Abstract
Purpose
To investigate the association between follicular fluid homocysteine levels and embryo quality and pregnancy rates in patients undergoing assisted reproduction.
Methods
Fifty infertile women who were admitted to our clinic were enrolled in the study. Ovulation induction was performed by using GnRH agonist and gonadotropins. For each patient, homocysteine level in the follicular fluid was measured by using nephelometric method after the oocyte pick-up. The association between the homocysteine concentration in the follicular fluid and the oocyte–embryo quality, pregnancy rates and hormone levels were investigated.
Results
Mean ± SD Hcy was 9.6 ± 2.02 μmol/L and 14.9 ± 2.93 μmol/L in pregnant and non-pregnant women, respectively (p < 0.0001). There were no statistically significant differences between pregnant and non-pregnant women in mean age, duration of infertility, body mass index, the oocyte–embryo quality parameters, and hormone levels. Homocystein did not have any correlation with M2, late M2, and total number of oocytes, number of fertilized oocytes and transferred embryos, and embryo quality grade. Area under curve (AUC) of hcy for prediction of pregnancy failure was 0.922 (p = 0.0001, 95% Confidence interval 0.85–0.99). A threshold of 11.9 μmol/L of hcy had a sensitivity of 82%, specificity of 100%, positive predictive value of 100% and negative predictive value of 91.6% for prediction of pregnancy failure. The subgroup analysis in male factor infertility group (n = 28), showed that mean homocystein was 9.9 ± 2.44 μmol/L and 14.1 ± 2.72 μmol/L in pregnant and non-pregnant women, respectively (p = 0.002).
Conclusion
Low follicular fluid homocysteine level is associated with a better chance of clinical pregnancy.
Keywords: Homocysteine, ICSI, Follicular fluid, Infertility
Introduction
Hyperhomocysteinemia may affect the reproductive process within various levels by poor oocyte quality, male infertility due to abnormal morphology, low sperm concentrations, and loss of motility, congenital malformation, miscarriage, hypertension and low birth weight [1--3].
Homocysteine (hcy) is an amino acid that is an intermediary product of methionine metabolism that is not incorporated into the structure of proteins. Methionine is an essential amino acid and the only source of hcy. Major causes of homocysteinemia include the imbalance in intake of folate, cobalamine, pridoxine and methionine or genetic variations [4]. Systemic Hcy levels reflect the follicular Hcy levels [1]. It is shown that the high hcy concentration (>14.7 μmol/L) in the follicular fluid decreases embryo quality in a study reported by Ebisch et al. [1].
Women receiving folic acid supplementation had significantly lower homocysteine concentrations in their follicular fluid [5]. A link between folic acid and ovarian function was noted in studies on rhesus monkeys in 1982 showing that a folate-restricted diet led to irregular menstrual cycles, whereas pre-ovulatory serum estradiol as well as midluteal progesterone concentrations decreased progressively when compared with animals under normal diet. Ovarian biopsies of folate-deprived monkeys demonstrated degeneration of graafian follicles, with an increase in atretic and cystic follicles, as well as a depletion of granulosa cells and a reduction or even an absence of corpus lutea [6].
Harmful effects due to hyperhomocysteinemia are caused in two ways. First, free radicals originating from oxidation of hcy are toxic to the vascular endothelium; on the other hand hcy disturbs the coagulation cascade and endothelium—that is predominantly anti-thrombotic under normal circumstances—becomes more thrombotic [7].
Another negative effect of hcy on the vascular endothelium is its decreasing effect on synthesis of nitric oxide (NO) and its bioavailability. Whereas NO is involved in nearly all steps of female reproduction such as ovulation, early embryonic cleavage, implantation, regulation of arterial pressure, uterine quiescence and, finally, labour contractions and cervical ripening. Importantly, physiological NO concentrations are within a narrow range and either excess or lack of NO will induce and adverse reproductive outcome [8].
Although there are only few publications about the relationship between hcy concentration in the follicular fluid and the parameters of fertility, we can say that high hcy levels have a toxic effect on gonadal cells by increasing production of oxygen radicals. Szymanski and Kazdepka–Zieminsko [9] found that mature oocyte count is high in patients with low hcy levels and that there is a relationship between hcy levels and oocyte maturity in their study. Other studies support that high hcy levels in the follicular fluid effects embryo quality by causing decreased division and increased fragmentation of cells [10–12]. In folliculogenesis, hyperhomocysteinemia may promote apoptosis, thereby leading to follicular atresia [13].
