Abstract
Although artificial prostheses for diseased heart valves have been around for several decades, viable heart valve replacements have yet to be developed due to their complicated nature. The majority of research in heart valve replacement technology seeks to improve decellularization techniques for porcine valves or bovine pericardium as an effort to improve current clinically used valves. The drawback of clinically used valves is that they are nonviable and thus do not grow or remodel once implanted inside patients. This is particularly detrimental for pediatric patients, who will likely need several reoperations over the course of their lifetimes to implant larger valves as the patient grows. Due to this limitation, additional biomaterials, both synthetic and natural in origin, are also being investigated as novel scaffolds for tissue engineered heart valves, specifically for the pediatric population. Here, we provide a brief overview of valves in clinical use as well as of the materials being investigated as novel tissue engineered heart valve scaffolds. Additionally, we focus on natural-based biomaterials for promoting cell behavior that is indicative of the developmental biology process that occurs in the formation of heart valves in utero, such as epithelial-to-mesenchymal transition or transformation (EMT). By engineering materials that promote native developmental biology cues and signaling, while also providing mechanical integrity once implanted, a viable tissue engineered heart valve may one day be realized. A viable tissue engineered heart valve, capable of growing and remodeling actively inside a patient, could reduce risks and complications associated with current valve replacement options and improve overall quality of life in the thousands of patients who received such valves each year, particularly for children.
Keywords: tissue engineered heart valves, epithelial-to-mesenchymal transition, polymer scaffolds, hydrogels, electrospinning
Introduction
Heart valve disease represents a leading cause of mortality and morbidity in today’s world. Nearly 300,000 valve replacement surgeries are performed each year, and this number is expected to triple as the aging population increases over the next 30 years [1]. While there are several valve replacement options available commercially, the currently available prostheses are not appropriate for pediatric patients due to size limitations and the need for reoperations as the patients grow [2]. Nearly 20,000 children worldwide are born each year with congenital heart defects, many of which require a heart valve replacement [3–4]. Tissue engineering has been proposed as a way to address the lack of a viable valve replacement, where complex scaffolds with biomimetic mechanical properties undergo growth, cellular invasion, and subsequent remodeling after being implanted in a patient. A particularly promising development is the rise of cell-directive biomaterials which have tunable mechanical properties and are bioactive in their ability to control cell growth and protein synthesis. In addition, the merging of developmental biology principles with tissue engineering innovations offers a chance to create an active biomaterial that will mimic the native environment of the developing heart valve and perhaps lead to the first viable tissue engineered heart valve replacements.
Drawbacks of Currently Used Heart Valve Replacements
The two types of clinically utilized prostheses are mechanical and bioprosthetic heart valve replacements. Of the roughly 90,000 annual heart valve replacements in the U.S., approximately 50% receive either porcine or bovine derived bioprosethetic vales, 43% receive mechanical valves, and 7% receive human valves (either cadaveric allografts or autografts via the Ross procedure) [5]. Mechanical valves are usually composed of metals, pyrolytic carbon, and expanded poly(tetraflouoroethylene) (ePTFE) and provide a significant product life-span of greater than 20 years [6]. Various models, each with its own advantages and drawbacks, have been developed over the years including ball and cage valves, tilted disk valves, and bileaflet valves [7–12]. Unfortunately, thrombosis is a significant risk after implantation of these devices, and a patient must remain on anti-coagulants for the remainder of his or her life. These valves also can experience hemodynamic failures, where the valve mechanism becomes ‘stuck’ in either the opened or closed position, a severe and potentially fatal complication. Most clinically used bioprosthetic valves are usually porcine valves or bovine pericardial tissues, decellularized to reduce antigenicity [7–22]. Porcine heart valves and bovine pericardium are decellularized through a variety of techniques and chemically crosslinked before sterilization and implantation. Usually the valve or valve construct is washed in enzyme solutions (DNAase, RNAase, etc.) to remove cells and cellular debris, followed by chemical crosslinking with glutaraldehyde or similar agents. Although this crosslinking process has been used since 1960, it leads to in vivo calcification and altered mechanical properties [8, 23–24]. For thorough treatments on the wide number of decellularization and crosslinking processes of xenograft valves, reviews are available [9, 11, 15–16]. Several promising animal studies using such valves are covered in these papers, although there are some reviews that report a lack of data and significant failure in preclinical trials [15, 25]. While patients who receive these bioprosthetic valves do not require anticoagulant treatment, the valves also exhibit shorter life spans, due to mechanical failures and extensive calcification, which can lead to more reoperations compared to patients receiving mechanical valves. Nearly 65% of patients under age 60 who receive a xenograft or allograft valve need reoperation(s) after 15 years [6]. Other studies indicate even shorter life spans for these valves, with patients needing a new replacement in less than 10 years [16]. Younger patients who receive bioprosthetic valves tend to live more active lifestyles than older recipients; thus, those who receive such implants have increased hemodynamic and metabolic demands on valve replacements that can hasten mechanical failure. The major disadvantage of both mechanical and most bioprosthetic valves available clinically is that they are nonviable. In other words, these valves are incapable of growing or remodeling after being implanted and essentially begin to ‘wear out’ from the moment they are implanted. This is a significant drawback, especially in pediatric patients who will require future operations to implant new, larger valves as the patients grow. Table 1 summarizes the clinically used valve replacements, their origins and materials. The field of tissue engineering has been promoted as a way to create a viable prosthetic heart valve, with improved biocompatibility, reduced antigenicity, and the ability to grow and remodel in vivo.
Table 1.
Clinically used heart valve prosthetics and those currently in pre-clinical testing.
| Valve Type | Pre-Treatment/ Materials | Animal Model | Clinical Trials (# of Patients) | References | |
|---|---|---|---|---|---|
| Mechanical | Ball and Cage | Metal, silicone elastomer ball | FDA Approved | [6, 123] | |
|
| |||||
| Tilted Disk | Metal, pyrolytic carbon, ePTFE | FDA Approved | [124] | ||
|
| |||||
| Bileaflet Valve | Titanium, pyrolytic carbon | FDA Approved | [125–128] | ||
|
| |||||
| Bioprosthetic | Porcine Valve | Glutaraldehyde, Various enzymes | FDA Approved
|
[8–9, 21–23] | |
| SynerGraft | DNAase, RNAase, Water | 23 | [7, 18–20, 129] | ||
| Matrix P | Deoxycholic Acid (DOA) | Sheep | 126, 23 autologous cells | [10–15] | |
|
| |||||
| Bovine Pericadium | Glutaraldehyde | FDA Approved | [130–133] | ||
How Tissue Engineering Can Improve Heart Valve Replacements
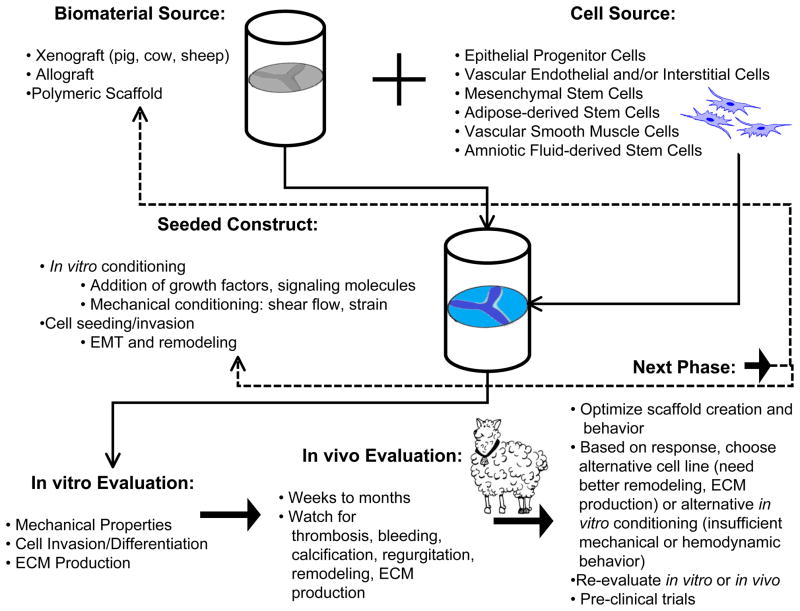
Heart valve tissue engineering follows one of a few methods of development (Figure 1). In one approach, synthetic or natural polymer scaffolds are created in a mold or platform. Other groups use xenografts as their scaffold starting material, chemically or physically altering the structure prior to additional work [26]. The primary function of heart valves is the unidirectional flow of blood, which places significant mechanical forces on the leaflets during a cardiac cycle. Specifically, when the valves are closed they are under significant transvalvular pressure to resistant retrograde blood flow [27–29]. Because of these loading demands in vivo, any tissue engineered heart valve must be ‘pre-conditioned’ to withstand mechanical loads in vitro. Numerous groups have designed protocols and bioreactors that improve both hemodynamic and mechanical properties of polymer scaffolds both with and without pre-seeded cells from a variety of sources [30–40]. Some studies pre-seed scaffold constructs with cells, while others rely on circulating cells to repopulate the scaffold in vitro. Such circulating cells may be responsible for replenishing cells within native heart valves [24]. Ideally, autologous cells should be used, which improve patient response to the valve as well as provide remodeling of the extracellular matrix (ECM) in the valve leaflets, improving biocompatibility. Finally the replacement valve is implanted, at which point it may undergo further in situ remodeling. This response can be a positive factor in that the valve becomes integrated into the patient’s heart and can be remodeled to resemble a naturally occurring valve. However, it is also possible that an implanted replacement valve may cause thrombosis or fibrotic tissue development. Currently available xenografts from porcine and bovine tissue can develop significant calcification, as non-human cell remnants promote formation of calcific nodules [9]. Another drawback of porcine and bovine xenografts is the possibility that non-human pathogens can be transmitted to the patient, including porcine endogenous retrovirus and bovine spongiform encephalopathy [41–42]. It is worth noting that the use of xeongrafts may not be considered tissue engineered valves, as they are already formed and only require processing before implantation. However, several tissue engineering research groups are using these approved valve replacements in combination with biomaterials or seeded-cells to create a TEHV platform, such cases will be discussed in later sections of this paper. Several scaffolds have been designed to promote endothelialization in vivo, so that acellular grafts encourage host cells to populate and remodel the valve scaffold once it has been implanted. The next generation of tissue engineered heart valves should have improved biocompatibility, hemodynamic/mechanical properties, as well as promote re-cellularization and remodeling all while reducing immunogenic responses, limiting calcification and thrombosis, and reducing stress-related failures associated with current clinical heart valve replacements.
