Introduction
Somatic chromosomal abnormalities are frequently found in infertile men, particularly in those with low sperm count [1–5]. Studies in infertile men have demonstrated that 2–14% carry constitutional chromosomal abnormalities [1] Research in this area has become more relevant in the past few years with the advent of assisted reproductive therapies (ART), especially intracytoplasmatic sperm injection (ICSI). ICSI has been extremely successful for the treatment of male infertility, but transmission of cytogenetic defects to offspring is a major concern and demonstrated to be reality [1], therefore it is recommended to investigate this. The incidence of chromosomal aberrations in infertile men depends on the definition of “infertility”, and is estimated to be approximately 5% in oligozoospermic men and 14% in azoospermic men [6]. Abnormalities mostly consist of numerical sex chromosome abnormalities (e.g. 47,XXY), balanced reciprocal translocations (reciprocal exchange between two chromosomes), inversions, Y-chromosome micro-deletions and balanced Robertsonian translocations (fusion of two acrocentric chromosomes, e.g. 13, 14, 15, 21, 22). Carriers of a balanced Robertsonian translocation show impaired gametogenesis to a variable degree and elevated levels of nullisomic or disomic sperm (3–40%, mean 15%) [1–5]. The fertilization with an aneuploid sperm results in monosomy or trisomy in the fetus [7, 8]. Furthermore fetal aneuploidies are a major cause of pregnancy loss and fetal malformations [9].
Here we report on a sperm aneuploidy screening of all chromosomes (1–22,X,Y) in an infertile patient showing low-frequency mosaicism of a balanced Robertsonian translocation involving both chromosomes 21.
Case report
Patient
The 36 years old man presented in our infertility clinic with sterility lasting for 2 years. His medical history showed no evidence of relevant diseases, medication or nicotin abuse and BMI (body mass index) was unremarkable. Hormone parameters of testosterone, follicule stimulating hormone (FSH), luteinising hormone (LH), prolactine and thyroid parameters were in the normal range. Serological testing (hepatitis B/C, HIV, mumps) showed inconspicuous results.
The sperm count revealed an oligo-astheno-teratozoospermia (Sperm concentration: 14,3 mio sperm per ml, sperm motility: WHO a (rapid progressive motility): 9%, WHO b (moderate progressive motility): 10%, WHO c (no progressive motility): 31%, WHO d (immotile): 50% and 98% abnormal sperm morphology). Somatic chromosome analysis from a blood sample revealed a low-frequency (4%) mosaic of a balanced Robertsonian translocation 45,XY,t(21;21)(q10;q10) in a normal 46,XY male background.
Materials and methods
We screened a sperm sample of this patient for elevated disomy levels of all chromosomes (1–22, X and Y) and for increased diploidy rates by multicolour interphase fluorescence in situ hybridisation (sperm-FISH). Patient sperm cells were prepared for FISH according to standard procedures [10]. In brief, cells were fixed with methanol/acetic acid (3/1 v/v) and then dropped onto glass slides. Subsequently, the sperm cell chromatin was decondensed by 10 mM LIS (Lithium-diiodosalicylat)/10 mM DTT (Dithiothreitol) treatment and finally the cells were permeabilized by pepsinization. FISH to sperm cell nuclei with chromosome-specific centromere (CEP, kindly provided by M. Rocchi, www. biologia.uniba.it/rmc) or BAC (LSI, obtained from http://bacpac.chori.org or Abbott Molecular) probes was performed for all human chromosomes (1–22, X and Y) as described in Williams 1993 [11] with minor modifications). Subsequently, the number of FISH-signals in each nucleus was determined by microscopic analysis according to standardized criteria [10].
At least 1000 nuclei per chromosome probe (>24.000 cell nuclei) were scored. Statistical analyses were performed with the Statistical Package for Social Sciences (SPSS for Windows 16.0, SPSS Inc., Chicago IL, USA). An increase of the aneuploidy rate in the patient by over two standard deviations compared to the mean baseline level in healthy controls described in the literature (see Results section) was considered statistically significant (P < =5%).
