Abstract
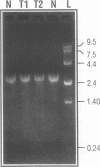
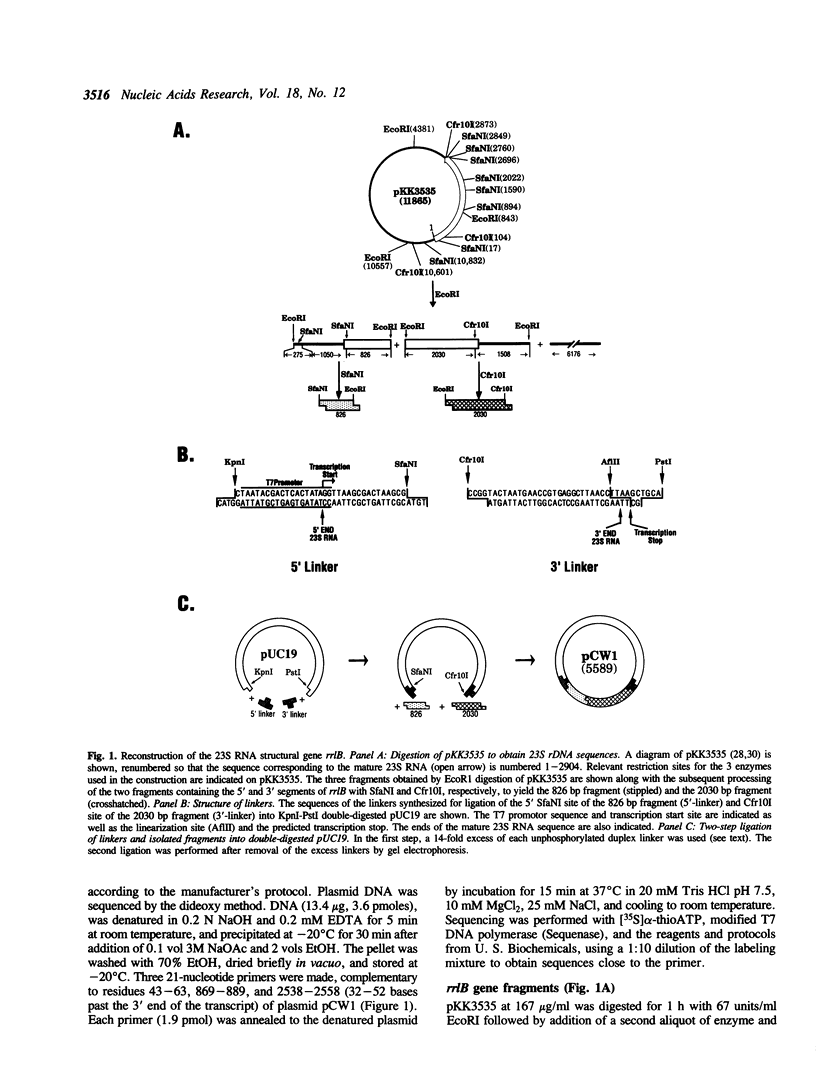
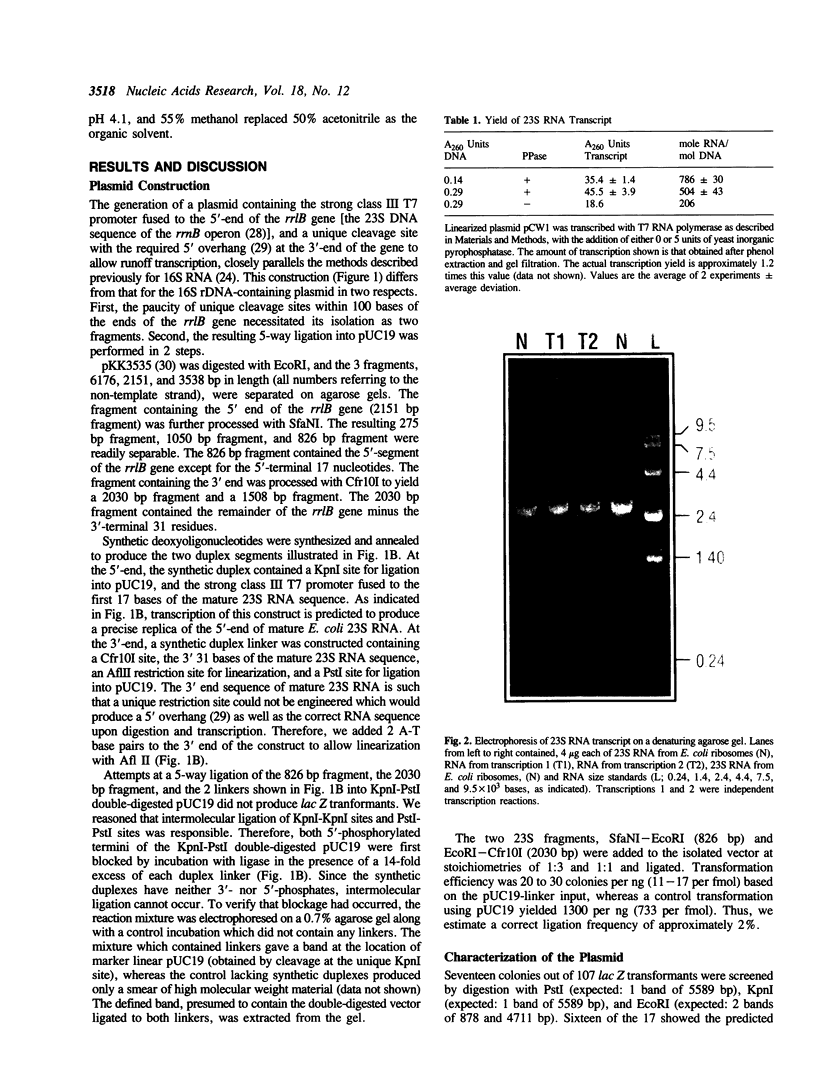
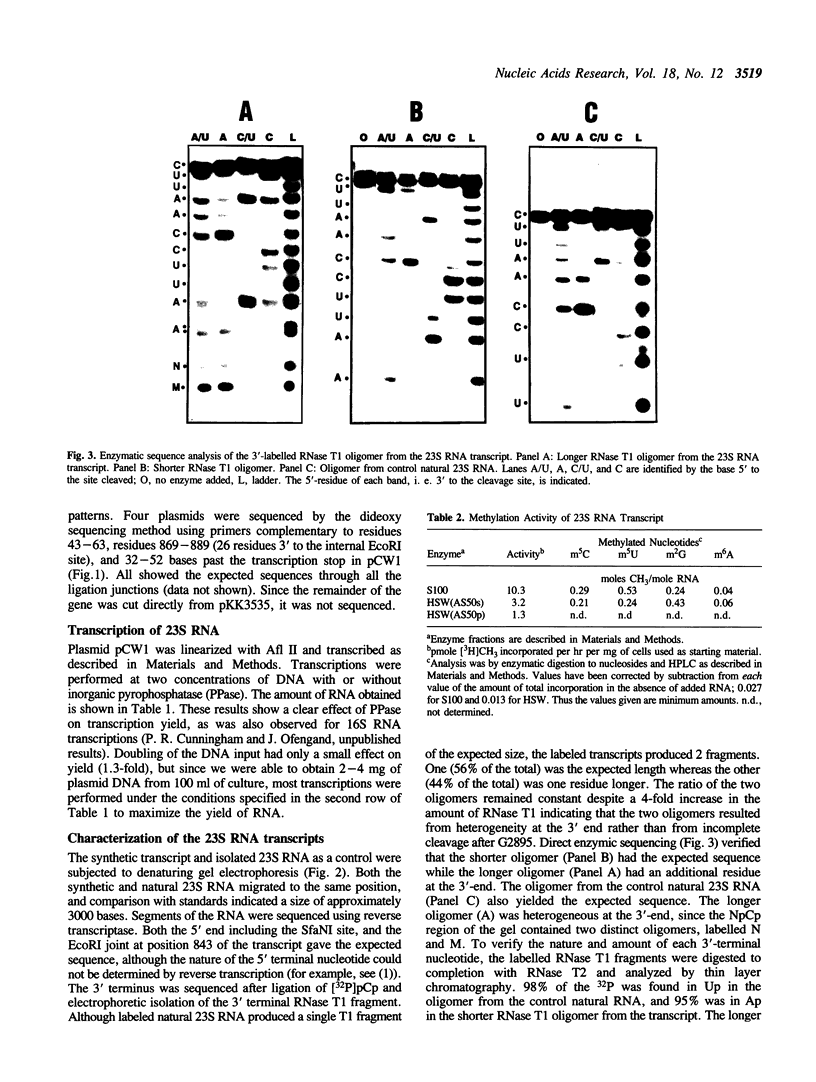
The 23S rRNA gene was excised from the rrnB operon of pKK3535 and ligated into pUC19 behind the strong class III T7 promoter so that the correct 5' end of mature 23S RNA was produced upon transcription by T7 RNA polymerase. At the 3' end, generation of a restriction site for linearization required the addition of 2 adenosine residues to the mature 23S sequence. In vitro runoff transcripts were indistinguishable from natural 23S RNA in size on denaturing gels and in 5'-terminal sequence. The length and sequence of the 3' terminal T1 fragment was also as expected from the DNA sequence, except that an additional C, A, or U residue was added to 21%, 18%, or 5% of the molecules, respectively. Typical transcription reactions yielded 500-700 moles RNA per mole template. This transcript was used as a substrate for methyl transfer from S-adenosyl methionine catalyzed by Escherichia coli cell extracts. The majority (50-65%) of activity observed in a crude (S30) extract appeared in the post-ribosomal supernatant (S100). Activities catalyzing formation of m5C, m5U, m2G, and m6A residues in the synthetic transcript were observed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Björk G. R., Kjellin-Stråby K. General screening procedure for RNA modificationless mutants: isolation of Escherichia coli strains with specific defects in RNA methylation. J Bacteriol. 1978 Feb;133(2):499–507. doi: 10.1128/jb.133.2.499-507.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branlant C., Krol A., Machatt M. A., Pouyet J., Ebel J. P., Edwards K., Kössel H. Primary and secondary structures of Escherichia coli MRE 600 23S ribosomal RNA. Comparison with models of secondary structure for maize chloroplast 23S rRNA and for large portions of mouse and human 16S mitochondrial rRNAs. Nucleic Acids Res. 1981 Sep 11;9(17):4303–4324. doi: 10.1093/nar/9.17.