Abstract
GPCR interacting scaffold protein (GISP) is a multi-domain brain-specific scaffold protein that can regulate GABAB receptor complexes by both enhancing their surface expression and by inhibiting their lysosomal degradation. GISP retards degradation of GABAB receptors through its interaction with tumour susceptibility gene 101 (TSG101), a member of the endosomal sorting complex required for transport (ESCRT) lysosomal sorting machinery. We show that in addition to GABAB, GISP exerts a more general role to increase the steady-state levels of several neurotransmitter receptors. Further, GISP delays TSG101-dependent agonist-induced EGFR down-regulation in human embryonic kidney (HEK) 293 cells whereas a mutant GISP lacking the TSG101 binding domain has no effect. These data suggest that GISP acts as a negative regulator of TSG101-dependent lysosomal degradation and plays an important role in determining the availability of neurotransmitter receptors.
Keywords: GISP, TSG101, AKAP, EGF, Receptor trafficking, Degradation, ESCRT, GPCR
G-protein coupled receptor interacting scaffolding protein (GISP) is a brain-specific protein with 90% cDNA homology with human AKAP450. We originally identified GISP as a binding partner of the GABAB receptor subunit GABAB1 [10]. Functional GABAB receptors comprise heterodimers of GABAB1 and GABAB2 subunits [3,9,13,24]. Whereas GABAB1 binds agonist, GABAB2 is responsible for G-protein coupling [7], and is required for forward trafficking of GABAB heterodimers via the masking of an endoplasmic reticulum (ER) retention motif in the C-terminus of GABAB1 [16]. One role of GISP is to facilitate the surface trafficking of GABAB receptor complexes by binding to GABAB1 [10]. Via a separate mechanism GISP also acts to regulate the stability of GABAB receptor complexes by retarding their lysosomal degradation via interaction with TSG101, a component of the lysosomal sorting machinery [11].
Membrane protein degradation occurs via their sorting into intralumenal vesicles of late endosomes. Subsequent maturation of this late endosome, or its fusion with lysosomes targets these intralumenal vesicles and the proteins contained on their surface to lysosomal degradation. This initial sorting event is primarily mediated by three large protein complexes termed the endosomal sorting complex required for transport (ESCRT)-I, -II and -III. These complexes act sequentially in the sorting of mono-ubiquitinated membrane proteins into subdomains on the endosomal surface prior to invagination and lumenal vesicle formation. TSG101, the mammalian homologue of yeast Vps23, is a central component of ESCRT-I. TSG101 binds ubiquitinated cargo protein prior to their passage to ESCRT-II and -III, and subsequent degradation (for reviews see [1,12,23]).
We have demonstrated that GISP binding to TSG101 inhibits its function in mediating the degradation of GABAB2 even in the absence of the GABAB1 subunit which binds GISP [11]. We therefore hypothesised that GISP may function as a general regulator of lysosomal degradation via its interaction with TSG101. Consistent with this, we report here that GISP upregulates the steady-state levels of several neurotransmitter receptor proteins expressed in HEK293 cells, despite the absence of a direct interaction between these receptor proteins and GISP. This effect of GISP is dependent on the interaction between GISP and TSG101 since a GISP mutant that is incapable of binding TSG101 has no effect on the steady-state levels of these proteins. We further confirm that the effect is mediated via the inhibition of lysosomal degradation by demonstrating that GISP retards the agonist-dependent degradation of the EGF receptor, a process well-established to be dependent on the lysosome, and that this effect leads to prolonged EGFR signalling. These results indicate that GISP functions both to facilitate the surface trafficking of GABAB receptors and has a more general role in the inhibition of lysosomal degradation through its interaction with TSG101.
Plasmid constructs
Full length GISP and a GISP mutant lacking the Δ8 region (GISPΔ8-Mut) in pcDNA3.1 were produced as described previously [10,11].