Results
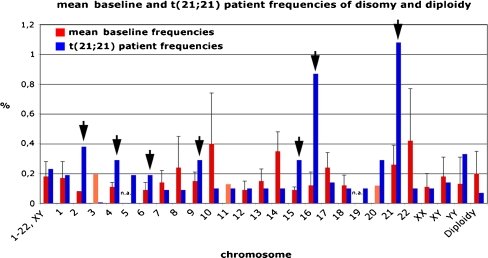
In order to obtain an updated overview about baseline aneuploidy rates in human sperm, we performed a meta-analysis of the current literature on sperm FISH aneuploidy analysis in healthy control individuals revealed 64 publications reporting on 620 individuals (complete reference list available upon request). It is of note that not all 24 chromosomes were analyzed in each individual, because most studies focussed on the clinically relevant chromosomes 13, 18, 21, X and Y. Therefore, the reliability of the presently available data may still vary considerably between chromosomes and for some chromosomes statistically meaningful data is still missing (e.g. chr. 3, 5, 11, 19 and 20). An average diploidy rate of 0.2% and a mean disomy frequency of 0.18% were described for healthy individuals. However, chromosome-specific differences are evident [12], for example when comparing chromosome 2 = 0.08% and chromosome 22 = 0.42%) (Fig. 1).
Fig. 1.
Summary of sperm aneuploidy analysis in healthy control individuals and in the mos t(21;21) patient. The investigated sperm cells of the t(21;21) patient showed significantly elevated level of disomy 21 (arrow). However, also other chromosomes showed significantly increased frequencies (arrows)
The investigated sperm cells of the mosaic t (21;21) patient showed a significantly elevated level of disomy 21 (1,08% of all cell nuclei analyzed). However, also for chromosomes 2 (0,38%), 4 (0,29%), 6 (0,19%), 9 (0,29%), 15 (0,29%) and most notably for chromosome 16 (0,87%) significantly increased disomy frequencies, compared to baseline frequencies from the literature, were observed (see Fig. 1). The remaining chromosomes showed no evidence of elevated disomy rates. The overall diploidy rate was also within the normal range.
Discussion
It is well established that patients with Robertsonian translocations may have higher aneuploidy rates in sperm cells. This can be associated with infertility, leading to an increased risk for miscarriages and fetal malformations [1, 13, 14]. In this case report we were able to extend this view by demonstrating that even low-frequency mosaic translocation carriers may be affected by increased germ cell aneuploidy rates and the resulting secondary symptoms.
Moreover, these aneuploidies may also involve other chromosomes than the translocation products. The occurrence and impact of this so-called interchromosomal effect (ICE) is controversially discussed, and some authors approved the effect [3, 15–19], while others did not [20–22]. It can be assumed that the ICE is the consequence of a general disturbance of meiotic processes caused by the translocation product, mainly the synapsis and disjunction of other chromosome pairs.
Chen et al. investigated meiotic segregation behaviour in spermatozoa from six males with Robertsonian translocations (t(13;14), t(14;22), t(13;21)) by FISH [23]. Furtheron, they studied chromosome 18, X and Y. They found a higher aneuploidy frequency for sex chromosomes in three patients and increased rates of diploidy in two t(13;14) carriers. Anton et al. also analysed the segregation of chromosomes 13 and 14 in 7 male t(13;14) carriers as well as chromosomes 18, 21, 22, X and Y [13]. They report a significant increase in the disomy rates for the sex chromosomes in two carriers and considered these findings correlated with an ICE.
However all previous studies investigated only a limited set of chromosomes. Most studies included only chromosomes 21, X and Y [13]. Less frequently, disomy of chromosomes 13 and 18 have been investigated. Some authors have also looked for aneuploidy of other chromosomal pairs, such as pairs 1, 4, 9, and 22. Others investigated chromosomes 7, 9, 13, 18, 21, X and Y in transolcation carriers [2]. Overall, only 10 or less individuals were analysed for chromosomes 3, 11 and 20. Moreover, there is no data available so far for chromosomes 5 and 19.