In this study we investigated whether or not follicular fluid hcy can predict oocyte and embryo quality and pregnancy rates in couples who undergo assisted reproductive technologies.
Materials and methods
Fifty infertile women who were referred to Istanbul University, Cerrahpasa School of Medicine, Department of Obstetrics and Gynecology, In Vitro Fertilization Unit were included in this prospective study. The study was approved by the Ethics Committee and Institutional Board of the Cerrahpasa School of Medicine.
Patient history and gynaecological exam, basic infertility tests (spermiogram, hormonal profile on the 3rd day of menstruation, hysterosalpingography) were made to evaluate the causes of infertility. The exclusion criteria were patient’s age >42, and women diagnosed with endometriosis or with the presence of hidrosalpinx. FSH >15, LH > 15 were excluded.
In the early follicular phase (the 3rd day of the cycle) and on day of oocyte pick-up (OPU), fasting venous blood samples were taken. Serum levels of day 3 follicle stimulating hormone (FSH), day 3 anti-Müllerian hormone (AMH) and estradiol (E2) levels on the HCG day were measured.
Gonadotropin releasing hormone (GnRH) analogues were started on the 21st day of the last cycle. On the 3rd day of the cycle, rec-FSH 75 unit: Gonal-f® (Merck Serono, Turkey), or Puregon® (Shering Ploug, Turkey) was added to the treatment. Step down protocol was used as described in our previous study [14]. When the largest follicle reached at least 18 mm in diameter and two follicles >16 mm was present 10,000 IU of human chorionic gonadotropin (hCG, Pregnyl®, Shering Ploug), was applied. OPU was done 36–38 h after HCG administration under transvaginal ultrasound guidance. Luteal phase supplementation of 600 mg/day micronized progesterone (Progestan® tb, Kocak, Turkey) intravaginally was started on the evening following oocyte pick-up and continued for 12 days thereafter.
Clinical pregnancy was defined as the presence of a gestational sac on ultrasound examination at 5 weeks after the embryo transfer.
During oocyte retrieval, follicular fluids of mature follicles (>17 mm) were aspirated and pooled for each patient. Follicle aspirates, which were not clear and contaminated with blood, were discarded.
After collecting the oocytes, follicular fluid was centrifuged in 2000 g for 10 min and to separate erythrocytes, leukocytes and granulosa cells. The samples were frozen at −80°C until assayed. Hcy levels of the collected follicular fluid samples were estimated by nephelometric method by using the Dade Behring BN2 device with latex nephelometric hcy kit (N Latex HCY, Siemens Healthcare Diagnostics, Marburg, Germany) at the Fikret Bial Central Research Laboratory of Cerrahpasa School of Medicine Hospital.
Hormone assays
AMH. All samples were assayed in duplicate using the AMH/MIS enzyme-linked immunosorbent assay kit (Diagnostic Systems Lab, Webster, Texas, USA). The sensitivity of the assay was 0.017 ng/mL. The intra- and inter- assay variations were <5% and <8%, respectively.
FSH, Estradiol, and LH. These were measured by Chemilluminescent Microparticle Immunoassay (Architect Abott Lab, IL, USA).
Classification of oocyte maturity was performed according to Veeck [15].
Oocyte Corona cumulus complexes (OCCC) were observed under the stereomicroscope and were evaluated according to their maturation and graded from zero to four. Oocytes with a large nucleus in their cytoplasm and a dense corona cumulus layer were regarded as immature “0”; oocytes which did not have a cytoplasmic germinal vesicle and a polar body, and had a corona layer that is relatively less dense and smaller than the total sizes of five oocytes were regarded as “1st degree”; oocytes which had radially placed crowded coronal cells in their vicinity, had cumulus cells spreading to a relatively wider area than the second group, contained a polar body and were easily removed from the cell group when manipulated were regarded as the “2nd degree”; oocytes which lost their tight adherence to the coronal cells and had cumulus cells which were quite scattered but still being cellular were regarded as “3rd degree”; oocytes which had pale cytoplasm and cumulus cells that lost their cellular image and became a gelatinous structure in its vicinity were regarded as “4th degree” mature in the classification (M2 and Late M2). (M2 oocytes have clear cytoplasm, normal cell size, normal zona pellucida, and non-fragmented polar body. Late M2 oocytes are post-mature oocytes which have dark cytoplasm with cytoplasmic granulations, abnormalities in zona pellucida, and fragmented polar bodies. M2 oocytes are considered to be better than late M2 oocytes) [15] In order to calculate mean oocyte degree; total maturation degrees were summed-up and the total was divided into total oocyte number.