Fig. 1.
Overview of common heart valve tissue engineering schemes, from selection of valve and cell sources to in vivo evaluation. Other factors considered in evaluation of valve construct: in vitro/in vivo loading environment and forces, material properties (degradation, geometry, fiber architecture), cellular invasion, protein synthesis/ECM production. Dashed lines represent the feedback of results into selection of new valve and/or cell sources or alterations to in vitro/in vivo conditioning, thus illustrating the reiterative nature of creating TEHVs. Adapted from [45, 136]
Importance of Valvulogenesis to Tissue Engineered Heart Valves
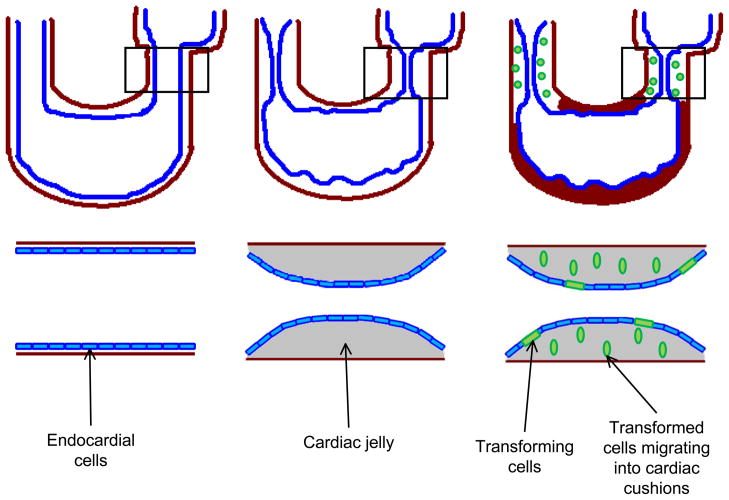
An emerging area of biomaterials research for TEHV seeks to fuse the techniques and approaches of tissue engineering with the essentials of developing heart valve biology. To better imitate the natural environment that leads to heart valves in utero, understanding the mechanical and biological signals that control valve development is necessary [43–47]. One important process in the embryonic development of the heart valve is epithelial-to- mesenchymal transition or transformation (EMT). In the developing heart valve, EMT occurs when cells from the endocardium differentiate into mesenchymal cells and migrate into the cardiac jelly that forms the developing pre-valve cardiac cushions, producing the ECM proteins that begin to form the leaflets (Figure 2) [48–56]. The cardiac cushion is primarily composed of glycosaminoglycans (GAGs), such as chondroitin sulfate and hyaluronic acid (HA), and filled with signaling molecules that regulate further valve development [49, 51]. Although the exact composition is unknown, some of the molecules that elicit or control EMT behavior include cytokines such as transforming growth factor β1 and β2 (TGF-β1, 2), vascular endothelial growth factor (VEGF), bone morphogenic protein 2 (BMP2), MEKK3, and Notch1 [47–49, 54–58]. Pathways including these EMT driving signals may be important to include and manipulate in developing in vitro TEHVs. The expression of signaling molecules to initiate EMT is not sufficient to control the process. Chiu et al. demonstrated that there is specific localization of TGF-β3, BMP2, and VEGFA in the overall process of EMT and valve remodeling [58]. The cardiac cushions regulate a complex synthesis of ECM molecules, leading to the elegant architecture that is heart valve leaflets and a fully functional valve. The properties of this ECM dictate short- and long-term function and durability of the valve; any defect present in the ECM architecture can lead to valve dysfunction or failure. Understanding how such a complex system naturally arises in the developing valve presents an opportunity to create a superior TEHV built on the same fundamental developmental cues that occur in utero. We believe that biomaterials designed to promote and control EMT will provide the next generation of TEHVs, synthesized including native signaling molecules and mechanical forces.
Fig. 2.
Illustration of EMT in the developing heart. Inserts are enlargement of atrioventricular canal (AVC). Left: Developing heart tube prior to cardiac cushion formation. Endocardial cells (shown in blue) line inside of u-shaped tubular heart separated from the myocardium (red) by a layer of cardiac jelly. Middle: The expansion of cardiac jelly, caused by secreted GAGs such as chondroitin sulfate and HA, leads to the development of cardiac cushion which contain signaling molecules (including, but not exclusive to TGF-β1 and β2, VEGF, BMP2, Notch1). Right: Endocardial cells undergoing EMT (shown in green) break down cell-cell junctions, elongate, and begin migrating into the cardiac cushions. Adapted from [137]
The development of heart valves is a complicated process that is not fully understood. EMT represents the initial step of valve formation which must be completed correctly before the endocardial cushion can remodel and eventually develop into a mature valve leaflet. Numerous studies show mutations of genes important in EMT, such as Has2 and MEKK3, result in lethalality. Camenisch et al. showed that mice lacking Has2 do not have HA present in cardiac jelly which prevents proper formation of cardiac cushions [59]. In a study with MEKK3 knockout mice, authors observed reduced endocardial cell proliferation, such that EMT could not occur [60]. Such studies indicate that although post-EMT remodeling of cardiac cushions may be important for proper mature valve formation and function, the initial process of EMT is the critical first step that may need to be focused on for future tissue engineered valve studies. Biomaterials capable of promoting or controlling these initial stages of valve formation may be key in creating de novo tissue engineered valve replacements, especially for pediatric patients who need valves with different physical characteristics than adult patients.
The difference in valve morphologies by patient age are an important consideration in the development of viable TEHVs. Several prominent research groups have discussed the role of valvular remodeling during disease, noting that a number of valvular defects may be caused by a genetic abnormality or functional defect related to the developmental process [60–61]. Fetal and post-natal valves demonstrate a high level of remodeling, due primarily to the activated myofibroblast-like phenotype of valvular interstitial cells which proliferate quickly in early stages of development but de-activate and become quiescent in adult valves [61–62]. Changes in mature valve characteristics are due to the alteration of ECM production or organization during valve maturation. Stephens et al. studied changes in material behavior of porcine mitral and aortic valves in animals ranging from 6 wks to 6 years (simulating child to adult behaviors) [63]. They observed that as the animals aged, there were significant increases in fibrosa thickness, leaflet stiffness, and loss of extensibility. The group also reported that there was an increase in collagen content as the animals aged and a marbling effect in the fibrosa with collagen fibers interspersed with GAGs. Although not directly related to EMT or valvulogenesis, considerations such as age of patient and “age” of heart valve will need to be considered when developing viable valve replacements. In this light, biomaterials which elicit EMT behavior may be more suitable for developing heart valves for neonates or other young patients who need heart valve replacements which exhibit characteristics of native neonatal or juvenile valves.
Custom Creations: Heart Valves Engineered from Synthetic Biomaterials
Since the rise of tissue engineering nearly 30 years ago, biomaterials have been an obvious answer to the question of how to create scaffolds to promote regeneration of natural tissues [64]. In this same time span, the rise of polymer science has created a plethora of materials with a wide range of mechanical, chemical, and biomedical properties. Several of these have received wide use in the clinic such as, poly(lactic acid) (PLA), poly(glycolic acid) (PGA) and poly(lactic-co-glycolic acid) (PLGA), primarily as biodegradable sutures. One of the advantages of synthetic biomaterials is the ability to vary the mechanical properties over a large range, alter the chemical properties to achieve numerous configurations, and tailor in vivo degradation times. In addition, since several of these materials have already been approved by the FDA for medical applications, numerous researchers have used them as a platform for new projects. Table 2 summarizes synthetic and natural polymer materials currently researched in TEHVs.
Table 2.