Here, we performed–for the first time- screening of a sperm sample from a low frequent t(21;21) patient for elevated disomy levels of all chromosomes (1–22, X and Y) by multicolour sperm-FISH. In this case, we see a clear indication for the presence of an ICE. We found significantly increased levels of chromosome 21 disomic sperm as well as elevated level of disomy for chromosomes 2, 4, 6, 9, 15 and 16. Therefore in our t(21;21) patient, an ICE may play a role and further increases the overall frequency of aneuploid sperm cells.
It is not known, if the increase of disomy rates in translocation-carriers is truly the result of ICE or if it is associated with other abnormal semen parameters. Indeed, several studies have shown increased frequencies of aneuploidy in spermatozoa of males with severe oligozoospermia or oligoasthenoteratozoospermia, despite having a normal leucocytic karyotype [3, 15, 16, 24, 25]. In our study elevated aneuploidy frequencies are obviously correleted with olio-astheno-teratozoospermia. The translocation product of this translocation carrier might further contribute to aneuploidy by ICE [26]. These data are in line with findings of this case report and suggest molecular cytogenetic analysis of spermatozoa to be a valuable tool in assessing a genetic basis of severe forms of male infertility.
In summary, even low level mosaic Robertsonian translocation carriers may show increased disomy frequencies in sperm cells. Moreover, these aneuploidies may also involve chromosomes not included in the translocation products and could be causally connected with the interchromosomal effect. The patient presented in this case report has a 1:100 risk for offspring with trisomy 21, which is considerably higher than the risk of women aged 35 (1:385). This needs to be considered in respect to recommending prenatal diagnosis.
It is recommended to offer standard karyotype analysis on somatic cells to men seeking fertility treatment by IVF/ICSI [27]. Our findings in this patient reinforce this recommendation, and emphasize the possibility of somatic mosaicism as a possible cause for male infertility. We conclude that analysis of sperm aneuploidy by FISH may be helpful technique in a comprehensive clinical work-up and may increase the accuracy of a prognostic prediction compared to standard karyotype analysis on somatic cells alone. Sperm-FISH screening of all 24 chromosome types is labour intensive, but the procedure can be automated. It can be used to clarify the significance of the so far elusive ICE, and the results obtained may direct further chromosome analysis during in vitro fertilization. Therefore, cytogenetic studies on spermatozoa could be integrated in the genetic exploration of the aetiology of infertility, and may give men with fertility problems a personalized assessment of the aneuploidy risk in their offspring.
Footnotes
Capsule
Sperm aneuploidy and Robertsonian translocation with possible interchromosomal effect.
References
- 1.Shi Q, Martin RH. Aneuploidy in human spermatozoa: FISH analysis in men with constitutional chromosomal abnormalities, and in infertile men. Reprod. 2001;121(5):655–666. doi: 10.1530/rep.0.1210655. [DOI] [PubMed] [Google Scholar]
- 2.Douet-Guilbert N, Bris MJ, Amice V, Marchetti C, Delobel B, Amice J, et al. Interchromosomal effect in sperm of males with translocations: report of 6 cases and review of the literature. Int J Androl. 2005;28(6):372–379. doi: 10.1111/j.1365-2605.2005.00571.x. [DOI] [PubMed] [Google Scholar]