HEK293 cell culture, transfection and lysis
HEK293 cells were cultured as previously described [6]. Cells were transfected using Lipofectamine 2000 (Invitrogen) and incubated for 24–48 h prior to harvesting. The transfection efficiency for GISP constructs was greater than 90% analysed by immunocytochemistry (Supplementary Fig. 1). In double transfections most cells expressing exogenous receptor proteins were also harbouring GISP constructs (Supplementary Fig. 2). Transfected cells were washed twice with phosphate buffered saline (PBS; Gibco) and scraped directly into sample buffer.
EGFR degradation assay
HEK293 cells transiently expressing GISP, GISPΔ8-Mut or control pCAT were incubated with media without serum for at least 6 h. Cells were then treated with EGF (50 ng) for 0, 15, 30, 60, 90 and 120 min. Cells were washed twice with PBS and scraped directly into sample buffer. Samples were sonicated and heated for 10 min prior to loading.
Immunoblotting
Proteins were resolved by SDS-PAGE and immunoblotting was performed using rabbit polyclonal antibodies to GISP (1 μg/ml), GFP (MolecularProbes 1:1000 dilution), CaSR (Affinity BioReagents; 1 μg/ml), guinea pig antibody to GABAB2 (Chemicon; 1:1000 dilution), a sheep polyclonal antibody to the EGFR (Abcam; 1 μg/ml) and mouse monoclonal antibodies to myc-tag (Sigma; 2 μg/ml), β-actin (Sigma; 1 μg/ml), transferrin receptor (Zymed; 1 μg/ml) and TSG101 (Abcam; 2 μg/ml). Quantitative densitometric analysis was performed using NIH Image J.
GISP increases expression of neurotransmitter receptors in HEK293 cells
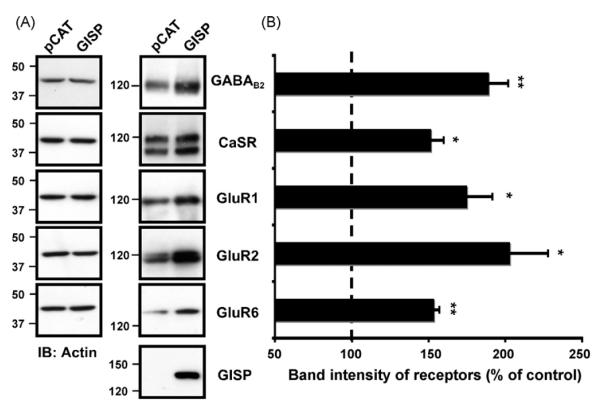
GISP enhances the expression of the GABAB2 subunit in the absence of GABAB1, despite the fact GISP and GABAB2 do not directly interact [10,11]. We therefore tested the effect of GISP, which is not expressed endogenously in HEK293 cells, on the steady state levels of other neurotransmitter receptors and receptor subunits (Fig. 1). First we co-expressed GISP with the calcium-sensing receptor (CaSR), another class C GPCR that does not directly bind GISP. Co-expression of GISP increased the total levels of the CaSR. Similarly, levels of the ionotropic AMPA receptor subunits GluR1 and GluR2, and the kainate receptor subunit GluR6 were also increased on co-expression with GISP. Again, none of these subunits bind GISP in yeast 2-hybrid assays. Significantly, GISP did not raise levels of β-actin, a cytosolic protein we used as control (Fig. 1). In addition, using immunocytochemical analysis, we observed that cells expressing high levels GISP stain more strongly for GABAB2 than cells expressing lower levels of GISP (Supplementary Fig. 2). These results suggest that the GISP-mediated increase in receptor levels does not depend on any direct interaction with the receptors themselves and appears to be membrane protein-specific. We conjectured that this effect could be due to GISP acting as a negative regulator of receptor degradation pathways via its interaction with TSG101 [11].
Fig. 1.