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius J., Dull T. J., Sleeter D. D., Noller H. F. Gene organization and primary structure of a ribosomal RNA operon from Escherichia coli. J Mol Biol. 1981 May 15;148(2):107–127. doi: 10.1016/0022-2836(81)90508-8. [DOI] [PubMed] [Google Scholar]
- Brosius J., Ullrich A., Raker M. A., Gray A., Dull T. J., Gutell R. R., Noller H. F. Construction and fine mapping of recombinant plasmids containing the rrnB ribosomal RNA operon of E. coli. Plasmid. 1981 Jul;6(1):112–118. doi: 10.1016/0147-619x(81)90058-5. [DOI] [PubMed] [Google Scholar]
- Burkard U., Willis I., Söll D. Processing of histidine transfer RNA precursors. Abnormal cleavage site for RNase P. J Biol Chem. 1988 Feb 15;263(5):2447–2451. [PubMed] [Google Scholar]
- Chu W. C., Horowitz J. 19F NMR of 5-fluorouracil-substituted transfer RNA transcribed in vitro: resonance assignment of fluorouracil-guanine base pairs. Nucleic Acids Res. 1989 Sep 25;17(18):7241–7252. doi: 10.1093/nar/17.18.7241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denman R., Nègre D., Cunningham P. R., Nurse K., Colgan J., Weitzmann C., Ofengand J. Effect of point mutations in the decoding site (C1400) region of 16S ribosomal RNA on the ability of ribosomes to carry out individual steps of protein synthesis. Biochemistry. 1989 Feb 7;28(3):1012–1019. doi: 10.1021/bi00429a014. [DOI] [PubMed] [Google Scholar]
- Denman R., Weitzmann C., Cunningham P. R., Nègre D., Nurse K., Colgan J., Pan Y. C., Miedel M., Ofengand J. In vitro assembly of 30S and 70S bacterial ribosomes from 16S RNA containing single base substitutions, insertions, and deletions around the decoding site (C1400). Biochemistry. 1989 Feb 7;28(3):1002–1011. doi: 10.1021/bi00429a013. [DOI] [PubMed] [Google Scholar]
