Abstract
Recent progress in the analysis of whole genome sequencing data has resulted in the emergence of paleogenomics, a field devoted to the reconstruction of ancestral genomes. Ancestral karyotype reconstructions have been used primarily to illustrate the dynamic nature of genome evolution. In this paper, we demonstrate how they can also be used to study individual gene families by examining the evolutionary history of relaxin hormones (RLN/INSL) and relaxin family peptide receptors (RXFP). Relaxin family hormones are members of the insulin superfamily, and are implicated in the regulation of a variety of primarily reproductive and neuroendocrine processes. Their receptors are G-protein coupled receptors (GPCR's) and include members of two distinct evolutionary groups, an unusual characteristic. Although several studies have tried to elucidate the origins of the relaxin peptide family, the evolutionary origin of their receptors and the mechanisms driving the diversification of the RLN/INSL-RXFP signaling systems in non-placental vertebrates has remained elusive. Here we show that the numerous vertebrate RLN/INSL and RXFP genes are products of an ancestral receptor-ligand system that originally consisted of three genes, two of which apparently trace their origins to invertebrates. Subsequently, diversification of the system was driven primarily by whole genome duplications (WGD, 2R and 3R) followed by almost complete retention of the ligand duplicates in most vertebrates but massive loss of receptor genes in tetrapods. Interestingly, the majority of 3R duplicates retained in teleosts are potentially involved in neuroendocrine regulation. Furthermore, we infer that the ancestral AncRxfp3/4 receptor may have been syntenically linked to the AncRln-like ligand in the pre-2R genome, and show that syntenic linkages among ligands and receptors have changed dynamically in different lineages. This study ultimately shows the broad utility, with some caveats, of incorporating paleogenomics data into understanding the evolution of gene families.
Introduction
Analyses of whole genome sequence data have confirmed that three rounds of whole genome duplication (WGD) are thought to have contributed immensely to the diversification of vertebrates [1], [2], [3]; two rounds of WGD (2R) occurred in early vertebrate evolution, probably before the divergence of agnathans and gnathostomes [4], while the third round (3R) occurred at the base of the teleostean lineage (Figure 1). Even though gene duplication has long been recognized as a major factor in the evolution of biological diversity [5], [6], determining the evolutionary relationships among members of gene families is not always easy because individual genes originate via both small-scale and whole genome duplication events, can be modified by selection or concerted evolution, and may experience differential loss across lineages [5], [6], [7], [8]. Although the ready availability of small-scale synteny data has facilitated the determination of orthologous and paralogous relationships among genes, some aspects of gene family evolution, such as their ancient origins and the timing and kind of duplication events they underwent, continue to be difficult to resolve using traditional bioinformatic approaches [9], [10].
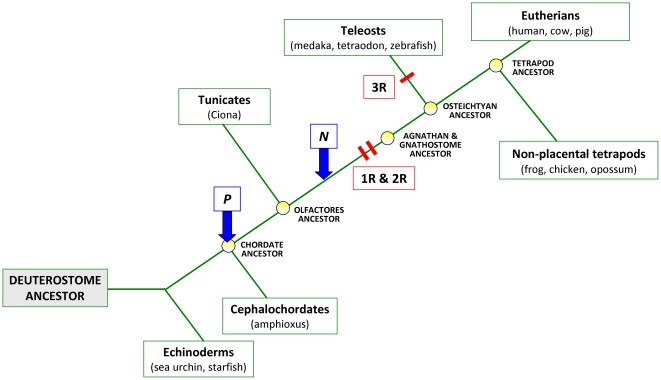
Figure 1. Simplified phylogenetic tree showing the evolutionary relationships among the major deuterostome taxa (with the common names of organisms in brackets) and their ancestors discussed in this paper.
The hypothetical ancestral genome predicted by Nakatani et al. (“N”) probably belongs to an organism that existed just before 2R in early vertebrates. On the other hand, Putnam et al. (“P”) reconstructed the karyotype of the common ancestor of amphioxus and the olfactores (tunicates+vertebrates). 1R, 2R and 3R mark the three rounds of whole genome duplication that happened throughout vertebrate evolution. Tree topology adapted from Putnam et al [20].
Recently, large scale synteny analyses comparing entire genomes of evolutionarily distant taxa have been employed to reconstruct the karyotypes of extinct ancestors and to look back at the events that shaped the appearance of modern genomes [11]. Ancestral genome reconstruction models depict metazoan chromosomes as composed of segments, originating from one or more linkage groups of a distant ancestor, which become united following repeated chromosomal fission and fusion events to ultimately form the karyotypes of modern taxa. By tracing the syntenic relationships among such chromosomal segments from two or more extant taxa, it is possible to reconstruct the linkage groups of their common ancestor at the time of taxon divergence. For example, comparison of the genomes of tetrapods and teleosts allows one to infer the chromosomes of the hypothetical ∼450 MY old gnathostome ancestor and to outline the linkage groups of the ∼500 MY old ancestor of all extant vertebrates (Figure 1) [12].
Reconstructions of ancestral genomes in the chordate lineage are particularly interesting, because they shed light on the role of WGD events and the intensive karyotype rearrangements that played key roles in the evolution of the vertebrate genetic portfolio. Although it has been suggested that genome reconstructions provide principally a heuristic tool for understanding genome evolution [11], in this study we show how such models can be used to trace the evolutionary history and linkage relationship of genes, thereby giving further power to elucidate both the origin and duplication history of gene families. To demonstrate the utility of this approach, we focus on the origins of a group of small peptide hormones and their receptors, whose evolutionary history has been a matter of debate.
The relaxin (RLN) and insulin-like (INSL) peptides mediate a broad variety of primarily reproductive and neuroendocrine functions and are hypothesized to have played important roles in mammalian evolution [13]. The relaxin-family hormones belong to the Insulin-Relaxin superfamily, which also includes Insulin (INS) and Insulin-like growth factors (IGF). However, distinct from both INS and IGF, which signal via receptor tyrosine kinases (RTK), RLN/INSL peptides interact with two very dissimilar classes of G protein-coupled receptors (GPCR). One class of Relaxin family peptide receptors (RXFPs), consisting of RXFP1 and RXFP2 (also known as LGR7 and LGR8 respectively), is closely related to glycoprotein hormone receptors and is hence distinguished by a large N-terminus containing leucine-rich repeats [14]. The other class of RXFPs, consisting of RXFP3 and RXFP4 (also known as SALPR and GPCR142), is related to small peptide (e.g. angiotensin or somatostatin) receptors, which have relatively short N-termini [14]. The promiscuous interaction of RLN/INSL with these 2 diverse classes of receptors is rare among GPCR ligands and its evolutionary significance has yet to be clarified [15].
Although it has been shown that orthologous copies of four RLN/INSL genes (RLN, INSL3, INSL5 and RLN3) are present in teleosts and mammals, the exact mechanisms giving rise to their diversification in non-placental vertebrates have remained elusive [13], [16], [17], [18]. For instance, it was proposed that the signaling system originated via duplication of the INS/IGF locus in the ancestor of olfactores (tunicates+vertebrates), and that the ancestral ligand originally functioned using an insulin RTK-like receptor, prior to switching to an RXFP3/4-type receptor in early vertebrates [19]. At the same time it was believed that RXFP1/2 receptors were not recruited into the system until after the emergence of mammals [13]. In addition, it has also been hypothesized that 3 vertebrate RLN/INSL genes are ohnologs, i.e. products of 2R that took place in the vertebrate ancestor, while the fourth gene arose from a local duplication [17].