(A) GISP increases levels of GPCRs and ion-channel receptors. A, GISP increases levels of GABAB2, CaSR, GluR1, GluR2 and GluR6 receptors in HEK293 cells. HEK293 cells were transfected with the receptor plasmid together with either a control vector (pCAT) or vector encoding GISP. 48 h post-transfection, the total receptor protein was determined by Western blotting with anti-receptor antibodies. The doublets for CaSR are due to mature (glycosylated) and immature (non-glycosylated) receptors. Immunoblots were probed with anti-β-actin antibody to ensure equal loading. (B) The bar charts show densiometric analysis of at least three blots and the dashed line indicates the control expression of each receptor (mean ± SEM). In each case, receptor protein expression was normalised to actin. The increase in receptor expression when co-transfected with GISP is significant *p < 0.01; **p < 0.001.
A non-TSG101 binding GISP mutant did not increase the steady state levels of neurotransmitter receptors
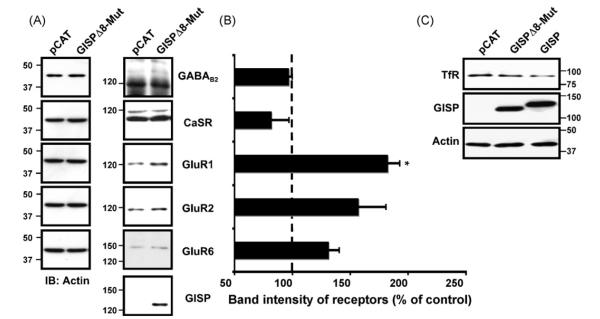
Using GISP truncations, we have shown previously that the Δ8 region of GISP (residues 780 to 850) is critical for GISP binding to TSG101. Importantly, a GISP mutant (GISPΔ8-Mut) lacking the region critical for TSG101 binding did not increase the steady state levels of any of the neurotransmitter receptors tested compared with control, with the exception of the AMPA receptor subunit GluR1 (Fig. 2A and B). These results suggest that the effect of GISP in enhancing receptor protein levels is dependent on the interaction between GISP and TSG101. The effect of the GISP mutant in upregulating steady-state GluR1 expression was unexpected. However, we interpret this to imply that GISP may also perform some other, as yet unknown, role to enhance GluR1 levels that will require further investigation. To further confirm that the effect of GISP on the upregulation of membrane proteins was due to its effect on the ESCRT system, we sought to determine whether GISP, or the GISPΔ8 mutant had any effect on the levels of the transferrin receptor (TfR), an integral membrane protein that does not undergo ESCRT-dependent degradation. TfR is endocytosed and sorted to early endosomes upon binding of diferric transferrin, and subsequently recycled to the cell surface [18] in an ESCRT-independent manner [19]. In contrast to the other receptors tested in this study levels of endogenous TfR were not enhanced in cells expressing GISP when compared to control cells or the GISPΔ8 mutant (Fig. 2C).
Fig. 2.
Effect of GISPΔ8-Mut protein on neurotransmitter receptor expression assessed by immunoblotting. (A) GISPΔ8-Mut does not increases levels of neurotransmitter receptors, apart from GluR1, in HEK293 cells compared to control pCAT. HEK293 cells were transfected with the receptor plasmid together with either a control vector (pCAT) or GISPΔ8-Mut. 48 h post-transfection, the total receptor protein was determined by immunoblotting and blots were probed with anti-receptor antibodies. Immunoblots were probed with anti-β-actin antibody to ensure equal loading and normalisation. (B) The bar charts show normalised densiometric analysis of three blots. The receptor expression when co-transfected with GISPΔ8-Mut is not significant compared to control, apart from in the case of GluR1 (*p < 0.01). (C) GISP does not increases levels of transferin receptor in HEK293 cells compared to GISP mutant. HEK293 cells were transfected with either a control vector (pCAT) or vector encoding GISP or GISPΔ8-Mut. 48 h post-transfection, the total receptor protein was determined by immunoblotting and blots were probed with anti-TfR antibodies.