- 3.Vegetti W, Assche E, Frias A, Verheyen G, Bianchi MM, Bonduelle M, et al. Correlation between semen parameters and sperm aneuploidy rates investigated by fluorescence in-situ hybridization in infertile men. Hum Reprod. 2000;15(2):351–365. doi: 10.1093/humrep/15.2.351. [DOI] [PubMed] [Google Scholar]
- 4.Härkönen K, Suominen J, Lähdetie J. Aneuploidy in spermatozoa of infertile men with teratozoospermia. Int J Androl. 2001;24(4):197–205. doi: 10.1046/j.1365-2605.2001.00280.x. [DOI] [PubMed] [Google Scholar]
- 5.Acar H, Kilinç M, Cora T, Aktan M, Taşkapu H. Incidence of chromosome 8,10, X and Y aneuploidies in sperm nucleus of infertile men detected by FISH. Urol Int. 2000;64(4):202–208. doi: 10.1159/000030531. [DOI] [PubMed] [Google Scholar]
- 6.Johnson MD. Genetic risks of intracytoplasmic sperm injection in the treatment of male infertility: recommendations for genetic counselling and screening. Fertil Steril. 1998;70:397–411. doi: 10.1016/S0015-0282(98)00209-X. [DOI] [PubMed] [Google Scholar]
- 7.Carrell DT, Wilcox AL, Udoff LC, Thorp C, Campbell B. Chromosome 15 aneuploidy in the sperm and conceptus of a sibling with variable familial expression of round-headed sperm syndrome. Fertil Steril. 2001;76(6):1258–1260. doi: 10.1016/S0015-0282(01)02904-1. [DOI] [PubMed] [Google Scholar]
- 8.Causio F, Fischetto R, Sarcina E, Geusa S, Tartagni M. Chromosome analysis of spontaneous abortions after in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI). Eur J Obstet Gynecol Reprod Biol. 2002;10;105(1):44–8. [DOI] [PubMed]
- 9.Li TC, Makris M, Tomsu M, Tuckerman E, Laird S. Recurrent miscarriage: aetiology, management and prognosis. Hum Reprod Update. 2002;8:463–481. doi: 10.1093/humupd/8.5.463. [DOI] [PubMed] [Google Scholar]
- 10.Griffin DK, Abruzzo MA, Millie EA, Sheean LA, Feingold E, Sherman SL, Hassold TJ. Non-disjunction in human sperm: evidence for an effect of increasing paternal age. Hum Mol Genet. 1995;4(12):2227–2232. doi: 10.1093/hmg/4.12.2227. [DOI] [PubMed] [Google Scholar]
- 11.Williams BJ, Ballenger CA, Malter HE, Bishop F, Tucker M, Zwingman TA, Hassold TJ. Non-disjunction in human sperm: results of fluorescence in situ hybridization studies using two and three probes. Hum Mol Genet. 1993;2(11):1929–1936. doi: 10.1093/hmg/2.11.1929. [DOI] [PubMed] [Google Scholar]
- 12.Templado C, Vidal F, Estop A. Aneuploidy in human spermatozoa. Cytogenet Genome Res. 2011;133(2–4):91–99. doi: 10.1159/000323795. [DOI] [PubMed] [Google Scholar]
- 13.Anton E, Blanco J, Egozcue J, Vidal F. Sperm FISH studies in seven male carriers of Robertsonian translocation t(13;14)(q10;q10) Hum Reprod. 2004;19:1345–1351. doi: 10.1093/humrep/deh232. [DOI] [PubMed] [Google Scholar]
- 14.Kirkpatrick G, Ferguson KA, Gao H, Tang S, Chow V, Yuen BH. A comparison of sperm aneuploidy rates between infertile men with normal and abnormal karyotypes. Hum Reprod. 2008;23(7):1679–1683. doi: 10.1093/humrep/den126. [DOI] [PubMed] [Google Scholar]
- 15.Pang MG, Hoegerman SF, Cuticchia AJ, Moon SY, Doncel GF, Acosta AA, Kearns WG. Detection of aneuploidy for chromosomes 4, 6, 7, 8, 9 10, 11, 12, 13, 17, 18, 21, X and Y by fluorescence in situ hybridization in spermatozoa from nine patients with oligoasthenoteratozoospermia undergoing intracytoplasmic sperm injection. Hum Reprod. 1999;14:1266–1273. doi: 10.1093/humrep/14.5.1266. [DOI] [PubMed] [Google Scholar]