Various biomaterials being used for heart valve tissue engineering with cell lines seeded in vitro and animal implants utilized.
| Biomaterial | Cell Line Useda | Implanted Into | References | |
|---|---|---|---|---|
| Synthetic Materials | PEG | hMSCs, Porcine VICs | na | [65–69] |
| PGA:PLA | Ovine fibroblasts, ECs, and valve cells Human fibroblasts, Bovine aortic ECs |
Lamb (2 weeks) | [71–72] | |
| PGA:P4HB | Ovine myofibroblasts, ECs Ovine vascular and SCs, Ovine EPCs, VECs Human AFSCs |
Lamb (20wks) Sheep (8 weeks) |
[74–79] | |
| PCL | Human myofibroblasts | na | [109–111] | |
| PGS:PCL | HUVECs | na | [115] | |
| PEUU | Rat SMCs | na | [112–114, 134] | |
| PDO | Sheep MSCs | Sheep (1, 4, 8 months) | [116] | |
| PCU-POSS | na | na | [70] | |
| Natural Materials | Collagen | na | Rats Beagles (84 days) | [84, 91–93] |
| Hyaluronic Acid (HA) | Porcine VICs Neonatal rat SMCs |
na | [73, 80, 85, 87] | |
| Collagen/HA Composites | Neonatal hDF 3T3s |
na | [82–83, 90] | |
| Gelatin | Porcine VICs | na | [135] | |
| Collagen/Chondroitin Sulfate Composite | Porcine VICs, VECs | na | [81] | |
| Fibrin/Fibronectin | hDF, Human aortic myofibroblasts, Ovine arterial SMCs and myofibroblasts Porcine VICs |
Sheep (3 months) | [89, 94–98] | |
| Hydroxyapatite (HAp) | HUVECs | na | [99] |
:Cell Line Abbreviations. Human mesenchymal stem cells (hMSCs), endothelial cells (ECs), stem cells (SCs), smooth muscle cells (SMCs), human dermal fibroblasts (hDF), human umbilical vein endothelial cells (HUVECs), endothelial progenitor cells (EPCs), valvular endothelial cells (VECs), valvular interstitial cells (VICs) amniotic fluid-derived stem cells (AFSCs).
na = not applicable
One of the most common polymers used in biomaterials research is poly(ethylene glycol) or PEG [65–69]. When implanted in the body, PEG chains become hydrated, presenting a hydrophilic surface rendering it bio-inert [65]. PEG gels can be modified by crosslinking, chemical modification via the addition of peptide chains or bioactive molecules, and copolymerization to alter mechanical properties. Recently, dynamically tunable PEG-based systems have been developed that respond to either mechanical or optical cues. Benton et al. investigated a highly crosslinked PEG hydrogel loaded with an MMP-sensitive peptide for studying the behavior of porcine valvular interstial cells (VICs) [65–66]. These cells populate native valves, and understanding their behavior in a 3D dynamic mechanical environment will be important in creating new valve replacements. Also, the interaction of VICs and valvular endothelial cells (VECs), has been shown to play a significant role in cellularization and matrix remodeling of valve scaffolds [6]. Similar work on a photosensitive PEG-based gel has been done to create a system with tunable mechanical properties. These gels show that encapsulated cells respond by differentiation due to gradients in substrate stiffness created using UV light [67–69]. Polyurethanes are another attractive option for heart valve tissue engineering, as several are incorporated into commercially available such as Elast-Eon medical products including plastic surgery implants or as insulation on pacemaker leads. These materials can withstand ~500 million cycles when tested in a flow system mimicking heart valve hemodynamics [70]. Composite scaffold leaflets made from polycarbonate soft segments (PCU, a soft polyurethane) and polyhedral oligomeric silsesquioxanes (POSS) nanoparticles showed improved mechanical properties compared to PCU control samples [70]. These same studies showed that the PCU-POSS leaflets had reduced platelet affinity, meaning these materials should be non-thrombogenic.
Biodegradation is another important property considered in TEHVs. Scaffolds that can be enzymatically or hydrolytically degraded as cells invade and remodel present an attractive option for potentially viable heart valve replacements. This is especially interesting for exploiting the developmental pathways, such as inducing EMT, to promote cellularization and ECM synthesis in valve replacements. Several groups use such biodegradable polymers as a base for their scaffolding system, incorporating additional polymers to provide enhanced mechanical properties. Because PLA and PGA were already accepted as biocompatible, biodegradable polymers, initial heart valve tissue engineering research focused on testing these materials. PLA and PGA composite scaffold leaflets were seeded with human fibroblasts and then had bovine aortic endothelial cells added to create a leaflet that mimicked native valve structure [71–72]. However, early non-woven versions of the scaffold system were too soft to be tested in animal models, and woven PLA:PGA scaffolds implanted in the pulmonary valve position in a lamb model had a failure rate of 75% due to infection and problems with implant shrinkage and deformation [71]. Because the traditional PGA-based scaffolds did not exhibit sufficient mechanical properties to promote further research, additives and other polymers have been added to PGA-systems to create tissue engineered valve replacements [2, 73]. One of the most common involves the addition of a polyhydroxyalkanoate called poly-4-hydroxybutyrate (P4HB), a bioabsorbable thermoplastic that can be molded into the complex shape of a heart valve and sealed together without the use of sutures [74]. In a seminal study, ovine VICs and VECs were seeded on PGA valve scaffolds coated with P4HB before implantation into a lamb model [75]. Results indicated ECM protein synthesis and mechanical properties similar to native valves after 20 weeks of study, while the polymer implant itself degraded within 8 weeks. More recently, another study where these valves were implanted in sheep using a catheter showed that the valves functioned up to 8 weeks. Thus these valves may pave the way for future preclinical trials in humans [76]. Another study utilized amniotic fluid-derived stem cells seeded onto the PGA:P4HB scaffolds and conditioned in vitro in a pulsatile system [77]. After conditioning, scaffolds exhibited endothlelialization, cell proliferation, and increased ECM components such as GAGs over control groups, indicating the potential of these cells in creation of a viable valve replacement. These cells were investigated as an alternative to bone marrow derived cells as a potential autologous cell source, although limited availability may limit their use to pediatric cases.
Despite what seems like initial success, this material has not been accepted for clinical use yet due to numerous uncertainties in how the valves function in vivo. Another study showed that the enhanced mechanical properties due to P4HB coatings on these valves degraded within a few weeks of in vitro conditioning in a bioreactor [32]. More work has been done on this polymer system to explore if cellularization can improve scaffold function. In 2003, Dvorin et al. showed that ovine VECS and endothelial progenitor cells (EPCs) respond to VEGF and TGF-β1 signaling when seeded onto PGA/P4HB gels [78]. The gels seeded with EPCs also promote increased DNA content and ECM protein synthesis; however, they also have much higher modulus than unseeded control scaffolds (7x) and native valves (40x) [79]. While these materials exhibit improved mechanical properties and have shown good hemodynamic properties in conditioning bioreactors, they are often not physiologically relevant in respect to presenting a scaffold that can be invaded and remodeled by cells. For this reason, another class of materials, one more familiar to the body, is being investigated to provide the biological cues to promote and control cell growth.
Nature Made: Controlling Cell Behavior in Engineered Heart Valves
Recently there has been a shift from synthetic polymeric materials to more natural ones based on ECM components. While they offer less flexibility in characterization than their synthetic counterparts, they do provide mechanical properties comparable to native tissues and the possibility of biological cues needed to direct and control cell growth. These materials also promote natural developmental processes, such as EMT, in order to achieve optimum scaffold function. Because they present cells with proteins present in native developing valves, it may be possible to ‘trick’ cells into undergoing valvulogenesis thereby growing a replacement heart valve in vitro for patients of all ages, including newborns with congenital heart defects. Gels made from collagen, fibronectin, HA, elastin, and other ECM components present cells with signals that promote in vitro and in vivo remodeling. This remodeling may be beneficial in creating a viable valve replacement. Thus, if the valve can remodel after implantation, its overall function may be improved, leading to a better quality of life for patients.
The two most examined natural biomaterials for tissue engineered heart valves are HA and collagen [73, 80–87]. Both of these materials are present in native valves at various developmental stages. The cardiac cushions in the developing heart valve contain a significant amount of HA, which plays a role in regulating ECM production and initiating/controlling EMT of endocardial cells [49, 88]. HA gels undergo slow biodegradation through hyaluronases, making them an excellent scaffold system for in vitro or in vivo remodeling. The spongiosa of native valves contains >90% HA and chondroitin sulfate, indicating these materials are non-thrombogenic and non-immunogenic. It is also hypothesized that utilizing HA in gel scaffolds may provide biological cues to cells and promote specific ECM production. For example in a study where neonatal rat smooth muscle cells were grown on HA gels, elastin production increased compared to cells grown on plastic after 4 weeks of culture [73]. Elastin is an important component of the mature valve, responsible for supporting the closed structure of the valve. Crosslinkable HA gels modified with methacrylate groups and co-polymerized with PEG-diacrylate showed encapsulated porcine VICs alter production of ECM proteins based on molecular weight of HA degradation products [80].
Collagen is one of the main ECM proteins produced in developing valves and maintained in mature valves, inducing anisotropic mechanical properties that the valve utilizes for proper function for up to three billion cardiac cycles. Because it provides a significant amount of mechanical strength and its alignment results in anisotropic strain response, collagen is often included in composite scaffold systems [84, 89]. However, on its own, collagen lacks the necessary mechanical properties to create a physiologically relevant heart valve replacement. Crosslinkable, methacrylated HA used in combination with collagen is another attractive choice for tissue engineered heart valves [90]. This composite material provides the fibrous structure of native valve leaflets, through collagen, with increased mechanical properties of HA and has been shown to promote proliferation of fibroblasts [82–83]. The addition of HA alters the formation of collagen fiber network, meaning that cells cannot significantly contract the composite gels compared with the collagen only networks. Collagen has also been combined with another GAG, chondroitin sulfate, to create mitral valve leaflet replacements [81]. These scaffolds showed in vitro development into similar architecture as native valves when seeded with porcine VICs and VECs.