Degradation of GluR2 and CaSR involves TSG101 in HEK293 cells
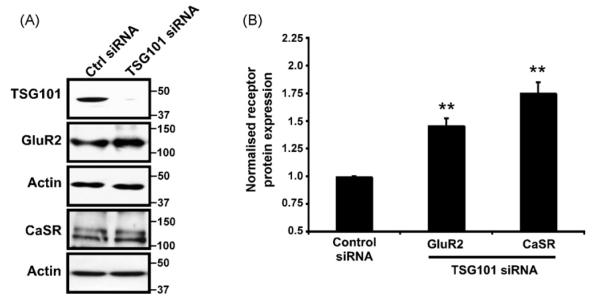
If GISP acts to increase receptor levels by interfering with TSG101-dependent lysosomal sorting this dictates that the affected receptors must be degraded at least in part by the lysosome. We have shown previously that GABAB2 undergoes lysosomal degradation in an ESCRT-dependent manner in HEK293 cells [8]. Furthermore, both GluR6 [17] and the epidermal growth factor receptor (EGFR) have been reported to undergo lysosomal degradation [2,4,8,15]. We therefore tested whether this was also the case for GluR2 and CaSR, the two other proteins investigated in this study whose levels are affected by the overexpression of GISP, but not the TSG101-binding deficient mutant. We determined the effects of depletion of endogenous HEK293 cell TSG101 using siRNA on the levels of exogenously expressed GluR2 and CaSR. As predicted, siRNA-mediated knockdown of endogenous TSG101 led to a significant increase in GluR2 and CaSR expression (Fig. 3A and B). Taken together these data suggest that GISP binding to TSG101 may down-regulate TGS101 protein function and consequent lysosomal degradation of the neurotransmitter receptors examined.
Fig. 3.
Effect of TSG101 siRNA on GluR2 and CaSR expression assessed by immunoblotting. (A) HEK293 cells were transfected with either GluR2 or CaSR in combination with a control siRNA or TSG101 siRNA. After 24 h the cells were again transfected with siRNA and left for further 24 h. 10 nM of siRNA was used for 1 ml of transfection mix. The data are representative of 3 separate experiments. Immunoblots were probed with anti-receptor antibodies, TSG101 antibody and anti-β-actin antibody to ensure equal loading. (B) Normalised densitometric analysis of the effect of TSG101 siRNA on the GluR2 or CaSR receptor protein analysed from 3 independent experiments. **p < 0.001.
GISP modulates TSG101 function in HEK293 cells
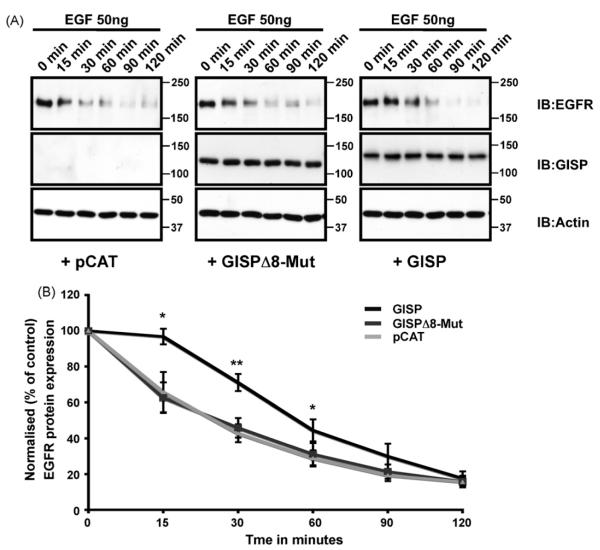
A standard assay for elucidating ESCRT complex function involves investigating agonist-induced EGF receptor degradation [2,4,8,15]. When stimulated, EGFRs are internalised and trafficked for degradation in the lysosome. This process requires functional ESCRT machinery, and is prevented by depletion of TSG101 [2,4,8]. We therefore tested if GISP could regulate this process. Using a well-established EGFR degradation assay in HEK293 cells [15], we found that transient expression of GISP significantly delayed agonist-induced EGFR degradation (Fig. 4). The level of EGFR expression after 2 h of EGF incubation was similar in the GISP and control cells suggesting that the down-regulation of lysosomal-mediated degradation by GISP is overridden after prolonged EGFR stimulation. Crucially, the GISP mutant lacking the TSG101 binding region had no effect on the EGF-induced EGFR degradation profile, suggesting that the GISP-mediated retardation in EGFR degradation is mediated by its interaction with TSG101.
