Abstract
For an animal to survive in a constantly changing environment, its behavior must be shaped by the complex milieu of sensory stimuli it detects, its previous experience and its internal state. Although taste behaviors in the fly are relatively simple, with sugars eliciting acceptance behavior and bitter compounds avoidance, these behaviors are also plastic and modified by intrinsic and extrinsic cues such as hunger and sensory stimuli. Here, we show that dopamine modulates a simple taste behavior, proboscis extension to sucrose. Conditional silencing of dopaminergic neurons reduces proboscis extension probability and increased activation of dopaminergic neurons increases extension to sucrose but not to bitter compounds or water. One dopaminergic neuron with extensive branching in the primary taste relay, the subesophageal ganglion, triggers proboscis extension and its activity is altered by satiety state. These studies demonstrate the marked specificity of dopamine signaling and provide a foundation to examine neural mechanisms of feeding modulation in the fly.
Introduction
Feeding behaviors are highly regulated, with sensory cues and internal state contributing to eating decisions. The nutrient content and palatability of the food source, current energy requirements of the animal and learned associations all factor into an animal's decision to eat. The complex regulation of feeding provides an excellent system to examine how neuronal circuits integrate information from the periphery with metabolic state to shape behavior.
In Drosophila, feeding begins with the proboscis extension response (PER). When gustatory neurons on the legs or the proboscis detect an acceptable taste compound, the fly extends its proboscis and initiates feeding (Dethier, 1976). Even this very simple component of feeding behavior is tightly regulated. The probability of extension depends on the nature of the taste compound; increasing sugar concentration increases the probability and increasing bitter concentration decreases it (Dethier, 1976; Meunier et al., 2003; Wang et al., 2004). The response is also modulated by hunger and satiety; flies that have recently consumed a meal are less likely to extend the proboscis than those that have not fed (Dethier, 1976). Associations with other stimuli also influence extension probability; for example, pairing sucrose with a noxious stimulus inhibits extension (Masek and Scott, 2010). How does the neural circuitry for proboscis extension allow for extensive plasticity in behavior?
The neural circuits from taste detection to proboscis extension are just beginning to be elucidated. Gustatory neurons are found in chemosensory sensilla on the proboscis, internal mouthparts and legs (Stocker, 1994). Each sensillum contains four gustatory neurons that recognize different taste modalities. One cell expresses a subset of gustatory receptor genes (GRs), including Gr5a, detects sugars and promotes proboscis extension (Thorne et al., 2004; Wang et al., 2004). A second expresses a different subset of GRs, including Gr66a, detects bitter compounds and inhibits extension (Thorne et al., 2004; Wang et al., 2004). A third cell, marked by the ion channel Ppk28, senses water (Cameron et al., 2010; Chen et al., 2010). The function of the fourth cell is unclear. Thus, similar to the mammalian gustatory system, there are just a few categories of sensory cells in the periphery that are tightly coupled to innate behavior.
Gustatory neurons from the proboscis, mouthparts and legs project to the fused subesophageal ganglion/ tritocerebrum (SOG) of the fly brain (Stocker, 1994). Unlike the primary olfactory relay, the SOG is not a dedicated taste area. Instead, there are approximately 6000 neurons associated with the SOG and it serves as a general relay for information flow between the brain and the ventral cord. Gustatory neurons that express different receptors or reside in different peripheral tissues terminate in different regions, suggesting that there are maps of taste modality and taste organ in the SOG (Thorne et al., 2004; Wang et al., 2004). Motor neurons that drive proboscis extension and feeding also reside in the SOG. For example, each of the 12 paired muscles that mediate proboscis extension is innervated by 1-3 motor neurons with cell bodies in the SOG (Stocker, 1994). Attempts to examine sensory-motor connectivity suggest that there are no direct connections (Gordon and Scott, 2009). Nevertheless, the proximity of sensory and motor neurons argues that there may be local circuits in the SOG for proboscis extension.
To begin to address how plasticity in this simple behavior is generated, we examined the role of candidate neuromodulatory neurons in regulating proboscis extension. We find that dopamine acts as a critical modulator of proboscis extension and identify a single dopaminergic neuron in the primary taste relay that governs modulation. These studies suggest that dopamine acts as a gain control system to alter the probability of proboscis extension to sucrose.