In this paper, we employ paleogenomics models to look at the origin and linkage relationships of RLN/INSL and RXFP genes and to determine the role of WGDs in their diversification. We provide evidence that WGDs played a central role, larger than previously appreciated, in the origination of these gene families and suggest that the system consisted of a trio of 2 receptors (one RXFP1/2-like and the other RXFP3/4-like) and a single ligand in the vertebrate ancestor. We find support for the hypotheses generated from the ancestral genome reconstruction models by employing small-scale synteny analyses and phylogenetic reconstructions performed on a broad repertoire of focal genes, and ultimately show the utility, with some caveats, of incorporating paleogenomics data into understanding the evolution of gene families.
Results
In the first part of this study, we inferred the origins of the RLN/INSL and RXFP gene sets by comparing the ancestry of large chromosomal fragments in a teleost fish (Japanese medaka), a bird (chicken) and human using a model of vertebrate genome evolution [12], the “N-model” (for a full explanation of the method, see Supporting Information S1). Since, with some exceptions, RLN/INSL and RXFP genes in non-mammals have been primarily characterized by automated gene scan tools and are poorly annotated, we searched a number of available vertebrate genomes (25 species) for our focal genes (235 total genes) to ensure that we considered all potential ligand and receptor ohnologs (see Dataset S1: Tables S4–S8). Thus for human, chicken and medaka, we mapped the genomic positions of 4, 3 and 6 ligand RLN/INSL and 6, 4 and 9 receptor RXFP genes (or pseudogenes) respectively onto the linkage groups contained in each of the 3 vertebrate genomes (according to the N-model) and “traced” their origins to the gnathostome ancestor chromosomes (GAC), i.e. linkage groups of the hypothetical post-2R ancestor of jawed (and possibly jawless, see [4]) vertebrates. According to the N-model, each of the 40 post-2R reconstructed GACs (A0-J1) originate from 10–13 Vertebrate Ancestral Chromosomes (VAC, A-J), i.e. linkage groups that existed in the hypothetical pre-2R genome. For 3 of the VACs (A, B and F), Nakatani et al. [12] were able to reconstruct the major chromosomal events that established chromosomal content of the post-1R and -2R vertebrate ancestor genomes. The occurrence of several of our genes-of-interest on these GACs allowed us to not only trace their pre-2R origins, but also to assess the number and linkage relationships of the RLN/INSL and RXFP genes in the intermediate post-1R vertebrate ancestor. In their work, Nakatani et al. [12] proposed two alternative scenarios for the duplication and rearrangement history of VAC “A” (found to host the predecessors of both RLN/INSL and RXFP3/4 genes, see below). We considered both scenarios (Figures 2 and S1) and adopted here the one presented in the main text of Nakatani et al [10], which also minimizes the number of linkage groups in the pre-2R vertebrate ancestor. As described in detail in Supporting Information S1, the primary difference of the main (“fusion”) and the alternative (“fission”) models concerns the syntenic linkage of AncRln-like and AncRxfp3/4 genes.
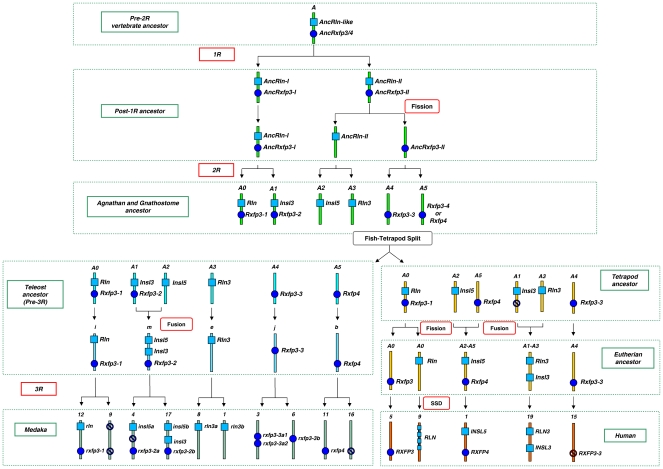
Figure 2. Reconstruction of the genetic events that led to the diversification of RXFP3-type receptors and RLN/INSL hormones in vertebrates based on the “fission” scenario of ancestral genome rearrangement.
The genomic origins of the hypothetical ancestral relaxin (AncRln-like) and Rxfp3/4 receptor (AncRxfp3/4) genes can be traced to a single chromosome in the vertebrate ancestor that had not yet been through 2R (Pre-2R vertebrate ancestor). The ancestral linkage group harboring AncRln-like and AncRxfp3/4-like sequentially underwent duplication, fission and another duplication yielding 5 distinct linkage groups (agnathan and gnathostome ancestor) harboring the ligand and receptor genes. Subsequently, tetrapods completely lost RXFP3-2 and often RXFP3-3 genes, but retained all of the post-2R ligand gene duplicates. Teleosts, on the other hand, retained both all of the ligand and receptor post-2R gene duplicates, suggesting that RXFP3-2 and RXFP3-3 acquired important functions in the pre-3R teleost ancestor. The duplicates of rxfp3-2 and rxfp3-3 were again retained in the post-3R teleost ancestor along with those of rln3 and insl5 (indicating their possible ligand-receptor relationships). Lastly, in vertebrates the RLN locus underwent multiple local duplications, resulting in the emergence of INSL4 in all eutherians, and INSL6 and RLN1 only in apes, whose RLN2 is orthologous to RLN of other eutherians. For simplicity, tetrapod and eutherian ancestor linkage groups are only shown to contain the fragments (e.g. A0, A2–A5) harboring the genes of interest; thus they should not be confused with actual chromosomes. Blue circles and squares represent receptor and their ligand genes respectively. Crossed circles represent pseudogenes (red, if they are verified in databases, blue if they are hypothetical). SSD: small-scale duplication. The first letter of ancestral gene names is capitalized.
RLN/INSL and RXFP3/4 originate from ancestral linkage group VAC “A”, while RXFP1/2 originates from another group, VAC “C”
Our analyses revealed that RLN, RLN3, INSL3, INSL5 and their orthologs in teleosts originated from one location in VAC “A” in the pre-2R vertebrate ancestor (Dataset S1: Table S1). Since each of the four RLN/INSL genes can be mapped to 4 distinct 2R-derived GACs (A0, A1, A2 and A3), we infer that modern vertebrate relaxin family genes arose from a single ancestral gene, AncRln-like, as a result of 2R (Figure 2).
The origins of the receptor RXFP3/4 genes in tetrapods and teleosts were traced to four GACs (A0, A1, A4 and A5; 2 of which, A0 and A1, are the same as those hosting RLN and INSL3), which suggests that they also originated from one gene, AncRxfp3/4-like, located on VAC “A” (Dataset S1: Table S1). This indicates that the ancestral genes for RLN/INSL and RXFP3/4 were physically linked before 2R took place (Figure 2; whereas in the alternative scenario, the ancestral ligand and receptor genes were located in separate linkage groups and half of their duplicates became linked in the post-1R descendants, Figure S2 and Supporting Information S1).