An alternative use of collagen as a scaffold material has been developed by Yamanami et al. who have pioneered a technique to create collagen-based valve scaffolds in vivo using the foreign body response of an organism [91–93]. Silicone rods were shaped into valve molds and wrapped in polyurethane or autologous connective tissue from Japanese white rabbits before implantation in subcutaneous cavities in the same animals. Over the course of 4 weeks, the host response to the implant was to secret connective tissue, primarily collagen, to coat the mold and fill in the aperture between rods. This created a tri-leaflet valve composed primarily of collagen that showed promising behavior including moderate (<20%) regurgitation in pulsatile conditioning systems mimicking pulmonary conditions [92–93]. An initial in vivo test using a beagle model over 84 days also showed promising results, although the authors noted that the study was on “allograft” valves implanted in the pulmonary position. Implants showed signs of neovascularization and minimal endothelial and myofibroblast cell attachment [91–93]. Future studies are going to focus on using “autograft” valves and further characterization of cellular response, in addition to long-term in vivo response. Because the grafts are composed almost exclusively of fibrous collagen, they may lack proper mechanical properties needed for long term function in humans.
Fibrin gels present another potentially viable alternative for TEHVs, since they can be derived from the patient thereby eliminating the worry of an adverse immunogenic response. Although studies of fibroblasts encapsulated in fibrin gels show increased collagen production and anisotropic mechanical properties, these materials do not have sufficient mechanical properties needed to be considered sustainable heart valve scaffold materials [89, 94–97]. Studies testing mechanical conditioning in the form of cyclic distension of fibrin scaffolds seeded with either porcine VICs or human dermal fibroblasts showed increased mechanical properties and collagen production compared to statically grown control samples [98]. This could indicate that fibrin-based scaffolds remodel and thus might generate sufficient mechanical properties for implantation, if conditioned correctly. Another approach for TEHVs involves improving clinically used mechanical valves. Hydroxyapaptite, a naturally occurring biomaterial present in bones, has been investigated as a coating for mechanical valves. The thought is that by increasing biocompatibility of the material presented in vivo of mechanical valves, that the problems of thrombogensis may be lessened eliminating the need for anticoagulant therapy [99]. While the preliminary results of natural biomaterials for TEHVs are promising, even with the advent of EMT-inducing and controlling materials, biocompatibility alone will not be enough to create the ‘perfect’ heart valve replacement. The valve substitute must also have sufficient mechanical properties to withstand the hemodynamic demands of a beating heart. The combination of cell-instructive biomaterials with other techniques that introduce mechanical strength could create such a scaffold system.
Another important consideration in future development of TEHV is the selection of a source of cells to seed onto scaffolds. Ideally a patient’s own cells could be harvested for this purpose, however the practicality of such options may be limited depending on the severity of the patient’s condition [100]. In one study, Hoffman-Kim et al. looked at three different cell lines as potential seeding sources for tissue engineering scaffolds. Sheep cells from the tricuspid heart valve leaflet were compared to cells obtained from jugular vein and carotid artery. Cells from the jugular veins showed higher initial production of collagen in vitro compared to the other lines. A final note from this study indicates that synthesis of collagen, elastin, and GAGs significantly decreased as passage number increased (up to passage 4, 28 days) in all cells lines. This trend may indicate a time limit for seeded cells to perform necessary protein synthesis and organization in TEHV scaffolds. Other research groups are focusing on a wide variety of cell lines as potential sources for TEHVs. Numerous groups have previously reported the use of MSCs, EPCs, or VICs and/or VECs to seed scaffolds in attempts to promote a specific type of cell behavior. A common behavior of all of these cells is that they exhibit remodeling potential that can be useful in controlling valve properties [101]. For example, Cebotari et al. demonstrated that allograft valves seeded with autologous EPCs in juveniles exhibited function and somatic growth, presumably due to cellular behavior within the valves [102]. However the feasibility and practicality of using autologous valve cells from a patient who needs a valve replacement is disputable. Also, it is uncertain that a sufficient number of valve cells could be grown in a suitable timeline for patients needing a valve replacement; although some initial work has been done to determine an appropriate seeding density of VICs [103]. In their study reports an in vitro conditioned human pulmonary valve seeded with autologous VICs showed significant cell proliferation and seeding within 4 days of culture, suggesting that it might be possible to generate valves seeded with autologous cells in a relatively short time frame. In another approach, researchers are attempting to use alternative cell types, and provide them with biological or mechanical signals necessary to generate valve-like cells. For example, Appleton et al. demonstrated that vascular smooth muscle cells harvested from rat aortas exhibit a myofibroblast-like phenotype when exposed to TGF-β1, basic fibroblast growth factor (bFGF), and platelet derived growth factor (PDGF) [104]. Although this treatment showed increased collagen, fibronectin, and versican, there is some uncertainty in its potential use as a cell source in valve replacements, since a prolonged active phenotype is also the hallmark of numerous disease states. Ideally, a cell source for TEHVs will exhibit phenotype plasticity, or stem cell-like behavior by being able to modulate between active and quiescent phenotypes based on flow regimes and soluble growth factors. Because initiating and controlling EMT is the proposed first key step in creating tissue engineered valves, a cell type with behaviors similar to EMT represent a promising source for scaffolds. A study by Paruchuri et al. demonstrated that human pulmonary valve progenitor cells show phenotypic plasticity that can be modulated between endothelial and mesenchymal states by introduction of VEGF or TGF-β2 treatment, respectively [105]. In these studies, clones of valve progenitor cells were shown to vary between endothelial markers and mesenchymal behavior (increase aSMA, migration, invasion, MMP-1 and 2). It is precisely this kind of controllable behavior and phenotype that is needed in an EMT-inducing platform for TEHVs. The inclusion of molecules that modulate cell phenotype, migration, and invasion will be key in promoting these behaviors in the development of a viable valve replacement.
Best of Both Worlds: Bioactivity and Mechanical Properties
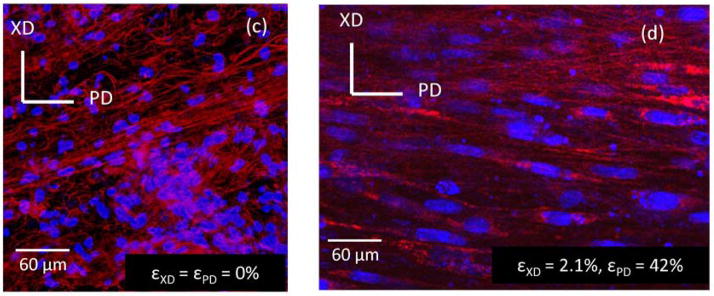
While the increasing interest in natural biomaterials is promising, ECM-derived polymer materials alone do not have sufficient mechanical properties to meet physiological demands as implantable heart valve constructs. Native heart valves are highly complex structures, with multiple levels of architecture and layers of mechanical and biological properties that have evolved over time into the fully functioning system. A more complete understanding of native heart valve properties is needed before we can create a living replacement heart valve. Additional insights into valve leaflet mechanical properties and how these relate to its function will enable researchers to define key elements to work on improving in engineered heart valves [106]. Ideally, the creation of a viable TEHV replacement will combine the enhanced bioactivity of EMT-promoting biomaterials with the improved mechanical properties of electrospun (ES) fibers. Electrospinning represents a unique technique which allows for the creation of nanoscale fibers that form fibrous scaffolds which mimic the architecture of natural ECM. Both synthetic and natural polymers are used in electrospinning for the creation of a wide variety of mechanical properties, structures, fiber diameters, and other characteristics. Since ES fibers result in increased surface area and interconnected three dimensional porous environments, the scaffolds represent a biomimetic system. These fibers also play an important role in modulation of mechanical properties. In light of this, there have been reports using electrospinning techniques for heart valve tissue engineering [107–111]. The ability to alter the alignment of ES fibers also allows for the creation of anisotropy in a sample’s mechanical properties. It is precisely this anisotropy control that makes electrospinning attractive to TEHV biomaterials research. Unfortunately, the pores of ES scaffolds may be too small to promote cellular invasion, therefore people have begun to create complex, composite ES scaffolds pre-seeded with cells. For instance, while initial results in creating an ES poly(ester urea urethane) (PEUU) scaffold were successful in creating anisotropic mechanical properties similar to native pulmonary valves, little cellular invasion into these systems was observed [112]. Further work has been done to create an ES system capable of integrating smooth muscle cells into the PEUU fibers during the electrospinning process [113]. Such a composite system exhibits physiologically interesting mechanical properties as well as the ability to control cell alignment with external mechanical stimulation, as exhibited by Stella et al. (Figure 3) [114]. Polycaprolactone fibers produced by electrospinning created a functional bioabsorbable scaffold that has leaflets which opened synchronously and exhibit correct coaptation in the diastolic phase although additional studies are needed [110]. Another research group created hybrid scaffolds of poly(glycerol sebacate) (PGS) and PCL [115]. By varying the ratio of PGS:PCL, the authors were able to achieve a wide range of fiber diameters. Results also demonstrated that increasing the ratio of PGS:PCL increase mechanical properties including elastic modulus, ultimate tensile strength, and ultimate elongation, exhibiting mechanical properties similar to native aortic valve tissues. HUVECs seeded on the hybrid scaffolds exhibited increased attachment and proliferation compared to cells seeded on PCL control scaffolds. Interestingly, a research group reported that mesenchymal stem cells seeded on polydioxaneone (PDO) electrospun bioabsorbable patches were implanted into the right ventricle outflow tract of 6 lambs for up to 8 months. They found that PDO scaffolds were completely degraded and replaced by endothelialized tissue with an ECM similar to native tissue [116].