Results
Inactivation of dopaminergic neurons reduces proboscis extension to sucrose
Several neuropeptide/neurotransmitter systems have been implicated in feeding regulation in Drosophila. Homologues of insulin, neuropeptide F, glucagon and neuromedin have been shown to participate in fasting behaviors and food deprived metabolic states (Leopold and Perrimon, 2007; Melcher et al., 2007). In addition, the biogenic amines serotonin, dopamine and octopamine influence feeding behavior in both vertebrates and invertebrates (Ramos et al., 2005; Srinivasan et al., 2008). We reasoned that as proboscis extension is an integral component of feeding behavior, it might be modulated by the same systems that affect food intake.
To identify neurons that modulate the proboscis extension response, we undertook a genetic approach to silence candidate modulatory neurons and examined the behavioral effect, using pre-existing Gal4 lines. An inward-rectifying potassium channel (Kir2.1) was expressed in modulatory neurons to prevent membrane depolarization, using the Gal4/UAS transgenic system (Baines et al., 2001). A pan-neural temperature-sensitive Gal80ts was used to repress Kir2.1 expression until adulthood and then Kir2.1 was induced by a 2-3 day temperature-shift to inactivate Gal80ts (McGuire et al., 2004). Genetically identical flies with and without Kir2.1 expression were examined for proboscis extension to 100mM sucrose after food deprivation for 24 hours. Most Gal4 lines showed similar behavior with and without Kir2.1 induction; however, the tyrosine hydroxylase-Gal4 (TH-Gal4) showed decreased extension probability only upon Kir2.1 expression (Figure 1). These flies sensed concentration differences but showed reduced sucrose sensitivity at high concentrations (Figure 1C). TH-Gal4 marks neurons that express tyrosine hydroxylase, the rate-limiting enzyme in the biosynthetic pathway for dopamine, thereby labeling dopaminergic neurons (Friggi-Grelin et al., 2003). Thus, the conditional silencing experiments suggest that dopaminergic neurons modulate PER.
Figure 1. Silencing TH-Gal4 neurons decreased the probability of proboscis extension.

A. Candidate neuromodulatory neurons in the fly brain are labeled with GFP. Each Gal4 line labels a unique neural subset. Candidate neuromodulatory neurons were chosen based on the availability of Gal4 lines. Scale bar is 50 μm.
B. Candidate neuromodulatory neurons were tested for their role in proboscis extension upon conditional expression of Kir2.1. When flies were raised at permissive temperature (black bars), Gal80ts was expressed, inhibiting expression of Kir2.1. When flies were shifted to restrictive temperature for 2-3 days (grey and red bars), Gal80ts was inactive, allowing expression of Kir2.1. Most flies showed a similar probability of proboscis extension upon 24hrs starvation under both rearing conditions. However TH-Gal4 flies showed reduced proboscis extension at restrictive temperature, showing that inactivating dopaminergic neurons inhibits the behavior (mean+/-CI, n=25-44, ***P<0.001, Fisher's exact test).
C. TH-Gal4, UAS-Kir2.1, tub-Gal80ts flies showed reduced proboscis extension for a range of sucrose concentrations upon Kir2.1 induction with 24hrs starvation. (mean+/-CI, 22°C: n=105, 30°C: n=104, ***P<0.001, Fisher's exact test).
Inducible activation of dopaminergic neurons triggers PER
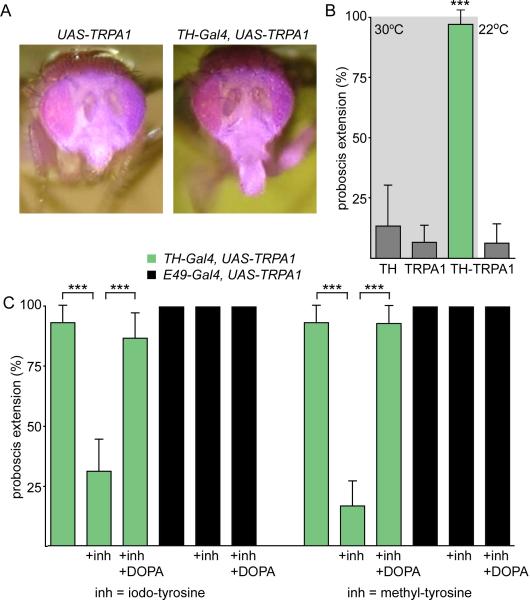
In Drosophila, as in mammals, dopamine serves many functions. In flies, it has primarily been shown to participate in arousal and sleep, as well as aversive and reward conditioning (Van Swinderen and Andretic, 2011; Waddell, 2010). Therefore, silencing these neurons may indirectly influence proboscis extension as a result of altered metabolic needs. Alternatively, decreased dopaminergic activity might directly reduce extension probability. If activity of dopaminergic neurons directly modulates PER, one expectation would be that increasing activity would promote extension. To test this, we monitored the behavioral effect of TH-Gal4 neuronal activation. The cation channel dTRPA1 is gated by temperature, opening at >25°C to depolarize cells (Hamada et al., 2008). Flies expressing dTRPA1 in TH-Gal4 neurons did not extend their proboscis at room temperature (2/32 extended) (22°C). However, the same flies showed proboscis extension when the temperature was elevated to 30°C by placement on a heating block (31/32 extended) (Figure 2AB).