After the 40th hour of the intracytoplasmic sperm injection (ICSI) process, morphology of the dividing embryo was observed and ‘embryo grading’ was done. Embryos with equally-sized blastomeres and without cytoplasmic fragmentation were regarded as “grade 1”, embryos with equally-sized blastomeres and minor cytoplasmic fragmentations were regarded as “grade 2”, embryos without equally-sized blastomeres and without cytoplasmic fragmentations were regarded as “grade 3”, embryos with or without equally-sized blastomeres and with major cytoplasmic fragmentations were regarded as “grade 4”, embryos with blastomeres that cannot be distinguished and with major cytoplasmic fragmentations were regarded as “grade 5” [16]. At least in the two-cell stage embryo transfer was done at third day after the oocyte collection.
Statistical evaluation
Statistical calculations were made with the consultation of Department of Biostatistics, Cerrahpasa School of Medicine. Statistical Packet for Social Sciences (SPSS) for Windows 10.0 statistical packet program was used for the calculations. Student’s t tests, Mann–Whitney U, Pearson and Spearman correlation analyses and receiver operator curves (ROC) were used. All the values were expressed as mean ± standard deviation (SD). P <0.05 was regarded as statistically significance limit. Power analysis was performed by MedCalc© (Free-trial version, Belgium). The most appropriate threshold of hcy to predict pregnancy failure was detected by MedCalc© (Free-trial version, Belgium).
Results
In our study the causes of infertility were male factor for 28 cases, ovulatory dysfunction for 6 cases, tubal factor without visible hidrosalpinx for 4 cases and unexplained infertility for 12 cases. The demographic, clinical and laboratory features of pregnant and non-pregnant women were demonstrated in Table 1. Seventeen women conceived and 33 women did not conceive. Mean ± SD Hcy was 9.6 ± 2.02 μmol/L and 14.9 ± 2.93 μmol/L in pregnant and non-pregnant women, respectively (p < 0.0001, with >99% power, type I error 0.01, type II error 0.01, minimal required sample size of each group = 11).
Table 1.
Patients’ characteristics in pregnant and non-pregnant women
| Pregnant (n = 17) | Non-pregnant (n = 33) | p value | |
|---|---|---|---|
| Female age (years) | 29.7 ± 4.07 | 32.3 ± 4.7 | 0.059 |
| Infertility duration (years) | 6.2 ± 3.3 | 8.8 ± 5.7 | 0.092 |
| BMI (kg/m2) | 25.1 ± 3.9 | 25.4 ± 4.7 | 0.796 |
| AMH (ng/ml) | 3.00 ± 2.07 | 3.78 ± 3.34 | 0.482 |
| FSH (mIU/ml) | 5.89 ± 1.86 | 7.28 ± 3.3 | 0.112 |
| E2 on HCG day (pg/ml) | 1137.3 ± 770.2 | 1162.6 ± 603.4 | 0.956 |
| Total gonadotropin dose (IU) | 2380.8 ± 1182 | 2235.9 ± 910.6 | 0.633 |
| Hcy (μmol/L) | 9.6 ± 2.02 | 14.9 ± 2.93 | <0.0001 |
** The values are given as mean ± SD or percent (numbers)
AMH anti-Müllerian hormone; BMI body mass index; FSH follicle stimulating hormone; E2 estradiol; HCG human chorionic gonadotropin; Hcy homocysteine
There was no significant difference between means of woman age, duration of infertility, total gonadotropin dose, mean BMI, AMH; mean FSH on the third day of cycle and serum estradiol on HCG day in the two groups.
There was no statistically significant difference in total number of oocytes; mean number of M2 and late M2 oocytes; mean oocyte degree; number of transferred grade 1, grade 2, grade 3 and total embryos between pregnant and non-pregnant women. (Table 2)
Table 2.