Fig. 3.
Fluorescent microscope images showing cell-scaffold constructs made via electrospinning PEUU and concurrently electrospraying rat vascular smooth muscle cells onto a rotating mandrel. (Left, c) Constructs in a static (non-strained) environment show rounded cell nuclei (blue) and random fiber orientation (red). (Right, d) Constructs under biaxial strain show elongated cell nuclei and PEUU fiber alignment, demonstrating enhanced mechanical properties of electrospun polymers compared with natural polymer systems. Reproduced with permission from [114]
As well as the classical ES techniques for heart valve tissue engineering, groups have used it in combination with other techniques such as fused deposition modeling and reactive electrospinning in a double syringe system. Chen et al. developed ES thermoplastic polyurethane (ES-TPU) scaffolds with a well-aligned fiber network using fused deposition modeling (FDM) for a dynamic design, which is important for heart valve constructs. They showed that such a combination of ES-TPU and FDM gave excellent elasticity and improved hemodynamic properties in bioreactors but have yet to complete in vitro or in vivo cell-seeding studies [117]. Such studies may offer key insights about to create systems with specific architectures like the complex valve environment. Work is needed to improve the ability to control the scale of composite systems, so that suitably sized valve replacements could be synthesized with the correct architecture.
Where Do We Go From Here?
The road to creating a usable TEHV replacement is a challenging one, filled with the need to improve chemical, biological, and mechanical properties of any proposed system. Advances in understanding the characteristics of the developing heart valve and the environment that creates it will allow researchers to pursue cell-directive materials for creation of a valve scaffold that can be invaded and remodeled after implantation in a patient. This prospective also provides a starting point for further biomaterials research, instead of the empirical approach currently used by selecting a material used in other applications and then testing its functionality as a heart valve scaffold. Biomaterials that induce EMT and other developmental behaviors offer the ability to control and direct cellularization of potential heart valve replacements, but these materials lack the needed mechanical properties to be physiologically relevant. ES fiber systems offer synthesis and control over enhanced mechanical properties of scaffold constructs but do not sufficiently promote or regulate required cellular activities to create a viable valve replacement. In view of these individual technical drawbacks, the next generation of biomaterials for TEHVs should look at combining EMT-inducing material properties with ES techniques. The combination of the two methods may allow for bioactive, viable valves with physiologically relevant mechanical properties needed to maintain in vivo function. As we get closer to living valve replacements, we will also have to make decisions about how to best evaluate their structure and function. Current regulations for heart valve replacements are relevant only for the non-viable mechanical and bioprosthetic valves used clinically [118]. Such guidelines will be important for screening potential scaffold candidates for preclinical and clinical trials. The studies highlighted above represent interesting advances in biomaterials for tissue engineered heart valves at different stages in their progress from bench to bedside. For the interested reader, additional reviews on heart valve tissue engineering are available [2, 15, 119–122].
Acknowledgments
YWC was supported by NIH Common Fund grants (EB008539 and HL092551). AK was supported by NIH (PE019024, HL092836, and HL099073). WDM was supported by the American Heart Association (0835496N and 09GRNT2010125), Wallace H. Coulter Foundation (Early Career Award), NSF (1055384), and NIH (HL094707).
List of Abbreviations and Acronyms
- bFGF
Basic fibroblast growth factor
- BMP2
Bone morphogenic protein 2
- DOA
Deoxycholic Acid
- ES
Electrospun
- EPC
Endothelial/Epithelial progenitor cell
- EMT
Epithelial-to-mesenchymal transition or transformation
- ePTFE
Expanded poly(tetraflouroethylene)
- ECM
Extracellular matrix
- FDM
Fused deposition modeling
- GAG
Glycosaminoglycan
- HA
Hyaluronic acid
- HAp
Hydroxyapatite
- MMP
Matrix metalloproteinase
- MSC
Mesenchymal stem cell
- MEKK3
Mitogen-activated protein 3 kinase
- PDGF
Platelet derived growth factor
- PEUU
Poly(ester urea urethane)
- PEG
Poly(ethylene glycol)
- PGS
Poly(glycerol sebacate)
- PGA
Poly(glycolic acid)
- PLA
Poly(lactic acid)
- PLGA
Poly(lactic-co-glycolic acid)
- P4HB
Poly-4-hyrdoxybutyrate
- PCL
Polycaprolactone
- PCU
Polycarbonate
- PDO
Polydioxaneone
- POSS
Polyhedral oligomeric silsesquioxanes
- TPU
Thermoplastic polyurethane
- TGF-β1
Transforming growth factor β1
- TGF-β2
Transforming growth factor β2
- VEC
Valvular endothelial cell
- VIC
Valvular interstitial cell
- VEGF
Vascular endothelial growth factor
References
- 1.Yacoub MH, Takkenberg JJ. Will heart valve tissue engineering change the world? Nat Clin Pract Cardiovasc Med. 2005;2(2):60–1. doi: 10.1038/ncpcardio0112. [DOI] [PubMed] [Google Scholar]
- 2.Mendelson K, Schoen FJ. Heart valve tissue engineering: concepts, approaches, progress, and challenges. Ann Biomed Eng. 2006;34(12):1799–819. doi: 10.1007/s10439-006-9163-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39(12):1890–900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- 4.Samanek M. Congenital heart malformations: prevalence, severity, survival, and quality of life. Cardiol Young. 2000;10(3):179–85. doi: 10.1017/s1047951100009082. [DOI] [PubMed] [Google Scholar]
- 5.Elkins R. Current Cardiology Reports. Vol. 5. 2003. Is Tissued-engineered Heart Vavle Replacement Clinically Applicable? pp. 125–128. [DOI] [PubMed] [Google Scholar]
- 6.Filova E, et al. Tissue-engineered heart valves. Physiol Res. 2009;58(Suppl 2):S141–58. doi: 10.33549/physiolres.931919. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein S, et al. Transpecies heart valve transplant: advanced studies of a bioengineered xeno-autograft. Ann Thorac Surg. 2000;70(6):1962–9. doi: 10.1016/s0003-4975(00)01812-9. [DOI] [PubMed] [Google Scholar]
- 8.Carpentier A, et al. Biological factors affecting long-term results of valvular heterografts. J Thorac Cardiovasc Surg. 1969;58(4):467–83. [PubMed] [Google Scholar]
- 9.Schmidt CE, Baier JM. Acellular vascular tissues: natural biomaterials for tissue repair and tissue engineering. Biomaterials. 2000;21(22):2215–31. doi: 10.1016/s0142-9612(00)00148-4. [DOI] [PubMed] [Google Scholar]
- 10.Dohmen PM, et al. Mid-term clinical results using a tissue-engineered pulmonary valve to reconstruct the right ventricular outflow tract during the Ross procedure. Ann Thorac Surg. 2007;84(3):729–36. doi: 10.1016/j.athoracsur.2007.04.072. [DOI] [PubMed] [Google Scholar]
- 11.Dohmen PM, Konertz W. Tissue-engineered heart valve scaffolds. Ann Thorac Cardiovasc Surg. 2009;15(6):362–7. [PubMed] [Google Scholar]
- 12.Dohmen PM, et al. Results of a decellularized porcine heart valve implanted into the juvenile sheep model. Heart Surg Forum. 2005;8(2):E100–4. doi: 10.1532/HSF98.20041140. discussion E104. [DOI] [PubMed] [Google Scholar]
- 13.Erdbrugger W, et al. Decellularized xenogenic heart valves reveal remodeling and growth potential in vivo. Tissue Eng. 2006;12(8):2059–68. doi: 10.1089/ten.2006.12.2059. [DOI] [PubMed] [Google Scholar]
- 14.Konertz W, et al. Hemodynamic characteristics of the Matrix P decellularized xenograft for pulmonary valve replacement during the Ross operation. J Heart Valve Dis. 2005;14(1):78–81. [PubMed] [Google Scholar]
- 15.Vesely I. Heart valve tissue engineering. Circ Res. 2005;97(8):743–55. doi: 10.1161/01.RES.0000185326.04010.9f. [DOI] [PubMed] [Google Scholar]
- 16.Neuenschwander S, Hoerstrup SP. Heart valve tissue engineering. Transpl Immunol. 2004;12(3–4):359–65. doi: 10.1016/j.trim.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 17.O’Brien MF, et al. The SynerGraft valve: a new acellular (nonglutaraldehyde-fixed) tissue heart valve for autologous recellularization first experimental studies before clinical implantation. Semin Thorac Cardiovasc Surg. 1999;11(4 Suppl 1):194–200. [PubMed] [Google Scholar]
- 18.Bechtel JEM, Stierle U, Sievers HH. Fifty-two months’ mean follow up of decellularized SynerGraft (TM)-treated pulmonary valve allografts. Journal of Heart Valve Disease. 2008;17(1):98–104. [PubMed] [Google Scholar]
- 19.Bechtel JF, et al. Evaluation of the decellularized pulmonary valve homograft (SynerGraft) J Heart Valve Dis. 2003;12(6):734–9. discussion 739–40. [PubMed] [Google Scholar]