Figure 2. Inducible activation of TH-Gal4 neurons triggers proboscis extension.
A. UAS-dTRPA1 flies stimulated with an infrared heat pulse to the head did not show proboscis extension. TH-Gal4, UAS-dTRPA1 flies showed proboscis extension to the same stimulus.
B. Quantification of inducible activation experiments. Experiments similar to A, except flies were exposed to a 30°C temperature shift for two minutes. TH-Gal4, UAS-dTRPA1 flies showed increased extension at 30°C but not at 22°C. (mean+/-CI, n=15-45, ***P<0.001, Fisher's exact test)
C. Competitive inhibitors of dopamine synthesis block dTRPA1 inducible activation. 1% iodo-tyrosine (left) or 1% methyl-tyrosine (right) was added to fly food for three days. TH-Gal4, UAS-dTRPA1 flies (green bars) were then tested for proboscis extension to heat as in B. Flies fed DA inhibitors showed significantly reduced proboscis extension and this defect was rescued by including 0.5% DOPA in the food (mean+/-CI, n=33-48 flies/condition, ***P<0.001, Fisher's exact test). Feeding iodo-tyrosine or methyl-tyrosine to flies expressing dTRPA1 in the E49 motor neuron (black bars) did not influence the probability of proboscis extension.
To test whether inducible activation requires dopamine, we carried out pharmacological treatments to reduce dopamine levels in the fly. Methyl-tyrosine and iodo-tyrosine are competitive inhibitors of tyrosine hydroxylase that decrease dopamine levels in the fly (Sitaraman et al., 2008). TH-Gal4, UAS-dTRPA1 flies were fed 1% methyl-tyrosine or iodo-tyrosine for 3 days and then tested for proboscis extension to heat. Upon drug exposure, TH-Gal4, UAS-dTRPA1 flies showed greatly reduced extension probability to heat (Figure 2C). This suggests that dopamine release from TH-Gal4 neurons is required to trigger extension. Consistent with this, feeding flies 0.5% dihydroxyphenylalanine (DOPA), the product of tyrosine hydroxylase, in addition to methyl-tyrosine or iodo-tyrosine, rescued heat-induced extension (Figure 2C). When dTRPA1 was expressed in proboscis motor neurons, the drugs did not adversely affect proboscis extension to heat, arguing that the tyrosine hydroxylase inhibitors do not block PER non-specifically, but rather act upstream of motor neuron activation.
As a second test of whether dopamine release from TH-Gal4 neurons drives extension, we examined whether extension required dopamine receptors. Four dopamine receptors have been identified in Drosophila, and previous studies have isolated mutants in the dopamine 1 receptor (DopR) (Gotzes et al., 1994; Sugamori et al., 1995) and the dopamine 2 receptor (D2R) (Bellen et al., 2004; Thibault et al., 2004). If proboscis extension upon activation of TH-Gal4 neurons requires specific dopamine receptors, then it should be inhibited in dopamine receptor mutant backgrounds. Indeed, TH-Gal4, UAS-dTRPA1 flies no longer showed proboscis extension to heat in a D2R mutant background (D2R homozygous mutants: 0/52 flies showed extension; D2R heterozygotes: 52/52 flies showed extension; P=1xe-30; Fisher's exact test) but still extended normally in a DopR background (DopR homozygous mutants: 48/56 flies showed extension). These experiments suggest that proboscis extension is triggered by dopamine release from TH-Ga4 neurons acting on D2R but not DopR.
Dopaminergic neurons shift the probability of PER to sucrose
To examine when dopamine is likely to regulate proboscis extension, flies with altered dopaminergic activity were stimulated with a range of sugar concentrations under different starvation conditions. Flies in which TH-Gal4 neurons were silenced by conditional expression of UAS-Kir2.1 in adults showed decreased probability of extension, as expected (Figure 1C, 3A). As starvation time increased, the response increased, arguing that these flies are still sensitive to other cues related to internal state. However, the response was blunted for the highest sugar concentrations, indicating that loss of dopaminergic activity decreases the gain of the response.