Comparison of oocyte and embryo quality in pregnant and non-pregnant women
| Pregnant (Mean ± SD) | Non-pregnant (Mean ± SD) | P value | |
|---|---|---|---|
| M2 (n) | 2.76 ± 2.53 | 2.00 ± 1.78 | 0.299 |
| Late M2 (n) | 3.88 ± 2.75 | 3.65 ± 3.82 | 0.789 |
| Total oocytes (n) | 8.58 ± 3.69 | 7.30 ± 4.84 | 0.343 |
| Fertilized oocyte (n) | 4.35 ± 2.14 | 3.48 ± 2.55 | 0.240 |
| Grade 1-2 Embryo (n) | 2.92 ± 2.26 | 2.15 ± 1.75 | 0.268 |
| Grade 3 Embryo (n) | 0.75 ± 0.46 | 1.41 ± 1.08 | 0.120 |
| Transferred embryos (n) | 2.76 ± 0.83 | 2.48 ± 1.12 | 0.373 |
Homocystein did not have any correlation with age of women, duration of infertility, total gonadotropin dose, BMI, serum AMH, FSH on the 3rd day of cycle, and E2 on HCG day; and any of the embryologic parameters, number of M2, late M2, total and fertilized oocytes, embryo grade and number of transferred embryos. (Data not shown on table)
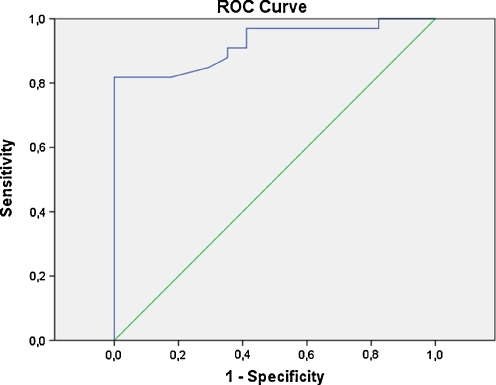
Area under curve (AUC) of hcy for prediction of pregnancy failure was 0.922 (p = 0.0001, 95% Confidence interval 0.85–0.99). A threshold of 11.9 μmol/L of hcy had a sensitivity of 82%, specificity of 100%, positive predictive value of 100% and negative predictive value of 91.6% for prediction of pregnancy failure. Different threshold levels of hcy and corresponding sensitivity, specificity, PPV and NPV are given in Table 3 and the hcy-ROC curve is presented in Fig. 1.
Table 3.
Sensitivity and specificity of hcy to predict “non-pregnancy” according various thresholds
| Threshold | Sensitivity | Specificity | Positive predictive value | Negative predictive value |
|---|---|---|---|---|
| 10.1 μmol/L | 93% | 58% | 53.3% | 95% |
| 11.0 μmol/L | 87% | 65% | 55.6% | 91.3% |
| 11.9 μmol/L* | 82% | 100% | 100% | 91.6% |
| 13.1 μmol/L | 72% | 100% | 100% | 86.8% |
| 14.0 μmol/L | 31% | 100% | 100% | 73.3% |
Fig. 1.
ROC curve of Hcy for prediciton of non-pregnancy
We also performed a subgroup analysis in male factor infertility group (n = 28), and we have seen that mean homocystein was 9.9 ± 2.44 μmol/L and 14.1 ± 2.72 μmol/L in pregnant and non-pregnant women, respectively (p = 0.002).
Discussion
In this study we have demonstrated a relationship between hcy levels of follicular fluid and pregnancy in women undergoing ICSI. Low follicular fluid hcy is related to a better chance of pregnancy.
Attaran et al. [17] reported that, more oxygen radicals are found in follicular fluid of women who conceived with in vitro fertilization (IVF) compared to women who did not conceive with IVF. The detrimental effects of high hcy levels are due to free radicals that are induced by hcy oxidation. [18] Ebisch et al. [1] reported a study which includes 156 cases, it was pointed that high hcy level in follicular fluid reduces embryo quality. It was shown that follicular fluid hcy levels and fertilization rate and oocyte and embryo qualities in PCOS patients undergoing assisted reproduction are negatively correlated.
In two different studies, Berker et al. [19] and Aitken et al. [20] showed that high hcy levels in follicular fluid caused decreased cell division and high fragmentation in embryo cultures, which means a decrease in oocyte and embryo quality. Moreover, mean hcy of grade 3 embryos was found to be higher than mean hcy of grade 1-2 embryos [19]. In another study, by Szymanski and Kazdepka-Zieminska [9], oocytes exposed to low hcy concentration present better quality and higher degree of maturity. There is a correlation between follicular fluid homocysteine concentration and oocyte maturity.