- 20.Tavakkol Z, et al. Superior durability of SynerGraft pulmonary allografts compared with standard cryopreserved allografts. Ann Thorac Surg. 2005;80(5):1610–4. doi: 10.1016/j.athoracsur.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 21.Steinhoff G, et al. Tissue engineering of pulmonary heart valves on allogenic acellular matrix conduits: in vivo restoration of valve tissue. Circulation. 2000;102(19 Suppl 3):III50–5. doi: 10.1161/01.cir.102.suppl_3.iii-50. [DOI] [PubMed] [Google Scholar]
- 22.Booth C, et al. Tissue engineering of cardiac valve prostheses I: development and histological characterization of an acellular porcine scaffold. J Heart Valve Dis. 2002;11(4):457–62. [PubMed] [Google Scholar]
- 23.Liao J, Joyce EM, Sacks MS. Effects of decellularization on the mechanical and structural properties of the porcine aortic valve leaflet. Biomaterials. 2008;29(8):1065–74. doi: 10.1016/j.biomaterials.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schoen FJ. Heart valve tissue engineering: quo vadis? Curr Opin Biotechnol. 2011 doi: 10.1016/j.copbio.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Simon P, et al. Early failure of the tissue engineered porcine heart valve SYNERGRAFT in pediatric patients. Eur J Cardiothorac Surg. 2003;23(6):1002–6. doi: 10.1016/s1010-7940(03)00094-0. discussion 1006. [DOI] [PubMed] [Google Scholar]
- 26.Hopkins RA. Tissue engineering of heart valves: decellularized valve scaffolds. Circulation. 2005;111(21):2712–4. doi: 10.1161/CIRCULATIONAHA.104.527820. [DOI] [PubMed] [Google Scholar]
- 27.Sacks MS, Merryman WD, Schmidt DE. On the biomechanics of heart valve function. J Biomech. 2009;42(12):1804–24. doi: 10.1016/j.jbiomech.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merryman WD, et al. Correlation between heart valve interstitial cell stiffness and transvalvular pressure: implications for collagen biosynthesis. Am J Physiol Heart Circ Physiol. 2006;290(1):H224–31. doi: 10.1152/ajpheart.00521.2005. [DOI] [PubMed] [Google Scholar]
- 29.Merryman WD. Mechano-potential etiologies of aortic valve disease. J Biomech. 2010;43(1):87–92. doi: 10.1016/j.jbiomech.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hildebrand DK, et al. Design and hydrodynamic evaluation of a novel pulsatile bioreactor for biologically active heart valves. Ann Biomed Eng. 2004;32(8):1039–49. doi: 10.1114/b:abme.0000036640.11387.4b. [DOI] [PubMed] [Google Scholar]
- 31.Dumont K, et al. Design of a new pulsatile bioreactor for tissue engineered aortic heart valve formation. Artif Organs. 2002;26(8):710–4. doi: 10.1046/j.1525-1594.2002.06931_3.x. [DOI] [PubMed] [Google Scholar]
- 32.Engelmayr GC, Jr, et al. A novel bioreactor for the dynamic flexural stimulation of tissue engineered heart valve biomaterials. Biomaterials. 2003;24(14):2523–32. doi: 10.1016/s0142-9612(03)00051-6. [DOI] [PubMed] [Google Scholar]
- 33.Hoerstrup SP, et al. New pulsatile bioreactor for in vitro formation of tissue engineered heart valves. Tissue Eng. 2000;6(1):75–9. doi: 10.1089/107632700320919. [DOI] [PubMed] [Google Scholar]
- 34.Mol A, et al. Tissue engineering of human heart valve leaflets: a novel bioreactor for a strain-based conditioning approach. Ann Biomed Eng. 2005;33(12):1778–88. doi: 10.1007/s10439-005-8025-4. [DOI] [PubMed] [Google Scholar]
- 35.Ruel J, Lachance G. A new bioreactor for the development of tissue-engineered heart valves. Ann Biomed Eng. 2009;37(4):674–81. doi: 10.1007/s10439-009-9646-9. [DOI] [PubMed] [Google Scholar]
- 36.Sierad LN, et al. Design and Testing of a Pulsatile Conditioning System for Dynamic Endothelialization of Polyphenol-Stabilized Tissue Engineered Heart Valves. Cardiovasc Eng Technol. 2010;1(2):138–153. doi: 10.1007/s13239-010-0014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Merryman WD. Development of a tissue engineered heart valve for pediatrics: a case study in bioengineering ethics. Sci Eng Ethics. 2008;14(1):93–101. doi: 10.1007/s11948-008-9053-x. [DOI] [PubMed] [Google Scholar]
- 38.Gottlieb D, et al. In vivo monitoring of function of autologous engineered pulmonary valve. J Thorac Cardiovasc Surg. 2010;139(3):723–31. doi: 10.1016/j.jtcvs.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 39.Ramaswamy S, et al. The role of organ level conditioning on the promotion of engineered heart valve tissue development in-vitro using mesenchymal stem cells. Biomaterials. 2010;31(6):1114–25. doi: 10.1016/j.biomaterials.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Engelmayr GC, Jr, et al. A novel flex-stretch-flow bioreactor for the study of engineered heart valve tissue mechanobiology. Ann Biomed Eng. 2008;36(5):700–12. doi: 10.1007/s10439-008-9447-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilson CA, et al. Type C retrovirus released from porcine primary peripheral blood mononuclear cells infects human cells. J Virol. 1998;72(4):3082–7. doi: 10.1128/jvi.72.4.3082-3087.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knight R, Collins S. Human prion diseases: cause, clinical and diagnostic aspects. Contrib Microbiol. 2001;7:68–92. doi: 10.1159/000060377. [DOI] [PubMed] [Google Scholar]
- 43.Riem Vis PW, et al. Environmental regulation of valvulogenesis: implications for tissue engineering. Eur J Cardiothorac Surg. 2011;39(1):8–17. doi: 10.1016/j.ejcts.2010.05.032. [DOI] [PubMed] [Google Scholar]
- 44.Lenas P, Luyten FP. An Emerging Paradigm in Tissue Engineering: From Chemical Engineering to Developmental Engineering for Bioartificial Tissue Formation through a Series of Unit Operations that Simulate the In Vivo Successive Developmental Stages. Industrial & Engineering Chemistry Research. 2011;50(2):482–522. [Google Scholar]
- 45.Ingber DE, et al. Tissue engineering and developmental biology: going biomimetic. Tissue Eng. 2006;12(12):3265–83. doi: 10.1089/ten.2006.12.3265. [DOI] [PubMed] [Google Scholar]
- 46.Ingber DE, Levin M. What lies at the interface of regenerative medicine and developmental biology? Development. 2007;134(14):2541–2547. doi: 10.1242/dev.003707. [DOI] [PubMed] [Google Scholar]
- 47.Combs MD, Yutzey KE. Heart valve development: regulatory networks in development and disease. Circ Res. 2009;105(5):408–21. doi: 10.1161/CIRCRESAHA.109.201566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7(2):131–42. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 49.Armstrong EJ, Bischoff J. Heart valve development - Endothelial cell signaling and differentiation. Circulation Research. 2004;95(5):459–470. doi: 10.1161/01.RES.0000141146.95728.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hinton RB, Yutzey KE. Heart Valve Structure and Function in Development and Disease. Annu Rev Physiol. 2010 doi: 10.1146/annurev-physiol-012110-142145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Person AD, Klewer SE, Runyan RB. Cell biology of cardiac cushion development. Int Rev Cytol. 2005;243:287–335. doi: 10.1016/S0074-7696(05)43005-3. [DOI] [PubMed] [Google Scholar]
- 52.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420–8. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thiery JP, et al. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–90. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 54.Barnett JV, Desgrosellier JS. Early events in valvulogenesis: a signaling perspective. Birth Defects Res C Embryo Today. 2003;69(1):58–72. doi: 10.1002/bdrc.10006. [DOI] [PubMed] [Google Scholar]
- 55.Olivey HE, et al. Transforming growth factor-beta stimulates epithelial-mesenchymal transformation in the proepicardium. Dev Dyn. 2006;235(1):50–9. doi: 10.1002/dvdy.20593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hay ED. An overview of epithelio-mesenchymal transformation. Acta Anat (Basel) 1995;154(1):8–20. doi: 10.1159/000147748. [DOI] [PubMed] [Google Scholar]
- 57.Stevens MV, et al. MEKK3 initiates transforming growth factor beta 2-dependent epithelial-to-mesenchymal transition during endocardial cushion morphogenesis. Circ Res. 2008;103(12):1430–40. doi: 10.1161/CIRCRESAHA.108.180752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chiu YN, et al. Transforming growth factor beta, bone morphogenetic protein, and vascular endothelial growth factor mediate phenotype maturation and tissue remodeling by embryonic valve progenitor cells: relevance for heart valve tissue engineering. Tissue Eng Part A. 2010;16(11):3375–83. doi: 10.1089/ten.tea.2010.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Camenisch TD, et al. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J Clin Invest. 2000;106(3):349–60. doi: 10.1172/JCI10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Markwald RR, et al. Developmental basis of adult cardiovascular diseases: valvular heart diseases. Ann N Y Acad Sci. 2010;1188:177–83. doi: 10.1111/j.1749-6632.2009.05098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aikawa E, et al. Human semilunar cardiac valve remodeling by activated cells from fetus to adult: implications for postnatal adaptation, pathology, and tissue engineering. Circulation. 2006;113(10):1344–52. doi: 10.1161/CIRCULATIONAHA.105.591768. [DOI] [PubMed] [Google Scholar]