- Ericson G., Chevli K., Wollenzien P. Structure of synthetic unmethylated 16S ribosomal RNA as purified RNA and in reconstituted 30S ribosomal subunits. Biochemistry. 1989 Jul 25;28(15):6446–6454. doi: 10.1021/bi00441a043. [DOI] [PubMed] [Google Scholar]
- Gehrke C. W., Kuo K. C. Ribonucleoside analysis by reversed-phase high-performance liquid chromatography. J Chromatogr. 1989 Jun 2;471:3–36. doi: 10.1016/s0021-9673(00)94152-9. [DOI] [PubMed] [Google Scholar]
- Gewirth D. T., Moore P. B. Exploration of the L18 binding site on 5S RNA by deletion mutagenesis. Nucleic Acids Res. 1988 Nov 25;16(22):10717–10732. doi: 10.1093/nar/16.22.10717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green C. J., Vold B. S. Structural requirements for processing of synthetic tRNAHis precursors by the catalytic RNA component of RNase P. J Biol Chem. 1988 Jan 15;263(2):652–657. [PubMed] [Google Scholar]
- Himeno H., Hasegawa T., Ueda T., Watanabe K., Miura K., Shimizu M. Role of the extra G-C pair at the end of the acceptor stem of tRNA(His) in aminoacylation. Nucleic Acids Res. 1989 Oct 11;17(19):7855–7863. doi: 10.1093/nar/17.19.7855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsuchen C. C., Dubin D. T. Methylation patterns of mycoplasma transfer and ribosomal ribonucleic acid. J Bacteriol. 1980 Dec;144(3):991–998. doi: 10.1128/jb.144.3.991-998.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaksson L. A. Partial purification of ribosomal RNA(m1G)- and rRNA(m2G)-methylases from Escherichia coli and demonstration of some proteins affecting their apparent activity. Biochim Biophys Acta. 1973 Jun 8;312(1):122–133. doi: 10.1016/0005-2787(73)90057-9. [DOI] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffries A. C., Symons R. H. A catalytic 13-mer ribozyme. Nucleic Acids Res. 1989 Feb 25;17(4):1371–1377. doi: 10.1093/nar/17.