The high number of receptor rxfp3-type genes in teleosts is explained by the post-2R retention of all four rxfp3/4 ohnologs in the teleost ancestor. Additionally, the fish-specific 3R coupled with a few local duplications increased the number of rxfp3-like genes in teleosts to 7 (Figure 2 and Dataset S1: Tables S7, S8). Interestingly, our data mining uncovered that a few tetrapods retained RXFP3-3, but RXFP3-2 appears to have been completely lost in the early tetrapod ancestor (Figure 2 and Dataset S1: Tables S4, S6). Using the available RXFP3-3 sequences from opossum, cow and pig we located the RXFP3-3 pseudogene in human and confirmed its common origin (GAC “A4”) with its medaka orthologs (Dataset S1: Table S1).
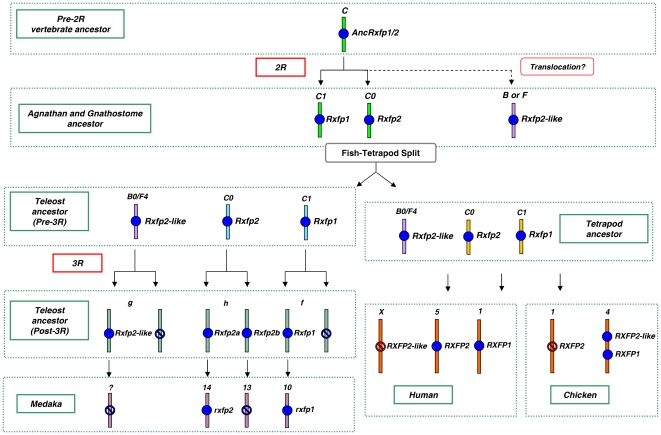
Our tracing of the ancestral origins of RXFP1 and RXFP2 receptors in human and medaka showed that both of these genes originated from VAC “C” (Dataset S1: Table S1). Thus we concluded that 2R led to the duplication of an ancestral gene, AncRxfp1/2, of which only 2 orthologs (RXFP1 and RXFP2) were retained in human and medaka (Figure 3 and also Figure S2). Interestingly, duplicates of rxfp1 and rxfp2 were also lost after 3R in stickleback (Gasterosteus aculeatus), tetraodon (Tetraodon nigroviridis) and fugu (Takifugu rubripes), but were partly retained in zebrafish, in which we found two rxfp2 orthologs (Figure S4).
Figure 3. Reconstruction of the genetic events that led to the diversification of RXFP1/2-type receptor genes in vertebrates.
Symbols and linkage group numbering same as in Figure 2 .
The two genes previously reported as RXFP1 and RXFP2 in chicken, turned out to have an evolutionary history that was slightly different from that of their counterparts in other vertebrates. Chicken “RXFP1” was traced to GAC “C1” (implying its orthology to the RXFP1 of human and medaka), but the chicken “RXFP2” gene was traced to a different ancestral linkage group (GAC “B0” or “F4”) than the expected GAC “C2” (Dataset S1: Table S1). Further analyses confirmed that this gene does not share synteny with either RXFP1 or RXFP2 human genes and we therefore rename it RXFP2-like. Subsequently, we identified an ortholog of this RXFP2-like gene in some other vertebrates, such as zebrafish and opossum, and found a pseudogene of the RXFP2-like gene on human chromosome X next to STARD8, its neighbouring gene in chicken (Dataset S1: Table S6). Convincingly, BLASTn searches also revealed a pseudogene of RXFP2 in the region of the chicken genome orthologous to that hosting RXFP2 in other vertebrates. To incorporate this information about the evolutionary relationship among genes, here we adopt an origin-based nomenclature for the novel genes identified in this study but retain, as much as possible, the traditional naming scheme for the RLN/INSL and RXFP gene families (Table 1).
Table 1. Explanation of the nomenclature used for the hypothetical ancestral genes that gave rise to the three gene families discussed in this study (receptors RXFP3/4, RXFP1/2 and their ligands RLN/INSL) via three rounds of WGD (1R, 2R and teleost-specific 3R).
| Origin | Gene Family: RXFP3/4 (SALPR/GPCR142) | |||||||
| Pre-2R | AncRxfp3/4 | |||||||
| Post-1R paralogs | AncRxfp3-I | AncRxfp3-II | ||||||
| Post-2R paralogs | RXFP3-1 | RXFP3-2 | RXFP3-3 | RXFP3-4 * | ||||
| Post-3R paralogs | rxfp3-1 | † | rxfp3-2a | rxfp3-2b | rxfp3-3a ‡ | rxfp3-3b | rxfp4 | † |
*We show that the gene known as “RXFP4” is one of the three ohnologs of RXFP3-1, hence based on its origin it should be termed “RXFP3-4”;
**The origins of RXFP2-like (present in zebrafish, amphibians, birds, reptiles and marsupials) remain controversial, it is possible that RXFP2-like is a post-2R descendant of AncRxfp2, in which case it should be called “RXFP2-2”, while the ortholog of human RXFP2 should be called “RXFP2-1”;
rxfp3-3a was locally duplicated in the Post-3R ancestor of zebrafish, medaka, stickleback and pufferfishes (hence rxfp3-3a and rxfp3-3b); in zebrafish there are three paralogous rxfp3-3a genes: 3-3a1, 3-3a2 and 3-3a3;
Gene loss
Linkage relationships among RLN/INSL and RXFP genes have changed over evolutionary time
According to the “fission” model of VAC “A” evolution, in the pre-2R vertebrate ancestor, the receptor AncRxfp3/4 gene was in the same linkage group as the ligand AncRln-like. Our reconstruction shows that two of the RXFP3 2R-ohnologs (RXFP3-1 and RXFP3-2) were linked to RLN and INSL3 (Figure 2), while the remaining ohnologs became unlinked. These ancestral genetic linkage relationships have mostly persisted in teleosts (Figure 4), but they have dynamically changed in tetrapods resulting in different combinations of linkage pairs such as INSL5-RXFP4, RLN3-INSL3 and RXFP1-RXFP2-like to name a few (Figure S5).
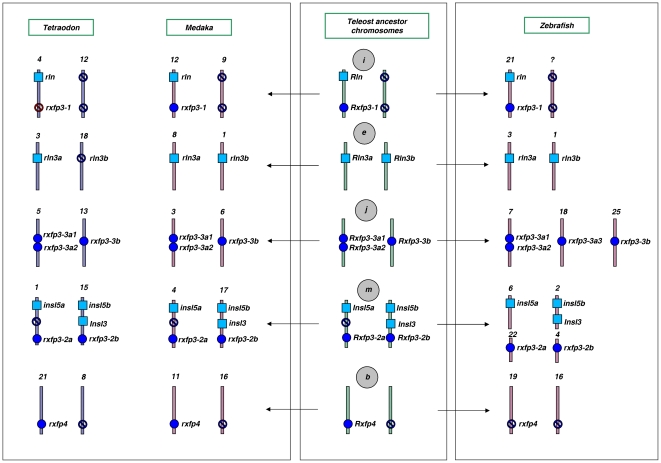
Figure 4. The evolution and genetic linkage of RLN/INSL (ligand) and RXFP3/4 (receptor) loci in the pre-3R teleost ancestor and three species of teleost fish.
