò M, Bonde JP, Hjøllund HI, Kolstad HA, Cordelli E, Leter G. Sperm chromatin damage impairs human fertility. The Danish First Pregnancy Planner Study Team. Fertil Steril. 2000;73:43–50. doi: 10.1016/S0015-0282(99)00462-8. [DOI] [PubMed] [Google Scholar]
- 43.WHO laboratory manual for the examination of human semen and semen-cervical mucus interaction. 4. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- 44.WHO laboratory manual for the examination and processing of human semen. 5. Geneva: WHO Press; 2010. [PubMed] [Google Scholar]
- 45.Wyrobek AJ, Eskenazi B, Young S, Arnheim N, Tiemann-Boege I, Jabs EW, et al. Advancing age has differential effects on DNA damage, chromatin integrity, gene mutations, and aneuploidies in sperm. Proc Natl Acad Sci U S A. 2006;103:9601–9606. doi: 10.1073/pnas.0506468103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang HB, Lu SM, Ma CY, Wang L, Li X, Chen ZJ. Early apoptotic changes in human spermatozoa and their relationships with conventional semen parameters and sperm DNA fragmentation. Asian J Androl. 2008;10:227–235. doi: 10.1111/j.1745-7262.2008.00295.x. [DOI] [PubMed] [Google Scholar]
- 47.Zhou XL, Sun PN, Huang TH, Xie QD, Kang XJ, Liu LM. Effects of hepatitis B virus S protein on human sperm function. Hum Reprod. 2009;24:1575–1583. doi: 10.1093/humrep/dep050. [DOI] [PubMed] [Google Scholar]
- 48.Zini A, Sigman M. Are tests of sperm DNA damage clinically useful? Pros and cons. J Androl. 2009;30:219–229. doi: 10.2164/jandrol.108.006908. [DOI] [PubMed] [Google Scholar]