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce G. F., Inoue T. Structure of the catalytic core of the Tetrahymena ribozyme as indicated by reactive abbreviated forms of the molecule. Nucleic Acids Res. 1987 Dec 10;15(23):9825–9840. doi: 10.1093/nar/15.23.9825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzyzosiak W. J., Denman R., Cunningham P. R., Ofengand J. An efficiently mutagenizable recombinant plasmid for in vitro transcription of the Escherichia coli 16 S RNA gene. Anal Biochem. 1988 Dec;175(2):373–385. doi: 10.1016/0003-2697(88)90560-x. [DOI] [PubMed] [Google Scholar]
- Krzyzosiak W., Denman R., Nurse K., Hellmann W., Boublik M., Gehrke C. W., Agris P. F., Ofengand J. In vitro synthesis of 16S ribosomal RNA containing single base changes and assembly into a functional 30S ribosome. Biochemistry. 1987 Apr 21;26(8):2353–2364. doi: 10.1021/bi00382a042. [DOI] [PubMed] [Google Scholar]
- Leffers H., Egebjerg J., Andersen A., Christensen T., Garrett R. A. Domain VI of Escherichia coli 23 S ribosomal RNA. Structure, assembly and function. J Mol Biol. 1988 Dec 5;204(3):507–522. doi: 10.1016/0022-2836(88)90351-8. [DOI] [PubMed] [Google Scholar]
- Melançon P., Gravel M., Boileau G., Brakier-Gingras L. Reassembly of active 30S ribosomal subunits with an unmethylated in vitro transcribed 16S rRNA. Biochem Cell Biol. 1987 Dec;65(12):1022–1030. doi: 10.1139/o87-134. [DOI] [PubMed] [Google Scholar]
- Milligan J. F., Groebe D. R., Witherell G. W., Uhlenbeck O. C. Oligoribonucleotide synthesis using T7 RNA polymerase and synthetic DNA templates. Nucleic Acids Res. 1987 Nov 11;15(21):8783–8798. doi: 10.1093/nar/15.21.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols L., Schmidt F. J. Dependence of M1 RNA substrate specificity on magnesium ion concentration. Nucleic Acids Res. 1988 Apr 11;16(7):2931–2942. doi: 10.1093/nar/16.7.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nierhaus K. H., Dohme F. Total reconstitution of 50 S subunits from Escherichia coli ribosomes. Methods Enzymol. 1979;59:443–449. doi: 10.1016/0076-6879(79)59106-x. [DOI] [PubMed] [Google Scholar]