In our study, there was no significant correlation between hcy levels and total oocyte number, maturity degree of oocyte, the number of Grade 1-2-3 embryos (p > 0.05). This may be attributed to the low number of patients in our study, and probably to the differences in population studied.
In the study of Ebisch et al. [1] hcy levels were higher in endometriosis patients known to suffer from low embryo quality; in our study we did not have any patient with endometriosis.
Bettahar et al. [21] showed that total hcy levels in the blood drops significantly while estradiol serum level increases during ovarian stimulation. In order to decrease the negative effects of hyperhomocysteinemia, administration of high amount of estrogen and lowering the level of homocysteine may be thought to be beneficial in menopausal women. However in ovarian hyperstimulation Bean et al. [22] showed that high doses of estrogen increased embryo aneuploidy. In our study there was no statistical difference in mean E2 on HCG day between the two groups. Similar to our results; Bettahar et al. [21] suggested that there was no relationship between estrogen and hcy. As a result, today we still do not know for sure whether or not estrogen affects hcy levels by a direct and/or an indirect mechanism.
Oxygen radical production can be responsible for the toxic effects of high hcy levels. Miyazaki et al. [23] have previously shown that certain amount oxygen radical is necessary for oocyte maturation. In a study by Bedawy et al. [24], oxidative damage was found to be related with decrease in division rate and increase in fragmentation rate in embryo cultures. If we consider recent studies on the effect of hcy on gonadal cells and oocyte-embryo development, a predictable relationship between hcy and pregnancy can be established.
Pasqualotto et al. [25] found that total antioxidant capacity (TAC) was positively correlated with pregnancy rate (31.7%) however follicular fluid TAC was not related with maturity of oocyte, oocyte fertilization and embryo quality. They concluded that certain amount of oxidative stress is necessary for achieving pregnancy. Contrary to this study, Oyawoye et al. [26] found that follicular fluid TAC of successfully fertilized oocytes was significantly higher than non-fertilized oocytes. Both Pasqualotto et al. [25] and Oyawoye et al. [26] reported that there are different effects of reactive oxygen species at different stages of embryonic development.
The plasma hcy levels correlate with follicular fluid hcy levels. High plasma hcy levels may be due to oxidative stress, and in return this may also affect the ovulation process and eventually cause infertility. In our study, AUC of hcy for prediction of pregnancy failure was 0.922, which is very high. A threshold of 11.9 μmol/L of follicular fluid hcy had a sensitivity of 82% and specificity of 100% for prediction of pregnancy failure. Pacchiarotti et al. [27] reported that implantation and pregnancy rates of women with low follicular Hcy levels were higher compared to women with high follicular fluid hcy (p < 0.05). Although the number of women were low in our study, the power analysis showed a rate of >99% for hcy, which indicates that the number of women is highly adequate to show the difference between two groups.
In order to exclude known factors for women such as PCOS, tubal factor or poor-responders; we performed a subgroup analysis in male factor infertility group (n = 28), and we have seen that mean homocystein was significantly lower in pregnant group compared to the non-pregnant women (p = 0.002). This is important because even in women who do not have any identified disorder homocystein is beneficial to predict pregnancy.
Good embryo quality does not always mean pregnancy. Even if we transfer the embryo in blastocyst stage implantation may not be accomplished. The clinical pregnancy may also be related to the environmental effects on the oocytes. We may hypothesize that the follicular fluid homocystein levels may be associated with the post-transfer stages such as the implantation process.
Conclusion
Our study shows a quite strong relationship between pregnancy and follicular fluid hcy levels. But there was no correlation between follicular fluid hcy levels and oocyte quality and embryo grading which was indicated by traditional morphologic methods. In connection with this result, we think that follicular fluid hcy value is an important marker of pregnancy potential.
Acknowledgments
Conflict of interest
Authors declare no conflict of interest.
Footnotes
Capsule
Low follicular fluid homocysteine level is associated with a better chance of clinical pregnancy.