Notice that among the three fish species analysed, medaka's genome and rln/insl-rxfp gene sets are the most preserved and resemble those of the teleost ancestor. Tetraodon experienced lineage-specific loss of two genes, rln3b and rxfp3-1, which may indicate their co-evolution as a ligand-receptor pair. The rxfp4 gene in zebrafish seems to have been replaced with an extra (zebrafish-specific) copy of an rxfp3-3 gene. The syntenic linkage between rln and insl3 (ligand) and rxfp3-1 and rxfp3-2(b) (receptor) genes has been conserved in all three teleosts since the post-2R ancestor (see Figure 2). Overall this scheme demonstrates that the rln/insl-rxfp system in teleosts has taken a slightly different, and seemingly more complicated, evolutionary pathway compared to other vertebrates. Chromosome numbers in extant species are shown as numbers and in the teleost ancestor as letters.
We additionally used an ancestral genome reconstruction model by Putnam et al. [20] (the “P” model, see Figure 1 and Supporting Information S1), to address the ancestry of human RLN/INSL/RXFP genes. Consistent with the results obtained using the N-model, the P-model places all human RLN/INSL genes together with RXFP3-1 in one ancestral linkage group. Likewise RXFP1 and RXFP2 were traced to one linkage group independent from the one harboring RXFP2-like. In disagreeement with the N-model, the ancestral linkage group predicted for RXFP3-3 differs from that harboring RXFP3-1, and RXFP4 could not be assigned to any ancestral region (Figure S3).
RXFP phylogenetic reconstruction supports strong role of WGDs in gene duplication events
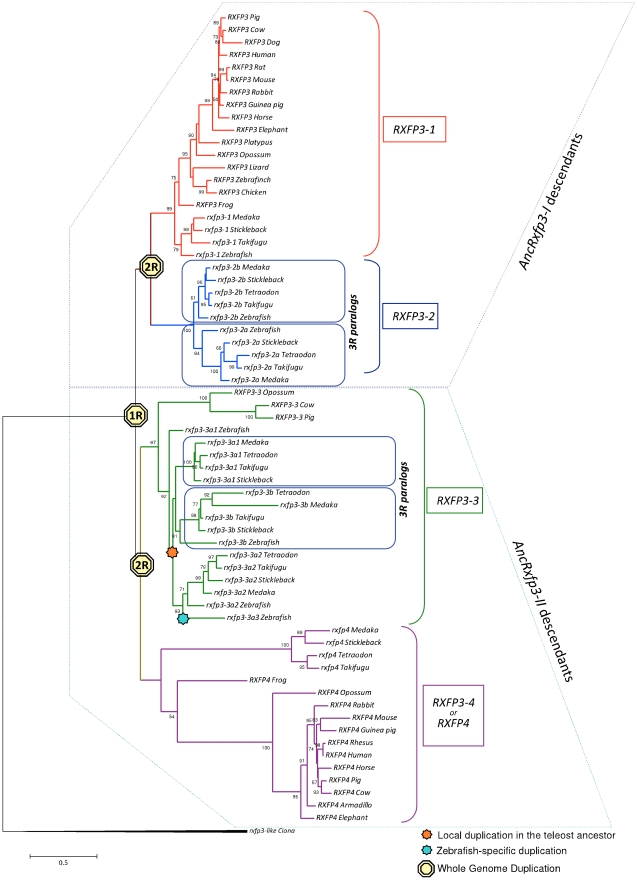
The second goal of this study was to use other types of analysis, such as phylogeny and small-scale synteny, to corroborate the above model of evolution of the vertebrate RLN/INSL-RXFP systems in a broader range of vertebrates. We created a protein database and subsequently phylogenetic trees of RLN/INSL and RXFP-type genes for vertebrates and a few pre-2R diverging taxa, based on publicly annotated genes and included a few that we identified de novo (Dataset S1: Tables S4–S11). Overall, we find that the phylogenetic relationship of the receptor RXFP3/4 sequences clearly recapitulates their proposed WGD-driven origination: the proposed 1R-descendants cluster into two groups, AncRxfp3-I versus AncRxfp3-II, while the proposed 2R-descendants are sister clades, i.e. RXFP3-1/RXFP3-2 and RXFP3-3/RXFP3-4 as expected (Figure 5). Because most tetrapods lost half of their post-2R RXFP3 ohnologs, the RXFP3-2 and RXFP3-3 clades mostly contain teleostean sequences.
Figure 5. Phylogenetic reconstruction of the evolutionary relationship among vertebrate RXFP3/4 protein sequences.
Reconstruction performed as outlined in methods with G = 0.91 and I = n/a. Numbers at each node indicate the bootstrap values (only values exceeding 50% shown). Teleost rxfp3-2 underwent duplication yielding two 3R-paralogs, rxfp3-2a and rxfp3-2b, while teleostean ancestral rxfp3-3 was duplicated giving rise to typically three rxfp3-3 loci in modern teleosts: 3R generated rxfp3-3a and rxfp3-3b, while a local duplication generated rxfp3-3a1 and rxfp3-3a2. Solely in zebrafish, rxfp3-3a2 duplicated again giving rise to rxfp3-3a3, an event which appears to have occurred coincidently with the exclusive loss of rxfp4 in zebrafish.
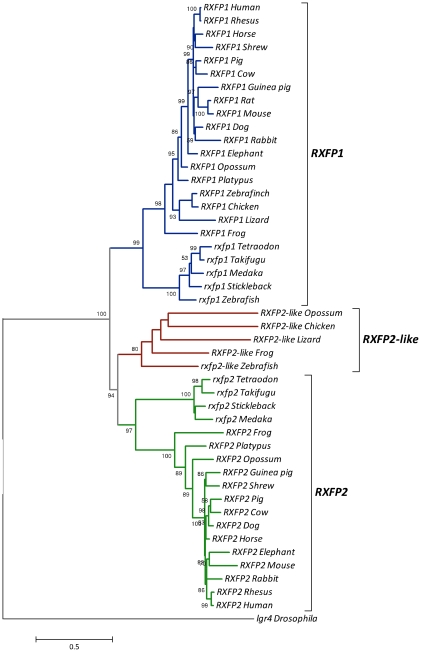
The RXFP1/2 phylogenetic tree (Figure 6) also generally supports the reconstruction model: there are 3 distinct clades for RXFP1, RXFP2 and RXFP2-like, and the RXFP2-like clade is sister to RXFP2, a clustering that supports the ohnologous nature of the relationship between RXFP2-like and RXFP1/2 genes, rather than a pre-2R origin of RXFP2-like. To examine this more closely, we analyzed several vertebrate RXFP1/2 and RXFP2-like protein sequences together with invertebrate rxfp1/2-type proteins, and found that all vertebrate sequences clustered together (Figure S6), indicating that all 3 genes (i.e. RXFP1, RXFP2 and RXFP2-like) originated after the divergence of protochordates.
Figure 6. Phylogenetic reconstruction of the evolutionary relationship among vertebrate RXFP1/2 protein sequences.
Phylogenetic tree reconstructed as outlined in methods with G = 0.958 and I = 0.034. Numbers at each node indicate the bootstrap values (only values exceeding 50% shown). Due to their incomplete nature, not all sequences from our created database (see Methods) were included in this tree (e.g. zebrafish rxfp2a and rxfp2b and medaka rxfp2).