Figure 3. Manipulating dopaminergic activity alters proboscis extension probability.
A. TH-Gal4, UAS-Kir2.1, tub-Gal80ts flies showed reduced proboscis extension upon Kir2.1 induction (30°C) upon 12 and 36 hours starvation, but not at 22°C (R.T.). The right panel shows the overlay of 12 and 36 hour responses. (mean+/-CI, n=40-45 flies/condition, *P<0.05, ***P<0.001, Fisher's exact test).
B. TH-Gal4, UAS-NaChBac, tub-Gal80ts flies showed increased proboscis extension upon NaChBac induction (30°C) but not at R.T. upon 0 and 24 hours starvation. The right panel shows the overlay. (mean+/-CI, n=50-58 flies/condition, *P<0.05, **P<0.01, ***P<0.001, Fisher's exact test). See also Figure S1 for responses to a bitter compound and water.
In the converse experiment, the electrical excitability of dopaminergic neurons was increased by conditional expression of UAS-NaChBac, a low threshold, slowly inactivating sodium channel. Unlike dTRPA1, this channel does not drive neural activity by exogenous cues but instead amplifies the cellular response to membrane depolarization (Nitabach et al., 2006). Expression of NaChBac in the adult increased the probability of response for all concentrations and starvation conditions (Figure 3B). Flies with altered dopaminergic activity did not differ in proboscis extension responses to denatonium, a bitter compound, or water, a non-nutritive but acceptable substance (Figure S1). This result argues that dopaminergic activity selectively alters the probability of proboscis extension to sucrose but not to non-nutritious compounds.
The probability of proboscis extension depends on sucrose concentration and satiety state. Previous studies have shown that the activity of gustatory sensory neurons dramatically increases with sucrose concentration (Hiroi et al., 2002; Marella et al., 2006). The concentration-dependent change in PER probability most likely reflects changes in sensory activity propagating through the circuit. The satiety state also acts to adjust probability of extension, with increased extension to a given concentration when the fly is food-deprived. Our behavioral studies argue that the activity of TH-Gal4 neurons serves to adjust the probability of extension to a given sucrose concentration. Thus, dopaminergic neural activity acts as a gain control mechanism to adjust the dynamic range for proboscis extension to sucrose, increasing extension probability when activity is high and decreasing it when it is low.
A single dopaminergic neuron in the SOG modulates PER
Tyrosine hydroxylase is expressed in a few hundred neurons in the fly brain, as demonstrated by TH-Gal4 expression and by Drosophila TH antisera (Friggi-Grelin et al., 2003; Nassel and Elekes, 1992). To identify the specific TH-Gal4 neurons that trigger proboscis extension, we employed a genetic mosaic analysis to restrict dTRPA1 expression to small subsets of TH-Gal4 neurons (Gordon and Scott, 2009). Briefly, the repressor Gal80 flanked by FRT recombination sites was expressed ubiquitously to inhibit Gal4-dependent expression. Induction of Flp recombinase under the control of a heat shock promoter led to the stochastic excision of Gal80 and the expression of UAS-dTRPA1 in different TH-Gal4 subpopulations (Gordon and Scott, 2009). The inclusion of UAS-mCD8-GFP allowed for visualization of cells expressing dTRPA1.
Mosaic animals were tested for proboscis extension to heat and classified as extenders and non-extenders. The neurons labeled in the brains and thoracic ganglia of extenders and non-extenders were compared to test whether specific TH-Gal4 neurons were associated with the extension phenotype. Eleven different cell populations were frequently labeled by this method (Figure 4). Most cell populations showed a similar frequency distribution in both behavioral categories; however, one cell was present in 93% of extenders (51/55) and rarely present in non-extenders (1%; 1/99). In addition, three extenders showed Gal4 expression in just two cells in the entire nervous system; each contained the cell found in 93% of extenders and a second cell that was different in each fly. These results argue that a single TH-Gal4 cell is sufficient to drive proboscis extension. Other cells may modestly influence proboscis extension but would not be uncovered by mosaic analyses. Instead, the mosaic analysis is biased towards identifying single neurons sufficient to activate proboscis extension.
Figure 4. Genetic mosaic analyses identify a single dopaminergic neuron that modulates PER.