References
- 1.Ebisch IMW, Peters WH, Thomas CM, Wetzels AM, Peer PG, Steegers RP. Homocysteine, glutathione and related thiols affect fertility parameters in the (sub) fertile couple. Hum Reprod. 2006;21:1725–1733. doi: 10.1093/humrep/del081. [DOI] [PubMed] [Google Scholar]
- 2.Steegers-Theunissen RP, Steegers EA, Thomas CMG, Hollanders HM, Peereboom-Stegeman JH, Trijbels FJ, et al. Study on the presence of homocysteine in ovarian follicular fluid. Fertil Steril. 1993;60:1006–1010. doi: 10.1016/s0015-0282(16)56401-2. [DOI] [PubMed] [Google Scholar]
- 3.Verkleij-Hagoort AC, Verlinde M, Ursem NT, Lindemans J, Helbing WA, Ottenkamp J, et al. Maternal hyperhomocysteinaemia is a risk factor for congenital heart disease. BJOG. 2006;113:1412–1418. doi: 10.1111/j.1471-0528.2006.01109.x. [DOI] [PubMed] [Google Scholar]
- 4.Cotter AM, Molloy AM, Scott JM, Daly SF. Elevated plasma homocysteine in early pregnancy: a risk factor for the development of severe preeclampsia. Am J Obstet Gynecol. 2001;185:781–5. doi: 10.1067/mob.2001.117304. [DOI] [PubMed] [Google Scholar]
- 5.Boxmeer JC, Brouns RM, Lindemans J, Steegers EA, Martini E, Macklon NS, Steegers-Theunissen RP. Preconception folic acid treatment affects the microenvironment of the maturing oocyte in humans. Fertil Steril. 2008;89(6):1766–70. doi: 10.1016/j.fertnstert.2007.06.036. [DOI] [PubMed] [Google Scholar]
- 6.Mohanty D, Das KC. Effect of folate deficiency on the reproductive organs of female rhesus monkeys: a cytomorphological and cytokinetic study. J Nutr. 1982;112:1565–76. doi: 10.1093/jn/112.8.1565. [DOI] [PubMed] [Google Scholar]
- 7.Raijmakers MT, Steegers EA, Peters WH. Glutathione S-transferases and thiol concentrations in embryonic and early fetal tissues. Hum Reprod. 2001;16:2445–2450. doi: 10.1093/humrep/16.11.2445. [DOI] [PubMed] [Google Scholar]
- 8.Maul H, Longo M, Saade GR, et al. Nitric oxide and its role during pregnancy: from ovulation to delivery. Curr Pharm Des. 2003;9:359–80. doi: 10.2174/1381612033391784. [DOI] [PubMed] [Google Scholar]
- 9.Szymanski W, Kazdepka-Zieminska A. Effect of homocysteine concentration in follicular fluid on a degree of oocyte maturity. Gynekol Pol. 2003;74:1392–6. [PubMed] [Google Scholar]
- 10.Riley JC, Behrman HR. Oxygen radicals and reactive oxygen species in reproduction. Proc Soc Exp Biol Med. 1991;198:781–791. doi: 10.3181/00379727-198-43321c. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez-Gonzalez E, Lopez-Bejar M, Mertens MJ, Paramio MT. Effects on in vitro embryo development and intracellular glutathione content of the presence of thiol compounds during maturation of prepubertal goat oocytes. Mol Reprod Dev. 2003;65:446–453. doi: 10.1002/mrd.10316. [DOI] [PubMed] [Google Scholar]
- 12.Shan XQ, Aw TY, Jones DP. Glutathione-dependent protection against oxidative injury. Pharmacol Ther. 1990;47:61–71. doi: 10.1016/0163-7258(90)90045-4. [DOI] [PubMed] [Google Scholar]
- 13.Forges T, Monnier-Barbarino P, Alberto JM, Gueant-Rodriguez RM, Daval JL, Gueant JL. Impact of folate and homocysteine metabolism on human reproductive health. Hum Reprod Update. 2007;13:225–38. doi: 10.1093/humupd/dml063. [DOI] [PubMed] [Google Scholar]
- 14.Irez T, Ocal P, Guralp O, Cetin M, Aydogan B, Sahmay S. Different serum anti-Müllerian hormone concentrations are associated with oocyte quality, embryo development parameters and IVF-ICSI outcomes. Arch Gynecol Obstet. 2011;284(5):1295–301. doi: 10.1007/s00404-011-1979-6. [DOI] [PubMed] [Google Scholar]