Discussion
Although it is now widely accepted that the two rounds of WGD that took place early in vertebrate evolution played a crucial role in the diversification of many vertebrate gene families [21], the processes by which WGD-driven gene family evolution occurred are not easy to determine. This has been shown to be true for the three vertebrate gene families encoding relaxin hormones and their receptors (RLN/INSL, RXFP1/2 and RXFP3/4), whose duplication history and invertebrate origins we analyzed here. By combining information from ancestral genome reconstructions with phylogenetic and syntenic data, we were able to elucidate the origin of the RLN/INSL and RXFP genes. While the phylogenetic and syntenic data confirm the paralogous nature of the RLN/INSL and RXFP genes, the ancestral genome reconstructions strongly suggest that the four RLN/INSL and four RXFP3/4-type genes present in the gnathostome ancestor arose during 2R, and are thus ohnologs, and that two of the three RXFP1/2 genes are similarly ohnologs, while the third RXFP1/2-like gene has an unclear origin. This is the first study to show the full evolutionary origin of the RLN/INSL and RXFP genes and the role of both 2R and 3R events in their diversification. Our study also supports the linkage of the ancestral RLN/INSL (ligand) and RXFP3/4 (receptor) loci in the pre-2R vertebrate ancestor genome as first suggested by Olinski et al. [22], and at the same time reveals how these linkage relationships have been retained in some lineages while new linkage relationships have arisen in other lineages.
The role of WGDs in the diversification of relaxin ligand-receptor systems
We based the reconstruction of the RLN/INSL and RXFP gene history principally on Nakatani et al.'s [12] model of vertebrate genome evolution. It has been proposed that the major vertebrate novelties, such as their structurally complex nervous, immune and reproductive systems, arose as a result of the massive amplification of genes that occurred during 2R [21], [23]. By making the necessary assumption that our focal genes remained in the given linkage groups since the pre-2R vertebrate ancestor, we deduced that the diversification of RLN/INSL and RXFP genes was coincidental with 2R events, suggesting that they probably played an important role in the establishment of neuroendocrine and reproductive regulation in early vertebrate evolution.
We also observe that the teleost-specific 3R, which strongly contributed to the genetic richness of teleosts and their biological success [24], [25], further increased the number of rln/insl and rxfp genes. However, in contrast to the 1R and 2R events, only those genes potentially involved in neuroendocrine regulation (rln3, insl5 and half of the rxfp3/4-type receptors), but not reproduction (rln, insl3 and rxfp1/2-type receptors) were retained after 3R in teleosts. The post-3R retention of rln3 and insl5 paralogs was paralleled by the retention of duplicates of rxfp3-2 and rxfp3-3 suggesting both co-functioning but also subfunctionalization of their neuroendocrine functions. Overall, we demonstrate that the large number of teleost receptor rxfp3 genes is only partly attributable to teleost-specific duplications (which was proposed as the sole factor driving their diversification in a previous study [26]), but rather also resulted from the massive loss of RXFP3 ohnologs in tetrapods.
The “tripartite” origins of relaxin signaling
With respect to the origin of the RXFP receptors, we find that while both amphioxus and sea urchin genomes seem to be devoid of rxfp3-type genes [27], [28] and the two rxfp3-type genes in C. intestinalis are very divergent from their vertebrate analogs (Figure 5), early deuterostome lineages witnessed many lineage-specific expansions of the RXFP1/2 locus (Figure S6). Intriguingly, three of the 5 amphioxus rxfp1/2-type genes appear orthologous to human RXFP1 and RXFP2 based on their shared ancestral chordate linkage groups as shown using the “P”-model (Figure S3 and Dataset S1: Table S3). Collectively, given the observation of multiple rln/insl and ins/igf [19], [22], [29], [30] and rxfp1/2-type genes, which are unmistakably evolutionarily related to their vertebrate counterparts, combined with the virtual absence of rxfp3-type genes in echinoderms and cephalochordates, we propose that the signaling of the ancestral RLN/INSL peptide in the chordate ancestor occurred via RXFP1/2-type receptors. Only at the onset of 2R, was the RXFP3/4-type receptor recruited to produce a signaling system encoded by 3 genes, composed of 2 receptors and a single ligand. It is tempting to hypothesize that this ancestral 2-receptor system had a dual function and played roles in both reproductive (using RXFP1/2-type receptor) and neuroendocrine processes (via RXFP3/4-type receptor).
The hypothesis that the AncRln-like locus exhibited co-expression in reproductive and neuroendocrine tissues and signaled via both RXFP1/2 and RXFP3/4 type receptors is supported by the following findings: 1) the dual functionality of human RLN3, considered to be the “oldest” member of the relaxin peptide family in vertebrates, a neuropeptide with the ability to trigger reproductive responses [31] and 2) the discovery of the relaxin-like nature of a starfish gonadotropin which is produced by the echinoderm's nervous system and directly influences the maturation of eggs in the ovary of starfish, probably via a GPCR receptor [32], [33]. This hypothesis also provides a rationale for the retention of the post-1R Rln/Insl duplicates and further helps to explain how the relaxin peptides have evolved to work with two divergent receptor types. If the neuroendocrine and reproductive actions of the pre-1R hormone were mediated by both RXFP1/2- and RXFP3/4-type receptors, then the post-1R loci (AncRln-I and AncRln-II) could have undergone subfunctionalization and specialization in terms of the tissues they targeted, i.e. either reproductive or neuroendocrine, and the receptors with which they functioned.
Issues with currently used nomenclature
By thus elucidating the origin of genes, our model underscores the somewhat artificial nature of both ligand and receptor nomenclature. For ligands, we show that all INSL (insulin-like) genes independently originated from RLN (relaxin-like) genes (and not from an ancestral INSL gene, as previously hypothesized [17]). For receptors, currently only 4 RXFP genes (RXFP1-4) are recognized, those present in humans and some other placentals, while, in fact, there are seven RXFP genes of independent origin in vertebrates (three RXFP1/2 and four RXFP3/4-type), at least six of which are ohnologs, and six of which are present in at least one copy in tetrapods. We also show that RLN3 and INSL3 are ohnologs, and not closely related genes that arose from a tandem duplication event as previously hypothesized [18]. Furthermore, all four RLN/INSL ohnologs were retained after 2R, which contradicts a less parsimonious scenario discussed by Hoffman and Opazo [17], in which one of RLN/INSL genes is lost in all vertebrates. Overall, our model for RLN/INSL evolution in vertebrates is consistent with the hypothesis postulating that INSL3 and RLN evolved as a subfamily distinct from that formed by RLN3 and INSL5 [13]. Our model, however, dates the diversification of the two subfamilies back to the agnathan and gnathostome ancestors, while Wilkinson and Bathgate [13] refer it to the more recent appearance of mammals.