Fig. 4.
GISP negatively regulates TSG101 function. (A) HEK293 cells were transfected with either GISP, GISPΔ8-Mut or control pCAT vector. 48 h post-transfection, the cells were serum-starved for at least 6 h and then incubated with 50 ng/ml concentration of EGF for the times indicated. Samples were then washed in ice-cold PBS, scraped directly into loading buffer and then separated by SDS-PAGE. Blots were probed with anti-EGFR antibody and also probed with anti-β-actin and anti-GISP antibodies to ensure equal loading. (B) Densitometric analysis showing that GISP slows the rate of EGFR degradation. Mean ± SEM from 4 independent experiments. *p < 0.02; **p < 0.004.
GISP delays EGFR degradation and prolongs receptor signalling
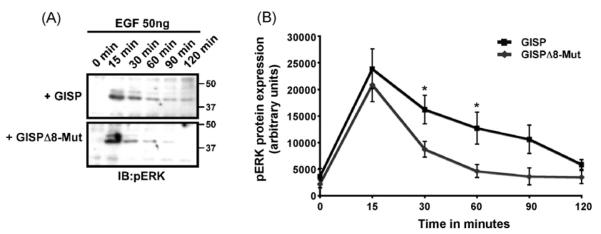
We next questioned the functional significance of the GISP-mediated delay in receptor degradation. We anticipated that increased receptor stability could potentially lead to prolonged receptor signalling. Samples were immunoblotted for phosphorylated (activated) ERK1/2, a pathway well-characterised to be activated by EGFR stimulation [12]. The duration of ERK1/2 activation was significantly prolonged in HEK293 cells transfected with GISP, as compared to cells transfected with mutant GISP incapable of binding TSG101 (Fig. 5). These results indicate that at least one functional consequence of the GISP-mediated delay in receptor degradation is prolonged receptor signalling, and that this effect is dependent on the ability of GISP to bind TSG101. Indeed, this is consistent with a role for GISP in the inhibition of TSG101, as TSG101-null fibroblasts also exhibit delayed EGFR degradation and prolonged ERK1/2 signalling [2].
Fig. 5.
GISP prolongs EGF-induced activation of the ERK1/2. (A) HEK293 cells were transfected with either GISP or GISPΔ8-Mut vector. 48 h post-transfection, the cells were serum-starved for at least 6 h and then incubated with 50 ng/ml EGF for the times indicated. Samples were then washed in ice-cold PBS, scraped directly into loading buffer and then separated by SDS-PAGE. Blots were probed with anti-pERK1/2 antibody and also probed with anti-β-actin to ensure equal loading. These are the same samples described and used in Fig. 4. (B) Quantitative representation of the pERK1/2 western blot in (A) determined by image J analysis. Mean ± SEM from 4 independent experiments. *p < 0.05.
While we cannot exclude some additional potential role for GISP in receptor protein synthesis, our results indicate that GISP is a regulator of TSG101-dependent lysosomal sorting. Firstly, the role of GISP in upregulating receptor levels is dependent on the Δ8 region, which has been previously characterised to bind TSG101, a protein well-established to be involved in the lysosomal targeting of receptor proteins [11]. Secondly, our previous data suggest that while GISP is widely expressed throughout neurons, it is excluded from the nucleus [10,11]. Finally, the effect of GISP in retarding the degradation of EGFR and prolonging its signalling can be seen as little as 15 min after receptor stimulation, a time course consistent with a role in receptor degradation, but likely too short to suggest a role in de novo receptor synthesis.
Protein degradation is of fundamental importance for neuronal function and perturbation of degradative pathways has been implicated in multiple neurodegenerative disorders, including Alzheimer’s, Parkinson’s and Huntington’s [20]. These disorders are characterised by significant neuronal inclusions that often over-whelm degradative pathways. In addition, correct functioning of the ESCRT machinery has also been implicated in neuropathological conditions [5,14,21,22]. It is therefore likely that these pathways are particularly tightly regulated in neurons and GISP may contribute to this process via down-regulation of TSG101.