- 62.Hinton RB, Jr, et al. Extracellular matrix remodeling and organization in developing and diseased aortic valves. Circ Res. 2006;98(11):1431–8. doi: 10.1161/01.RES.0000224114.65109.4e. [DOI] [PubMed] [Google Scholar]
- 63.Stephens EH, et al. Age-related changes in material behavior of porcine mitral and aortic valves and correlation to matrix composition. Tissue Eng Part A. 2010;16(3):867–78. doi: 10.1089/ten.tea.2009.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260(5110):920–6. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 65.Benton JA, Fairbanks BD, Anseth KS. Characterization of valvular interstitial cell function in three dimensional matrix metalloproteinase degradable PEG hydrogels. Biomaterials. 2009;30(34):6593–603. doi: 10.1016/j.biomaterials.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Benton JA, Kern HB, Anseth KS. Substrate properties influence calcification in valvular interstitial cell culture. J Heart Valve Dis. 2008;17(6):689–99. [PMC free article] [PubMed] [Google Scholar]
- 67.Kloxin AM, et al. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science. 2009;324(5923):59–63. doi: 10.1126/science.1169494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kloxin AM, Benton JA, Anseth KS. In situ elasticity modulation with dynamic substrates to direct cell phenotype. Biomaterials. 2010;31(1):1–8. doi: 10.1016/j.biomaterials.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kloxin AM, Tibbitt MW, Anseth KS. Synthesis of photodegradable hydrogels as dynamically tunable cell culture platforms. Nat Protoc. 2010;5(12):1867–87. doi: 10.1038/nprot.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kidane AG, et al. A novel nanocomposite polymer for development of synthetic heart valve leaflets. Acta Biomater. 2009;5(7):2409–17. doi: 10.1016/j.actbio.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 71.Shinoka T, et al. Tissue engineering heart valves: valve leaflet replacement study in a lamb model. Ann Thorac Surg. 1995;60(6 Suppl):S513–6. doi: 10.1016/0003-4975(95)00733-4. [DOI] [PubMed] [Google Scholar]
- 72.Zund G, et al. The in vitro construction of a tissue engineered bioprosthetic heart valve. Eur J Cardiothorac Surg. 1997;11(3):493–7. doi: 10.1016/s1010-7940(96)01005-6. [DOI] [PubMed] [Google Scholar]
- 73.Ramamurthi A, Vesely I. Evaluation of the matrix-synthesis potential of crosslinked hyaluronan gels for tissue engineering of aortic heart valves. Biomaterials. 2005;26(9):999–1010. doi: 10.1016/j.biomaterials.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 74.Sodian R, et al. Fabrication of a trileaflet heart valve scaffold from a polyhydroxyalkanoate biopolyester for use in tissue engineering. Tissue Eng. 2000;6(2):183–8. doi: 10.1089/107632700320793. [DOI] [PubMed] [Google Scholar]
- 75.Hoerstrup SP, et al. Functional living trileaflet heart valves grown in vitro. Circulation. 2000;102(19 Suppl 3):III44–9. doi: 10.1161/01.cir.102.suppl_3.iii-44. [DOI] [PubMed] [Google Scholar]
- 76.Schmidt D, et al. Minimally-invasive implantation of living tissue engineered heart valves: a comprehensive approach from autologous vascular cells to stem cells. J Am Coll Cardiol. 2010;56(6):510–20. doi: 10.1016/j.jacc.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 77.Schmidt D, et al. Prenatally fabricated autologous human living heart valves based on amniotic fluid derived progenitor cells as single cell source. Circulation. 2007;116(11 Suppl):I64–70. doi: 10.1161/CIRCULATIONAHA.106.681494. [DOI] [PubMed] [Google Scholar]
- 78.Dvorin EL, et al. Quantitative evaluation of endothelial progenitors and cardiac valve endothelial cells: proliferation and differentiation on poly-glycolic acid/poly-4-hydroxybutyrate scaffold in response to vascular endothelial growth factor and transforming growth factor beta1. Tissue Eng. 2003;9(3):487–93. doi: 10.1089/107632703322066660. [DOI] [PubMed] [Google Scholar]
- 79.Sales VL, et al. Endothelial progenitor cells as a sole source for ex vivo seeding of tissue-engineered heart valves. Tissue Eng Part A. 2010;16(1):257–67. doi: 10.1089/ten.tea.2009.0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Masters KS, et al. Crosslinked hyaluronan scaffolds as a biologically active carrier for valvular interstitial cells. Biomaterials. 2005;26(15):2517–25. doi: 10.1016/j.biomaterials.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 81.Flanagan TC, et al. A collagen-glycosaminoglycan co-culture model for heart valve tissue engineering applications. Biomaterials. 2006;27(10):2233–46. doi: 10.1016/j.biomaterials.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 82.Kreger ST, Voytik-Harbin SL. Hyaluronan concentration within a 3D collagen matrix modulates matrix viscoelasticity, but not fibroblast response. Matrix Biol. 2009;28(6):336–46. doi: 10.1016/j.matbio.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Suri S, Schmidt CE. Photopatterned collagen-hyaluronic acid interpenetrating polymer network hydrogels. Acta Biomater. 2009;5(7):2385–97. doi: 10.1016/j.actbio.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 84.Tedder ME, et al. Stabilized collagen scaffolds for heart valve tissue engineering. Tissue Eng Part A. 2009;15(6):1257–68. doi: 10.1089/ten.tea.2008.0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Masters KS, et al. Designing scaffolds for valvular interstitial cells: cell adhesion and function on naturally derived materials. J Biomed Mater Res A. 2004;71(1):172–80. doi: 10.1002/jbm.a.30149. [DOI] [PubMed] [Google Scholar]
- 86.Merryman WD, et al. Differences in tissue-remodeling potential of aortic and pulmonary heart valve interstitial cells. Tissue Eng. 2007;13(9):2281–9. doi: 10.1089/ten.2006.0324. [DOI] [PubMed] [Google Scholar]
- 87.Rodriguez KJ, Piechura LM, Masters KS. Regulation of valvular interstitial cell phenotype and function by hyaluronic acid in 2-D and 3-D culture environments. Matrix Biol. 2011;30(1):70–82. doi: 10.1016/j.matbio.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zoltan-Jones A, et al. Elevated hyaluronan production induces mesenchymal and transformed properties in epithelial cells. J Biol Chem. 2003;278(46):45801–10. doi: 10.1074/jbc.M308168200. [DOI] [PubMed] [Google Scholar]
- 89.Robinson PS, et al. Functional tissue-engineered valves from cell-remodeled fibrin with commissural alignment of cell-produced collagen. Tissue Eng Part A. 2008;14(1):83–95. doi: 10.1089/ten.a.2007.0148. [DOI] [PubMed] [Google Scholar]
- 90.Brigham MD, et al. Mechanically robust and bioadhesive collagen and photocrosslinkable hyaluronic acid semi-interpenetrating networks. Tissue Eng Part A. 2009;15(7):1645–53. doi: 10.1089/ten.tea.2008.0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hayashida K, et al. Development of an in vivo tissue-engineered, autologous heart valve (the biovalve): preparation of a prototype model. J Thorac Cardiovasc Surg. 2007;134(1):152–9. doi: 10.1016/j.jtcvs.2007.01.087. [DOI] [PubMed] [Google Scholar]
- 92.Yamanami M, et al. Preparation of in-vivo tissue-engineered valved conduit with the sinus of Valsalva (type IV biovalve) J Artif Organs. 2010;13(2):106–12. doi: 10.1007/s10047-010-0491-2. [DOI] [PubMed] [Google Scholar]
- 93.Yamanami M, et al. Development of a completely autologous valved conduit with the sinus of Valsalva using in-body tissue architecture technology: a pilot study in pulmonary valve replacement in a beagle model. Circulation. 2010;122(11 Suppl):S100–6. doi: 10.1161/CIRCULATIONAHA.109.922211. [DOI] [PubMed] [Google Scholar]
- 94.Ye Q, et al. Fibrin gel as a three dimensional matrix in cardiovascular tissue engineering. Eur J Cardiothorac Surg. 2000;17(5):587–91. doi: 10.1016/s1010-7940(00)00373-0. [DOI] [PubMed] [Google Scholar]
- 95.Jockenhoevel S, et al. Fibrin gel -- advantages of a new scaffold in cardiovascular tissue engineering. Eur J Cardiothorac Surg. 2001;19(4):424–30. doi: 10.1016/s1010-7940(01)00624-8. [DOI] [PubMed] [Google Scholar]
- 96.Flanagan TC, et al. The in vitro development of autologous fibrin-based tissue-engineered heart valves through optimised dynamic conditioning. Biomaterials. 2007;28(23):3388–3397. doi: 10.1016/j.biomaterials.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 97.Flanagan TC, et al. In Vivo Remodeling and Structural Characterization of Fibrin-Based Tissue-Engineered Heart Valves in the Adult Sheep Model. Tissue Engineering Part A. 2009;15(10):2965–2976. doi: 10.1089/ten.TEA.2009.0018. [DOI] [PubMed] [Google Scholar]