Genetic linkage of RXFP receptors and their ligands
To the best of our knowledge, this is the first study to reveal the dynamic nature of the changing linkage relationships among relaxin family peptides and their receptor genes. The association of RXFP3/4 genes with the RLN/INSL paralogon was first documented by Olinski et al. [22] and the linkage of human INSL5 and RXFP4 on one chromosome also mirrors their ligand-receptor interaction [34]. Nevertheless, we show that INSL5 and RXFP4 occupied different linkage groups in the gnathostome and tetrapod ancestors and only became linked in the eutherian ancestor (Figure 2). Chromosomal linkage of receptor and ligand genes, and other functionally linked or interacting genes, has been known for a number of unrelated gene families and is more common in the human genome than expected by chance [35], [36]. To explain this phenomenon, it has been proposed that receptor-ligand linkage could be advantageous for the creation of new receptor-ligand pairs when they result from block duplications [35]. However, this beneficial effect of linkage would not pertain to genes duplicated via WGDs, as is the case of RLN/INSL and RXFP loci. Instead, we propose that the ancestral RXFP3/4-RLN/INSL linkage was fortuitous and only the subsequent retention of linkage in descendants may have been subject to selection for an advantageous topology, which may have caused the co-expression of ligands and receptors thereby increasing the frequency of their interaction. Although the original linkage was disrupted for one of the post-1R ohnolog pairs, linkage of certain RLN/INSL-RXFP3 pairs has been conserved in some organisms, e.g. in medaka, while not in others, such as in rat (Figure 3 and Figure S6). In this regard, it is interesting that the chromosomal sections harboring the INSL/RLN paralogons contain many other conserved gene families, such as the major histocompatibility complex genes, whose origins are traceable to singular pre-2R ancestor genes [21]. This suggests that conservation of the linkage relationship among the RLN/INSL and RXFP genes may result from conservation of synteny at a larger scale. At the same time, vertebrates have also acquired novel and lineage-specific gene linkages, such as that of RLN3-INSL3 in opossum, human and pig and RXFP1-RXFP2-like in chicken (Figure S6), which could be explained by other factors such as recurrent evolutionary chromosomal breaks in the fragile parts of genomes containing these genes [37].
Difficulties encountered using paleogenomics models
Finally, it should be noted that there can be difficulties in resolving the origin of some genes using ancestral genome reconstructions. This was observed here for the RXFP2-like gene. When a gene maps to a different ancestral linkage group than expected (VAC “F” versus “C” in the case of RXFP2-like), it is difficult to determine if 1) this gene has independent origins from its expected ohnologs or 2) it underwent a single-gene translocation that caused it to move from its authentic chromosomal fragment after duplication or whether 3) the ancestral linkage groups are inaccurately reconstructed. For RXFP2-like it seems possible that there were 2 genes present in the ancestral pre-2R genome, because there are many rxfp1/2-type genes in primitive chordates. However, the RXFP2-like genes from vertebrates cluster closely within the vertebrate RXFP1/2 sequences (Figure S6), suggesting that RXFP2-like is an ohnolog of RXFP1 and RXFP2 genes. Hence RXFP2-like was probably either translocated from its authentic position in the gnathostome ancestor or we are confronted with an inaccurate reconstruction of VAC “C” and “F”. Interestingly, there is a documented example of a single gene translocation of a duplicated insulin (INS) gene in rodents [38]. When traced using the N-model (not shown here) the murid-specific INS gene maps to an ancestral linkage group different from the expected VAC “D” owing to a single gene-translocation that took place early in the evolution of mice and rats [38].
Conclusions
In summary, the origination of relaxin hormones and their two distinct classes of receptors in vertebrates was strongly driven by whole genome duplication events. We postulate that the relaxin hormone-receptor signaling system in the pre-2R ancestor consisted of three components, one ligand and two receptors, and had a dual (reproductive and neuroendocrine regulatory) function. The genetic linkage of RLN/INSL and RXFP3/4 genes, which has been highly conserved in teleosts since the post-2R ancestor, probably played a role in the original establishment of ligand-receptor interactions between ancestral RLN/INSL and RXFP3/4 proteins. We show that most of the ligand and receptor genes duplicated during 2R (or 3R) and that, compared to tetrapods, teleosts have had significantly higher post-2R retention rates of RXFP genes. Our findings about the evolution of relaxin hormones and their receptors should facilitate further research on this system in various vertebrates, including both placental and non-placental taxa. For instance, the discussed 2R-driven model of evolution should raise questions about the number of involved genes in early diverging vertebrates, such as jawless fish, whose status in relation to 2R has until recently been debated [21].
Overall, our study highlights the utility of incorporating ancestral genome data into investigations of the origin, linkage relationship and duplication history of gene families. The methodology employed here will hopefully be useful in similar studies, where traditional approaches may fail to clearly resolve the origin of genes due to their small size, strong roles of selection or insufficient synteny data. Presently, however, a major drawback of the method is the absence of a unified scheme, which would avoid having to perform the time consuming and tedious manual inspection of multiple ancestral genome reconstruction models. In the future this problem could be resolved by designing appropriate computer software. Thus, rather than being viewed as a primarily heuristic tool for studying large scale genome evolution, ancestral genome reconstructions have a potential to form the basis of an instrument that could be routinely consulted to supplement traditional bioinformatic analyses.
Methods
Tracing of the duplication history of RLN/INSL, INS/IGF and RXFP genes
Detailed methods used to trace the evolutionary history of genes are provided in Supporting Information S1. A brief overview of the procedure is given here: First, using their exact map positions, we mapped the RLN/INSL, RXFP and INS/IGF genes found in human, medaka and chicken to their corresponding chromosomal segments. These chromosomal segments were then matched to the linkage groups in ancestral genomes primarily according to Nakatani et al.'s [12] model, but we also invoked other vertebrate genome reconstructions [39], [40], in particular Putnam et al's [20], as needed. Finally we compared the results obtained for each of the three taxa to resolve the positions of the focal genes at consecutive stages of the vertebrate genome evolution. Where discrepancies arose and the genes reported as “orthologous” were traced to different ancestral linkage groups, we performed small-scale synteny analyses (details below) to clarify the relationship of individual genes among taxa.
Identification of RLN/INSL and RXFP(-like) sequences across vertebrates
All annotated RLN/INSL and RXFP coding sequences with their genomic positions were retrieved from the Ensembl v.60 database (http://ensembl.org) for 13 mammals (11 placentals, opossum and platypus), 3 reptiles (anole lizard, chicken and zebrafinch), 2 amphibians (clawed frog and edible frog) and 5 teleosts (Dataset S1: Tables S4–S8). The annotated sequences for rhesus monkey were obtained from NCBI (http://ncbi.nlm.nih.gov/gene). For RXFP1/2 genes, when multiple splice variants were available, the longer variant was chosen, unless shorter variants had been confirmed to be functional.
Using the more or less complete sets of human, mouse, zebrafish and medaka sequences as reference, we performed searches of the databases at both Ensembl and NCBI to look for unannotated and/or yet unidentified genes in other tetrapods and teleosts using the NCBI BLAST package [41]. Additionally, to either confirm the identity of sequences obtained using the above procedure or to search for other difficult to identify genes, we searched the paralogons syntenic to previously determined human/teleost RLN/INSL [16] or RXFP genes in Ensembl by using the Genscan tool or the MIT Genscan server (http://genes.mit.edu/GENSCAN.html) in combination with the conserved-domain search tool (http://ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi), or by blasting the entire syntenic regions via BLASTn in NCBI with an appropriate query. The synteny analysis for the RXFP genes was done using either the Genomicus v.60.01 server (http://dyogen.ens.fr/genomicus-60.01/cgi-bin/search.pl), the appropriate Ensembl tools and/or manual identification of orthologous regions through subjecting genes to BLASTp at NCBI.