While it is currently unclear precisely how GISP influences ESCRT-mediated degradation, we have shown previously that GISP and TSG101 co-immunoprecipitate from microsomal fractions, suggesting their interaction takes place at membranes [11]. It is possible that GISP could directly inhibit TSG101 function by blocking the binding of TSG101 to other members of the ESCRT machinery or by preventing TSG101 from binding ubiquitinated cargo. Alternatively, GISP may be acting as a scaffold, recruiting some other inhibitory factor to TSG101. Further work will be required to distinguish these possibilities.
Supplementary Material
Acknowledgements
We are grateful to the Wellcome Trust, the MRC and the EU (GRIPPANT, PL 005320) for financial support. We also thank Philip Rubin, Patrick Tidball and Yasuko Nakamura for technical assistance.
Footnotes
Appendix A. Supplementary data Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.neulet.2009.01.011.
References
- [1].Babst M. A protein’s final ESCRT. Traffic. 2005;6:2–9. doi: 10.1111/j.1600-0854.2004.00246.x. [DOI] [PubMed] [Google Scholar]
- [2].Babst M, Odorizzi G, Estepa EJ, Emr SD. Mammalian tumor susceptibility gene 101 (TSG101) and the yeast homologue, Vps23p, both function in late endosomal trafficking. Traffic. 2000;1:248–258. doi: 10.1034/j.1600-0854.2000.010307.x. [DOI] [PubMed] [Google Scholar]
- [3].Bettler B, Kaupmann K, Bowery NG. GABAB receptors: drugs meet clones. Curr Opin Neurobiol. 1998;8:345–350. doi: 10.1016/s0959-4388(98)80059-7. [DOI] [PubMed] [Google Scholar]
- [4].Bishop N, Horman A, Woodman P. Mammalian class E vps proteins recognize ubiquitin and act in the removal of endosomal protein-ubiquitin conjugates. J Cell Biol. 2002;157:91–101. doi: 10.1083/jcb.200112080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bohren KM, Gabbay KH, Owerbach D. Affinity chromatography of native SUMO proteins using His-tagged recombinant UBC9 bound to Co(2+)-charged talon resin. Protein Expr Purif. 2007;54:289–294. doi: 10.1016/j.pep.2007.03.013. [DOI] [PubMed] [Google Scholar]
- [6].Bouschet T, Martin S, Henley JM. Receptor-activity-modifying proteins are required for forward trafficking of the calcium-sensing receptor to the plasma membrane. J Cell Sci. 2005;118:4709–4720. doi: 10.1242/jcs.02598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Couve A, Moss SJ, Pangalos MN. GABAB receptors: a new paradigm in G protein signaling. Mol Cell Neurosci. 2000;16:296–312. doi: 10.1006/mcne.2000.0908. [DOI] [PubMed] [Google Scholar]
- [8].Doyotte A, Russell MR, Hopkins CR, Woodman PG. Depletion of TSG101 forms a mammalian “Class E” compartment: a multicisternal early endosome with multiple sorting defects. J Cell Sci. 2005;118:3003–3017. doi: 10.1242/jcs.02421. [DOI] [PubMed] [Google Scholar]
- [9].Jones KA, Borowsky B, Tamm JA, Craig DA, Durkin MM, Dai M, Yao WJ, Johnson M, Gunwaldsen C, Huang LY, Tang C, Shen Q, Salon JA, Morse K, Laz T, Smith KE, Nagarathnam D, Noble SA, Branchek TA, Gerald C. GABA(B) receptors function as a heteromeric assembly of the subunits GABA(B)R1 and GABA(B)R2. Nature. 1998;396:674–679. doi: 10.1038/25348. [DOI] [PubMed] [Google Scholar]