- 15.Veeck LL. Oocyte assessment and biological performance. Ann NY Acad Sci. 1999;541:259–74. doi: 10.1111/j.1749-6632.1988.tb22263.x. [DOI] [PubMed] [Google Scholar]
- 16.Gardner DK, Lane M, Stevens J. Non-invasive assessment of human embryo nutrient consumption as a measure of developmental potential. Fertil Steril. 2001;76:1175–1180. doi: 10.1016/S0015-0282(01)02888-6. [DOI] [PubMed] [Google Scholar]
- 17.Attaran M, Pasqualotto E, Falcone T, Goldberg JM, Miller KF, Agarwal A, et al. The effect of follicular fluid reactive oxygen species on the outcome of in vitro fertilization. Int J Fertil Womens Med. 2000;45:314–320. [PubMed] [Google Scholar]
- 18.Kim IH, Langendonckt A, Soom A, Vanroose G, Casi AL, Hendriksen PJ, et al. Effect of exogenous glutathione on the in vitro fertilization of bovine oocytes. Theriogenology. 1999;52:537–547. doi: 10.1016/S0093-691X(99)00150-8. [DOI] [PubMed] [Google Scholar]
- 19.Berker B, Kaya C, Aytac R, Satiroglu H. Homocysteine concentrations in follicular fluid are associated with poor oocyte and embryo qualities in polycystic ovary syndrome patients undergoing assisted reproduction. Hum Reprod. 2009;24(9):2293–302. doi: 10.1093/humrep/dep069. [DOI] [PubMed] [Google Scholar]
- 20.Aitken RJ, Irvine DS, Wu FC. Prospective analysis of sperm-oocyte fusion and reactive oxygen species generation as criteria for the diagnosis of infertility. Am J Obstet Gynecol. 1991;164:542–551. doi: 10.1016/s0002-9378(11)80017-7. [DOI] [PubMed] [Google Scholar]
- 21.Bettahar LK, Feugeas O, Wittemer C, Ohl J, Rongieres C, Nisand I. Evolution of homocysteine during ovarian stimulation for IVF or ICSI. Gynecol Obstet Fertil. 2002;30(2):121–8. doi: 10.1016/S1297-9589(01)00278-8. [DOI] [PubMed] [Google Scholar]
- 22.Bean CJ, Hassold TJ, Judis L, Hunt PA. Fertilization in vitro increases non-disjunction during early cleavage divisions in a mouse model system. Hum Reprod. 2002;17(9):2362–7. doi: 10.1093/humrep/17.9.2362. [DOI] [PubMed] [Google Scholar]
- 23.Miyazaki T, Sueoka K, Dharmarajan AM, Atlas SJ, Bulkley GB, Wallach EE. Effect of inhibition of oxygen free radical on ovulation and progesterone production by the in-vitro perfused rabbit ovary. J Reprod Fertil. 1991;91:207–212. doi: 10.1530/jrf.0.0910207. [DOI] [PubMed] [Google Scholar]
- 24.Bedawy MA, Miller K, Goldberg JM, Nelson DR, Agarwal A, Falcone T. Assessment of the predictive value of follicular fluid cytokines and reactive oxygen species in IVF cycles. Fertil Steril. 2002;78:5–6. doi: 10.1016/S0015-0282(02)03392-7. [DOI] [Google Scholar]
- 25.Pasqualotto EB, Agarwal A, Sharma RK, Izzo VM, Pinotti JA, Joshi NJ, et al. Effect of oxidative stress in follicular fluid on the outcome of assisted reproductive procedures. Fertil Steril. 2004;81:973–976. doi: 10.1016/j.fertnstert.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 26.Oyawoye O, Abdel GA, Gamer A, Constantinovici N, Perrett C, Hardiman P. Antioxidants and reactive oxygen species in follicular fluid of women undergoing IVF: relationship to outcome. Hum Reprod. 2003;18:2270–2274. doi: 10.1093/humrep/deg450. [DOI] [PubMed] [Google Scholar]
- 27.Pacchiarotti A, Mohamed MA, Micara G, Linari A, Tranquilli D, Espinola SB, Aragona C. The possible role of hyperhomocysteinemia on IVF outcome. Assist Reprod Genet. 2007;24:459–62. doi: 10.1007/s10815-007-9165-2. [DOI] [PMC free article] [PubMed] [Google Scholar]