- Noller H. F. Structure of ribosomal RNA. Annu Rev Biochem. 1984;53:119–162. doi: 10.1146/annurev.bi.53.070184.001003. [DOI] [PubMed] [Google Scholar]
- Nègre D., Weitzmann C., Ofengand J. In vitro methylation of Escherichia coli 16S ribosomal RNA and 30S ribosomes. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4902–4906. doi: 10.1073/pnas.86.13.4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldermans B., Roza L., Van Knippenberg P. H. Studies on the function of two adjacent N6,N6-dimethyladenosines near the 3' end of 16 S ribosomal RNA of Escherichia coli. III. Purification and properties of the methylating enzyme and methylase-30 S interactions. J Biol Chem. 1979 Sep 25;254(18):9094–9100. [PubMed] [Google Scholar]
- Reyes V. M., Abelson J. A synthetic substrate for tRNA splicing. Anal Biochem. 1987 Oct;166(1):90–106. doi: 10.1016/0003-2697(87)90551-3. [DOI] [PubMed] [Google Scholar]
- Ryan P. C., Draper D. E. Thermodynamics of protein-RNA recognition in a highly conserved region of the large-subunit ribosomal RNA. Biochemistry. 1989 Dec 26;28(26):9949–9956. doi: 10.1021/bi00452a012. [DOI] [PubMed] [Google Scholar]
- Sampson J. R., Uhlenbeck O. C. Biochemical and physical characterization of an unmodified yeast phenylalanine transfer RNA transcribed in vitro. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1033–1037. doi: 10.1073/pnas.85.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson T., Borén T., Johansen T. I., Lustig F. Properties of a transfer RNA lacking modified nucleosides. J Biol Chem. 1988 Sep 25;263(27):13692–13699. [PubMed] [Google Scholar]
- Schulman L. H., Pelka H. Anticodon switching changes the identity of methionine and valine transfer RNAs. Science. 1988 Nov 4;242(4879):765–768. doi: 10.1126/science.3055296. [DOI] [PubMed] [Google Scholar]
- Uhlenbeck O. C. A small catalytic oligoribonucleotide. Nature. 1987 Aug 13;328(6131):596–600. doi: 10.1038/328596a0. [DOI] [PubMed] [Google Scholar]