- 98.Syedain ZH, Weinberg JS, Tranquillo RT. Cyclic distension of fibrin-based tissue constructs: evidence of adaptation during growth of engineered connective tissue. Proc Natl Acad Sci U S A. 2008;105(18):6537–42. doi: 10.1073/pnas.0711217105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sha JM, et al. In-vitro seeding of human umbilical cord vein endothelial cells on hydroxyapatite for mechanical heart valve applications. J Heart Valve Dis. 2010;19(4):506–12. [PubMed] [Google Scholar]
- 100.Hoffman-Kim D, et al. Comparison of three myofibroblast cell sources for the tissue engineering of cardiac valves. Tissue Eng. 2005;11(1–2):288–301. doi: 10.1089/ten.2005.11.288. [DOI] [PubMed] [Google Scholar]
- 101.Smith S, et al. Force generation of different human cardiac valve interstitial cells: relevance to individual valve function and tissue engineering. J Heart Valve Dis. 2007;16(4):440–6. [PubMed] [Google Scholar]
- 102.Cebotari S, et al. Clinical application of tissue engineered human heart valves using autologous progenitor cells. Circulation. 2006;114(1 Suppl):I132–7. doi: 10.1161/CIRCULATIONAHA.105.001065. [DOI] [PubMed] [Google Scholar]
- 103.Frank BS, et al. Determining Cell Seeding Dosages for Tissue Engineering Human Pulmonary Valves. J Surg Res. 2010 doi: 10.1016/j.jss.2010.11.911. [DOI] [PubMed] [Google Scholar]
- 104.Appleton AJ, et al. Vascular smooth muscle cells as a valvular interstitial cell surrogate in heart valve tissue engineering. Tissue Eng Part A. 2009;15(12):3889–97. doi: 10.1089/ten.TEA.2009.0031. [DOI] [PubMed] [Google Scholar]
- 105.Paruchuri S, et al. Human pulmonary valve progenitor cells exhibit endothelial/mesenchymal plasticity in response to vascular endothelial growth factor-A and transforming growth factor-beta2. Circ Res. 2006;99(8):861–9. doi: 10.1161/01.RES.0000245188.41002.2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Merryman WD, et al. Defining biomechanical endpoints for tissue engineered heart valve leaflets from native leaflet properties. Prog Pediat Cardiol. 2006;21(2):153–60. [Google Scholar]
- 107.Hong H, et al. Fabrication of biomatrix/polymer hybrid scaffold for heart valve tissue engineering in vitro. ASAIO J. 2008;54(6):627–32. doi: 10.1097/MAT.0b013e31818965d3. [DOI] [PubMed] [Google Scholar]
- 108.Hong H, et al. Fabrication of a novel hybrid heart valve leaflet for tissue engineering: an in vitro study. Artif Organs. 2009;33(7):554–8. doi: 10.1111/j.1525-1594.2009.00742.x. [DOI] [PubMed] [Google Scholar]
- 109.Del Gaudio C, et al. Electrospun bioresorbable heart valve scaffold for tissue engineering. Int J Artif Organs. 2008;31(1):68–75. doi: 10.1177/039139880803100110. [DOI] [PubMed] [Google Scholar]
- 110.Del Gaudio C, Bianco A, Grigioni M. Electrospun bioresorbable trileaflet heart valve prosthesis for tissue engineering: in vitro functional assessment of a pulmonary cardiac valve design. Ann Ist Super Sanita. 2008;44(2):178–86. [PubMed] [Google Scholar]
- 111.van Lieshout MI, et al. Electrospinning versus knitting: two scaffolds for tissue engineering of the aortic valve. J Biomater Sci Polym Ed. 2006;17(1–2):77–89. doi: 10.1163/156856206774879153. [DOI] [PubMed] [Google Scholar]
- 112.Courtney T, et al. Design and analysis of tissue engineering scaffolds that mimic soft tissue mechanical anisotropy. Biomaterials. 2006;27(19):3631–8. doi: 10.1016/j.biomaterials.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 113.Stankus JJ, et al. Microintegrating smooth muscle cells into a biodegradable, elastomeric fiber matrix. Biomaterials. 2006;27(5):735–44. doi: 10.1016/j.biomaterials.2005.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stella JA, et al. Tissue-to-cellular level deformation coupling in cell micro-integrated elastomeric scaffolds. Biomaterials. 2008;29(22):3228–36. doi: 10.1016/j.biomaterials.2008.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sant S, et al. Hybrid PGS-PCL microfibrous scaffolds with improved mechanical and biological properties. J Tissue Eng Regen Med. 2011;5(4):283–91. doi: 10.1002/term.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kalfa D, et al. A polydioxanone electrospun valved patch to replace the right ventricular outflow tract in a growing lamb model. Biomaterials. 2010;31(14):4056–63. doi: 10.1016/j.biomaterials.2010.01.135. [DOI] [PubMed] [Google Scholar]
- 117.Chen R, et al. A novel approach via combination of electrospinning and FDM for tri-leaflet heart valve scaffold fabrication. Front Mater Sci China. 2009;3(4):359–366. [Google Scholar]
- 118.Hjortnaes J, et al. Translating autologous heart valve tissue engineering from bench to bed. Tissue Eng Part B Rev. 2009;15(3):307–17. doi: 10.1089/ten.TEB.2008.0565. [DOI] [PubMed] [Google Scholar]
- 119.Apte SS, et al. Current developments in the tissue engineering of autologous heart valves: moving towards clinical use. Future Cardiol. 2011;7(1):77–97. doi: 10.2217/fca.10.120. [DOI] [PubMed] [Google Scholar]
- 120.Sacks MS, Schoen FJ, Mayer JE. Bioengineering challenges for heart valve tissue engineering. Annu Rev Biomed Eng. 2009;11:289–313. doi: 10.1146/annurev-bioeng-061008-124903. [DOI] [PubMed] [Google Scholar]
- 121.Butcher JT, Mahler GJ, Hockaday LA. Aortic valve disease and treatment: The need for naturally engineered solutions. Adv Drug Deliv Rev. 2011 doi: 10.1016/j.addr.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 122.Bouten CV, et al. Substrates for cardiovascular tissue engineering. Adv Drug Deliv Rev. 2011 doi: 10.1016/j.addr.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 123.Murray G. Homologous aortic-valve-segment transplants as surgical treatment for aortic and mitral insufficiency. Angiology. 1956;7(5):466–71. doi: 10.1177/000331975600700509. [DOI] [PubMed] [Google Scholar]
- 124.Bjork VO, Henze A, Hindmarsh T. Radiopaque marker in the tilting disc of the Bjork-Shiley heart valve. Evaluation of in vivo prosthetic valve function by cineradiography. J Thorac Cardiovasc Surg. 1977;73(4):563–9. [PubMed] [Google Scholar]
- 125.Emery RW, et al. Clinical and hemodynamic results with the St. Jude medical aortic valve prosthesis. Surg Forum. 1979;30:235–8. [PubMed] [Google Scholar]
- 126.Emery RW, Mettler E, Nicoloff DM. A new cardiac prosthesis: the St. Jude Medical cardiac valve: in vivo results. Circulation. 1979;60(2 Pt 2):48–54. doi: 10.1161/01.cir.60.2.48. [DOI] [PubMed] [Google Scholar]
- 127.Emery RW, Nicoloff DM. St. Jude Medical cardiac valve prosthesis: in vitro studies. J Thorac Cardiovasc Surg. 1979;78(2):269–76. [PubMed] [Google Scholar]
- 128.Zilla P, et al. Prosthetic heart valves: catering for the few. Biomaterials. 2008;29(4):385–406. doi: 10.1016/j.biomaterials.2007.09.033. [DOI] [PubMed] [Google Scholar]
- 129.O’Brien MF, et al. The synergraft valve: A new acellular (nonglutaraldehyde-fixed) tissue heart valve for autologous recellularization first experimental studies before clinical implantation. Semin Thorac Cardiovasc Surg. 1999;11(4):194–200. [PubMed] [Google Scholar]
- 130.Cribier A, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation. 2002;106(24):3006–8. doi: 10.1161/01.cir.0000047200.36165.b8. [DOI] [PubMed] [Google Scholar]
- 131.Reul GJ, et al. Valve Failure with the Ionescu-Shiley Bovine Pericardial Bioprosthesis -Analysis of 2680 Patients. Journal of Vascular Surgery. 1985;2(1):192–204. [PubMed] [Google Scholar]
- 132.Dellgren G, et al. Late hemodynamic and clinical outcomes of aortic valve replacement with the Carpentier-Edwards Perimount pericardial bioprosthesis. Journal of Thoracic and Cardiovascular Surgery. 2002;124(1):146–154. doi: 10.1067/mtc.2002.121672. [DOI] [PubMed] [Google Scholar]
- 133.O’Brien TK, et al. Immunological reactivity to a new glutaraldehyde tanned bovine pericardial heart valve. Trans Am Soc Artif Intern Organs. 1984;30:440–4. [PubMed] [Google Scholar]
- 134.Stella JA, et al. On the biomechanical function of scaffolds for engineering load-bearing soft tissues. Acta Biomater. 2010;6(7):2365–81. doi: 10.1016/j.actbio.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Benton JA, et al. Photocrosslinking of gelatin macromers to synthesize porous hydrogels that promote valvular interstitial cell function. Tissue Eng Part A. 2009;15(11):3221–30. doi: 10.1089/ten.tea.2008.0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Butler DL, et al. Functional tissue engineering parameters toward designing repair and replacement strategies. Clin Orthop Relat Res. 2004;(427 Suppl):S190–9. doi: 10.1097/01.blo.0000144858.65450.d2. [DOI] [PubMed] [Google Scholar]
- 137.Delaughter DM, et al. What chick and mouse models have taught us about the role of the endocardium in congenital heart disease. Birth Defects Res A Clin Mol Teratol. 2011 doi: 10.1002/bdra.20809. [DOI] [PMC free article] [PubMed] [Google Scholar]