RXFP-type genes in pre-2R taxa
For C. intestinalis, 8 rxfp1/2-type genes were retrieved from Ensembl and 2 candidate rxfp3/4-type genes were obtained from ANISEED (http://crfb.univ-mrs.fr/aniseed). Five amphioxus rxfp1/2-type and were retrieved from GenBank. The 6 ilp genes from the amphioxus database were previously analyzed and shown to have syntenically shared genes with the vertebrate Insulin-Relaxin loci [29]. Our searches in the sea urchin database (http://www.spbase.org/SpBase/) yielded 27 rxfp1/2-like sequences. 2 rxfp1/2-like genes (lgr3 and lgr4) were obtained from Ensembl Metazoa (http://metazoa.ensembl.org) for fruit fly (Drosophila melanogaster).
Phylogenetic reconstruction of the relationships among RXFP genes
The alignment of RXFP sequences (available upon request) was accomplished using MUSCLE [42] as implemented in MEGA v. 5.01 [43] and through manual adjustments. Phylogenetic reconstruction of protein sequences was carried out in Phyml [44] using: for RXFP genes, the LG model of sequence evolution and with estimated or fixed values for G, the shape parameter for the gamma distribution, and I, the proportion of invariant sites, depending on what was determined to be the best model of amino acid sequence evolution based on AIC as implemented in ProtTest [45]. Confidence in the phylogenetic reconstruction was assessed using 1000 replicate bootstrap samples. The phylogenetic relationship among invertebrate rxfp1/2-type genes was reconstructed separately following the same method.
Supporting Information
The alternative (“fusion”) scenario of duplication and rearrangement history for VAC “A” according to the N-model.
(PDF)
Two alternative scenarios for the 2R-driven duplication of the AncRxfp1/2 gene.
(PDF)
Comparison of the results obtained using two ancestral genome reconstructions. Top: Tracing of human RLN/INSL and RXFP-like genes in chordate linkage groups (CLG) using P-model; bottom: Tracing of human RLN/INSL and RXFP genes in pre-2R vertebrate ancestor chromosomes.
(PDF)
Chromosomal location of rxfp1/2-type genes in three species of teleosts and in the post-3R teleost ancestor.
(PDF)
Dynamic changes in the chromosomal linkage relationships of RLN/INSLRXFP genes in tetrapods.
(PDF)
Phylogenetic reconstruction of RXFP1/2 proteins from vertebrates, protochordates and an echinoderm.
(PDF)
The detailed methods explaining the use of ancestral genome reconstructions to trace the evolutionary history of individual focal genes.
(PDF)
All accession numbers and map locations for genes used in this study (includes Tables S1–S12).
(PDF)
Acknowledgments
We thank Naoko Takezaki, Jan Bogerd and Jens Franck for comments on an earlier version of this manuscript. We also thank two anonymous reviewers whose comments significantly improved this paper.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by an NSERC Discovery Grant to SVG (www.nserc.ca), and graduate fellowships to SY provided for by both the province of Manitoba and the University of Winnipeg. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Abi-Rached L, Gilles A, Shiina T, Pontarotti P, Inoko H. Evidence of en bloc duplication in vertebrate genomes. Nat Genet. 2002;31:100–105. doi: 10.1038/ng855. [DOI] [PubMed] [Google Scholar]
- 2.Dehal P, Boore JL. Two rounds of whole genome duplication in the ancestral vertebrate. PLoS Biol. 2005;3:e314. doi: 10.1371/journal.pbio.0030314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaillon O, Aury JM, Brunet F, Petit JL, Stange-Thomann N, et al. Genome duplication in the teleost fish Tetraodon nigroviridis reveals the early vertebrate proto-karyotype. Nature. 2004;431:946–957. doi: 10.1038/nature03025. [DOI] [PubMed] [Google Scholar]
- 4.Kuraku S, Meyer A, Kuratani S. Timing of genome duplications relative to the origin of the vertebrates: did cyclostomes diverge before or after? Mol Biol Evol. 2009;26:47–59. doi: 10.1093/molbev/msn222. [DOI] [PubMed] [Google Scholar]
- 5.Ohno S. Evolution by gene duplication. Berlin, New York: Springer-Verlag. 1970:160. [Google Scholar]
- 6.Taylor JS, Raes J. Duplication and divergence: the evolution of new genes and old ideas. Annu Rev Genet. 2004;38:615–643. doi: 10.1146/annurev.genet.38.072902.092831. [DOI] [PubMed] [Google Scholar]
- 7.Nei M, Gu X, Sitnikova T. Evolution by the birth-and-death process in multigene families of the vertebrate immune system. Proc Natl Acad Sci U S A. 1997;94:7799–7806. doi: 10.1073/pnas.94.15.7799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolfe KH. Yesterday's polyploids and the mystery of diploidization. Nat Rev Genet. 2001;2:333–341. doi: 10.1038/35072009. [DOI] [PubMed] [Google Scholar]
- 9.Maere S, De Bodt S, Raes J, Casneuf T, Van Montagu M, et al. Modeling gene and genome duplications in eukaryotes. Proc Natl Acad Sci U S A. 2005;102:5454–5459. doi: 10.1073/pnas.0501102102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Makino T, Hokamp K, McLysaght A. The complex relationship of gene duplication and essentiality. Trends in Genet. 2009;25:152–155. doi: 10.1016/j.tig.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Muffato M, Roest Crollius H. Paleogenomics in vertebrates, or the recovery of lost genomes from the mist of time. Bioessays. 2008;30:122–134. doi: 10.1002/bies.20707. [DOI] [PubMed] [Google Scholar]
- 12.Nakatani Y, Takeda H, Kohara Y, Morishita S. Reconstruction of the vertebrate ancestral genome reveals dynamic genome reorganization in early vertebrates. Genome Res. 2007;17:1254–1265. doi: 10.1101/gr.6316407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilkinson TN, Bathgate RA. The evolution of the relaxin peptide family and their receptors. Adv Exp Med Biol. 2007;612:1–13. doi: 10.1007/978-0-387-74672-2_1. [DOI] [PubMed] [Google Scholar]
- 14.Halls ML, van der Westhuizen ET, Bathgate RA, Summers RJ. Relaxin family peptide receptors--former orphans reunite with their parent ligands to activate multiple signalling pathways. Br J Pharmacol. 2007;150:677–691. doi: 10.1038/sj.bjp.0707140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gloriam DE, Foord SM, Blaney FE, Garland SL. Definition of the G protein-coupled receptor transmembrane bundle binding pocket and calculation of receptor similarities for drug design. J Med Chem. 2009;52:4429–4442. doi: 10.1021/jm900319e. [DOI] [PubMed] [Google Scholar]
- 16.Good-Avila SV, Yegorov S, Harron S, Bogerd J, Glen P, et al. Relaxin gene family in teleosts: phylogeny, syntenic mapping, selective constraint, and expression analysis. BMC Evol Biol. 2009;9:293. doi: 10.1186/1471-2148-9-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffmann FG, Opazo JC. Evolution of the relaxin/insulin-like gene family in placental mammals: implications for its early evolution. J Mol Evol. 2011;72:72–79. doi: 10.1007/s00239-010-9403-6. [DOI] [PubMed] [Google Scholar]