- 16.Rives N, Saint Clair A, Mazurier S, Sibert L, Simeon N, Joly G, Mace B. Relationship between clinical phenotype, semen parameters and aneuploidy frequency in sperm nuclei of 50 infertile males. Hum Genet. 1999;105:266–272. doi: 10.1007/s004390051100. [DOI] [PubMed] [Google Scholar]
- 17.Blanco J, Egozcue J, Vidal F. Interchromosomal effect for chromosome 21 in carriers of structural chromosome reorganizations determined by fluorescence in situ hybridization on sperm nuclei. Hum Genet. 2000;106:500–505. doi: 10.1007/s004390000295. [DOI] [PubMed] [Google Scholar]
- 18.Palermo GD, Colombero LT, Hariprashad JJ, Schlegel PN, Rosenwaks Z. Chromosome analysis of epipidymal and testicular sperm in azoospermic patients undergoing ICSI. Hum Reprod. 2002;17:570–575. doi: 10.1093/humrep/17.3.570. [DOI] [PubMed] [Google Scholar]
- 19.Roux C, Tripogney C, Morel F, Joanne C, Fellmann F, Clavequin MC, et al. Segregation of chromosomes in sperm of Robertsonian translocation carriers. Cytogenet Genome Res. 2005;111(3–4):291–296. doi: 10.1159/000086902. [DOI] [PubMed] [Google Scholar]
- 20.Honda H, Miharu N, Ohashi Y, Honda N, Hara T, Ohama K. Analysis of segregation and aneuploidy in two reciprocal translocation carriers, t(3;9)(q26.2;q32) and t(3;9)(p25;q32), by triple-color fluorescence in situ hybridization. Hum Genet. 1999;105:428–436. doi: 10.1007/s004390051126. [DOI] [PubMed] [Google Scholar]
- 21.Estop AM, Cieply K, Munne S, Surti U, Wakim A, Feingold E. Is there an interchromosomal effect in reciprocal translocation carriers? Sperm FISH studies. Hum Genet. 2000;106:517–524. doi: 10.1007/s004390000275. [DOI] [PubMed] [Google Scholar]
- 22.Oliver-Bonet M, Navarro J, Codina-Pascual M, Abad C, Guitart M, Egozcue J, et al. From spermatocytes to sperm: meiotic behaviour of human male reciprocal translocations. Hum Reprod. 2004;19:2515–2522. doi: 10.1093/humrep/deh492. [DOI] [PubMed] [Google Scholar]
- 23.Chen Y, Huang J, Liu P, Qiao J. Analysis of meiotic segregation patterns and interchromosomal effects in sperm from six males with Robertsonian translocations. J Assist Reprod Genet. 2007;24(9):406–411. doi: 10.1007/s10815-007-9137-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pfeffer J, Pang MG, Hoegerman SF, Osgood CJ, Stacey MW, Mayer J, et al. Aneuploidy frequencies in semen fractions from ten oligoasthenoteratozoospermic patients donating sperm for intracytoplasmic sperm injection. Fertil Steril. 1999;72:472–478. doi: 10.1016/S0015-0282(99)00279-4. [DOI] [PubMed] [Google Scholar]
- 25.Calogero AE, Palma A, Grazioso C, Barone N, Romeo R, Rappazzo G, et al. Aneuploidy rate in spermatozoa of selected men with abnormal semen parameters. Hum Reprod. 2001;16:1172–1179. doi: 10.1093/humrep/16.6.1172. [DOI] [PubMed] [Google Scholar]
- 26.Pellestor F, Imbert I, Andreo B, Lefort G. Study of the occurrence of interchromosomal effect in spermatozoa of chromosomal rearrangement carriers by fluorescence in-situ hybridization and primed in-situ labelling techniques. Hum Reprod. 2001;16:1155–1164. doi: 10.1093/humrep/16.6.1155. [DOI] [PubMed] [Google Scholar]
- 27.Dohle GR, Colpi GM, Hargreave TB, Papp GK, Jungwirth A, Weidner W. EAU working group on male infertility. EAU Guidelines on male infertility. Eur Urol. 2005;48(5):703–711. doi: 10.1016/j.eururo.2005.06.002. [DOI] [PubMed] [Google Scholar]