- [10].Kantamneni S, Correa SA, Hodgkinson GK, Meyer G, Vinh NN, Henley JM, Nishimune A. GISP: a novel brain-specific protein that promotes surface expression and function of GABA(B) receptors. J Neurochem. 2007;100:1003–1017. doi: 10.1111/j.1471-4159.2006.04271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kantamneni S, Holman D, Wilkinson KA, Correa SA, Feligioni M, Ogden S, Fraser W, Nishimune A, Henley JM. GISP binding to TSG101 increases GABA(B) receptor stability by down-regulating ESCRT-mediated lysosomal degradation. J Neurochem. 2008;107:86–95. doi: 10.1111/j.1471-4159.2008.05580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Katzmann DJ, Odorizzi G, Emr SD. Receptor downregulation and multivesicular-body sorting. Nat Rev Mol Cell Biol. 2002;3:893–905. doi: 10.1038/nrm973. [DOI] [PubMed] [Google Scholar]
- [13].Kuner R, Kohr G, Grunewald S, Eisenhardt G, Bach A, Kornau HC. Role of heteromer formation in GABAB receptor function. Science. 1999;283:74–77. doi: 10.1126/science.283.5398.74. [DOI] [PubMed] [Google Scholar]
- [14].Lee JA, Gao FB. Roles of ESCRT in autophagy-associated neurodegeneration. Autophagy. 2008;4:230–232. doi: 10.4161/auto.5384. [DOI] [PubMed] [Google Scholar]
- [15].Lu Q, Hope LW, Brasch M, Reinhard C, Cohen SN. TSG101 interaction with HRS mediates endosomal trafficking and receptor down-regulation. Proc Natl Acad SciUSA. 2003;100:7626–7631. doi: 10.1073/pnas.0932599100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Margeta-Mitrovic M, Jan YN, Jan LY. A trafficking checkpoint controls GABA(B) receptor heterodimerization. Neuron. 2000;27:97–106. doi: 10.1016/s0896-6273(00)00012-x. [DOI] [PubMed] [Google Scholar]
- [17].Martin S, Henley JM. Activity-dependent endocytic sorting of kainate receptors to recycling or degradation pathways. EMBO J. 2004;23:4749–4759. doi: 10.1038/sj.emboj.7600483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- [19].Progida C, Malerod L, Stuffers S, Brech A, Bucci C, Stenmark H. RILP is required for the proper morphology and function of late endosomes. J Cell Sci. 2007;120:3729–3737. doi: 10.1242/jcs.017301. [DOI] [PubMed] [Google Scholar]
- [20].Ross CA, Poirier MA. Protein aggregation and neurodegenerative disease. Nat Med. 2004;(Suppl. 10):S10–17. doi: 10.1038/nm1066. [DOI] [PubMed] [Google Scholar]
- [21].Rusten TE, Filimonenko M, Rodahl LM, Stenmark H, Simonsen A. ESCRTing autophagic clearance of aggregating proteins. Autophagy. 2007;4 [Google Scholar]
- [22].Skibinski G, Parkinson NJ, Brown JM, Chakrabarti L, Lloyd SL, Hummerich H, Nielsen JE, Hodges JR, Spillantini MG, Thusgaard T, Brandner S, Brun A, Rossor MN, Gade A, Johannsen P, Sorensen SA, Gydesen S, Fisher EM, Collinge J. Mutations in the endosomal ESCRTIII-complex subunit CHMP2B in frontotemporal dementia. Nat Genet. 2005;37:806–808. doi: 10.1038/ng1609. [DOI] [PubMed] [Google Scholar]
- [23].Slagsvold T, Pattni K, Malerod L, Stenmark H. Endosomal and non-endosomal functions of ESCRT proteins. Trends Cell Biol. 2006;16:317–326. doi: 10.1016/j.tcb.2006.04.004. [DOI] [PubMed] [Google Scholar]
- [24].White JH, Wise A, Main MJ, Green A, Fraser NJ, Disney GH, Barnes AA, Emson P, Foord SM, Marshall FH. Heterodimerization is required for the formation of a functional GABA(B) receptor. Nature. 1998;396:679–682. doi: 10.1038/25354. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.