- 18.Park JI, Semyonov J, Chang CL, Yi W, Warren W, et al. Origin of INSL3-mediated testicular descent in therian mammals. Genome Res. 2008;18:974–985. doi: 10.1101/gr.7119108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olinski RP, Dahlberg C, Thorndyke M, Hallbook F. Three insulin-relaxin-like genes in Ciona intestinalis. Peptides. 2006;27:2535–2546. doi: 10.1016/j.peptides.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 20.Putnam NH, Butts T, Ferrier DE, Furlong RF, Hellsten U, et al. The amphioxus genome and the evolution of the chordate karyotype. Nature. 2008;453(7198):1064–1071. doi: 10.1038/nature06967. [DOI] [PubMed] [Google Scholar]
- 21.Kasahara M. The 2R hypothesis: an update. Curr Opin Immunol. 2007;19:547–552. doi: 10.1016/j.coi.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 22.Olinski RP, Lundin LG, Hallbook F. Conserved synteny between the Ciona genome and human paralogons identifies large duplication events in the molecular evolution of the insulin-relaxin gene family. Mol Biol Evol. 2006;23:10–22. doi: 10.1093/molbev/msj002. [DOI] [PubMed] [Google Scholar]
- 23.Huminiecki L, Heldin CH. 2R and remodeling of vertebrate signal transduction engine. BMC Biol. 2010;8:146. doi: 10.1186/1741-7007-8-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoegg S, Brinkmann H, Taylor JS, Meyer A. Phylogenetic timing of the fish-specific genome duplication correlates with the diversification of teleost fish. J Mol Evol. 2004;59:190–203. doi: 10.1007/s00239-004-2613-z. [DOI] [PubMed] [Google Scholar]
- 25.Meyer A, Van de Peer Y. From 2R to 3R: evidence for a fish-specific genome duplication (FSGD). Bioessays. 2005;27:937–945. doi: 10.1002/bies.20293. [DOI] [PubMed] [Google Scholar]
- 26.Wilkinson TN, Speed TP, Tregear GW, Bathgate RA. Evolution of the relaxin-like peptide family. BMC Evol Biol. 2005;5:14. doi: 10.1186/1471-2148-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nordstrom KJ, Fredriksson R, Schioth HB. The amphioxus (Branchiostoma floridae) genome contains a highly diversified set of G protein-coupled receptors. BMC Evol Biol. 2008;8:9. doi: 10.1186/1471-2148-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sodergren E, Weinstock GM, Davidson EH, Cameron RA, Gibbs RA, et al. The genome of the sea urchin Strongylocentrotus purpuratus. Science. 2006;314:941–952. doi: 10.1126/science.1133609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holland LZ, Albalat R, Azumi K, Benito-Gutierrez E, Blow MJ, et al. The amphioxus genome illuminates vertebrate origins and cephalochordate biology. Genome Res. 2008;18:1100–1111. doi: 10.1101/gr.073676.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McRory JE, Sherwood NM. Ancient divergence of insulin and insulin-like growth factor. DNA Cell Biol. 1997;16:939–949. doi: 10.1089/dna.1997.16.939. [DOI] [PubMed] [Google Scholar]
- 31.McGowan BM, Stanley SA, Donovan J, Thompson EL, Patterson M, et al. Relaxin-3 stimulates the hypothalamic-pituitary-gonadal axis. Am J Physiol Endocrinol Metab. 2008;295:E278–286. doi: 10.1152/ajpendo.00028.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mita M, Yamamoto K, Nagahama Y. Interaction of Relaxin-Like Gonad-Stimulating Substance with Ovarian Follicle Cells of the Starfish Asterina pectinifera. Zoolog Sci. 2011;28:764–769. doi: 10.2108/zsj.28.764. [DOI] [PubMed] [Google Scholar]
- 33.Mita M, Yoshikuni M, Ohno K, Shibata Y, Paul-Prasanth B, et al. A relaxin-like peptide purified from radial nerves induces oocyte maturation and ovulation in the starfish, Asterina pectinifera. Proc Natl Acad Sci U S A. 2009;106:9507–9512. doi: 10.1073/pnas.0900243106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu C, Kuei C, Sutton S, Chen J, Bonaventure P, et al. INSL5 is a high affinity specific agonist for GPCR142 (GPR100). J Biol Chem. 2005;280:292–300. doi: 10.1074/jbc.M409916200. [DOI] [PubMed] [Google Scholar]
- 35.Hurst LD, Lercher MJ. Unusual linkage patterns of ligands and their cognate receptors indicate a novel reason for non-random gene order in the human genome. BMC Evol Biol. 2005;5:62. doi: 10.1186/1471-2148-5-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Makino T, McLysaght A. Interacting Gene Clusters and the Evolution of the Vertebrate Immune System. Molecular Biology and Evolution. 2008;25:1855–1862. doi: 10.1093/molbev/msn137. [DOI] [PubMed] [Google Scholar]
- 37.Bailey JA, Baertsch R, Kent WJ, Haussler D, Eichler EE. Hotspots of mammalian chromosomal evolution. Genome Biol. 2004;5:R23. doi: 10.1186/gb-2004-5-4-r23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shiao MS, Liao BY, Long M, Yu HT. Adaptive evolution of the insulin two-gene system in mouse. Genetics. 2008;178:1683–1691. doi: 10.1534/genetics.108.087023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kasahara M, Naruse K, Sasaki S, Nakatani Y, Qu W, et al. The medaka draft genome and insights into vertebrate genome evolution. Nature. 2007;447:714–719. doi: 10.1038/nature05846. [DOI] [PubMed] [Google Scholar]
- 40.Kemkemer C, Kohn M, Cooper DN, Froenicke L, Hogel J, et al. Gene synteny comparisons between different vertebrates provide new insights into breakage and fusion events during mammalian karyotype evolution. BMC Evol Biol. 2009;9:84. doi: 10.1186/1471-2148-9-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, et al. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, et al. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol. 2010;59:307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- 45.Abascal F, Zardoya R, Posada D. ProtTest: selection of best-fit models of protein evolution. Bioinformatics. 2005;21:2104–2105. doi: 10.1093/bioinformatics/bti263. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The alternative (“fusion”) scenario of duplication and rearrangement history for VAC “A” according to the N-model.
(PDF)
Two alternative scenarios for the 2R-driven duplication of the AncRxfp1/2 gene.
(PDF)
Comparison of the results obtained using two ancestral genome reconstructions. Top: Tracing of human RLN/INSL and RXFP-like genes in chordate linkage groups (CLG) using P-model; bottom: Tracing of human RLN/INSL and RXFP genes in pre-2R vertebrate ancestor chromosomes.
(PDF)
Chromosomal location of rxfp1/2-type genes in three species of teleosts and in the post-3R teleost ancestor.
(PDF)
Dynamic changes in the chromosomal linkage relationships of RLN/INSLRXFP genes in tetrapods.
(PDF)
Phylogenetic reconstruction of RXFP1/2 proteins from vertebrates, protochordates and an echinoderm.
(PDF)
The detailed methods explaining the use of ancestral genome reconstructions to trace the evolutionary history of individual focal genes.
(PDF)
All accession numbers and map locations for genes used in this study (includes Tables S1–S12).
(PDF)