A. Animals expressing dTRPA and CD8-GFP in subsets of dopaminergic neurons were tested for proboscis extension to heat. The graph plots the frequency distribution of different neural classes in flies that extended the proboscis to heat (black bars, n=55) and flies that did not extend (grey bars, n=99) (mean +/- CI). The frequency distribution is similar for both behavioral categories, except for cell-type one that is nearly always present in extenders and not in non-extenders (Fisher's exact test, P=8xe-35). Three other cell-types showed statistical differences in distribution (Fisher's exact test): cell-type 9 (P=0.0003), 10, (P=0.03), 11 (P=0.003); however, these cells were often found in both behavioral categories.
B. Eleven different cell-types were frequently identified in TH-Gal4 mosaic animals, including 8 in the brain (1-8) and three in thoracic ganglia (9-11). The conditions for generating mosaic animals led to sparse labeling of a subset of TH-Gal4 neurons. Arrows point to the cell bodies. The numbers correspond to the cell-types numbered in A. Scale bar = 100 μm.
C. Example brains from flies showing no extension; the right brain is from a fly showing extension to heat. Scale bar = 100 μm.
The TH-Gal4 neuron that generates extension shows broad arborizations in the ventral anterior subesophageal ganglion (SOG), the primary taste relay (Figure 5 and Figure S2). This brain region receives gustatory axons from the proboscis, mouthparts and legs, and motor neuron dendrites that drive proboscis extension (Figure S2). Previous studies characterizing the anatomy of TH-Gal4 neurons have classified this neuron as a ventral unpaired medial neuron based on cell body position (Nassel and Elekes, 1992). We name this neuron TH-VUM. As expected, TH-VUM expresses tyrosine hydroxylase by immunohistochemistry (Figure 5C), demonstrating that it is indeed a dopaminergic neuron. To determine whether processes are dendrites or axons, a marker for pre-synaptic terminals, Synaptobrevin-GFP (Syn-GFP) (Estes et al., 2000), was expressed in single cell TH-Gal4 clones. Syn-GFP labeled all arbors of TH-VUM, suggesting that the neuron releases transmitter throughout the SOG (Figure 5D). Based on its localization in the primary taste region and its extensive arborizations, the TH-VUM neuron is well situated to modulate taste behaviors.
Figure 5. The dopaminergic neuron triggering extension is a wide-field interneuron in the SOG.
A. A brain (top) plus thoracic ganglia (below) showing expression of GFP in a ventral unpaired medial interneuron in the SOG, named TH-VUM. This mosaic animal expressed dTRPA1 and showed heat-induced proboscis extension. Two cells were labeled: TH-VUM in the SOG and a neuron in the thoracic ganglion (arrowhead).
B. High resolution image of TH-VUM arborizations in the SOG, shown in a confocal projection image (top) and a volume rendered three-dimensional reconstruction using Amira (version 5.4) (below).
C. A brain from a mosaic animal expressing GFP in a subset of TH-Gal4 neurons including TH-VUM (arrow) co-labeled with TH antisera. The GFP clone is in green (left) and TH antisera is in magenta (middle). The overlay is on right, showing that the TH-VUM expresses TH antigen. TH antisera labels all dopaminergic neurons in the SOG. Thus, several cells and punctae in addition to TH-VUM are labeled, including 3 other TH-positive neurons in the ventral SOG.
D. A brain from a mosaic animal expressing nSynaptobrevin-GFP and CD8-RFP in the TH-VUM neuron. The nSyb-GFP label is in green (left), the CD8-RFP label is in magenta (middle). The overlay is on right, showing that the vast majority of TH-VUM processes are axonal. Brightness/contrast of single channels were adjusted for the entire image in each panel using ImageJ software. Scale bar = 50 μm in all panels. See Figure S2 for 3D reconstructions of TH-VUM in other orientations and TH-VUM proximity to sugar-sensing axons.
The SOG dopaminergic neuron is modulated by hunger and satiety
Adjusting the activity of TH-Gal4 neurons alters the probability of proboscis extension, arguing that TH-Gal4 neurons are directly or indirectly involved in processing gustatory information. If TH-VUM were directly part of the taste processing pathway, then it should be activated in response to taste cues. If it were a modulatory neuron that impinged on the taste processing pathway, then it may not be directly activated by taste cues but should modulate taste behavior.
We tested whether TH-VUM activity was elicited by taste compounds by monitoring calcium changes with the genetically-encoded indicator G-CaMP during sucrose stimulation of the proboscis (Marella et al., 2006). The neuron did not respond to 1 M sucrose in fed animals or animals that were food-deprived for 24 hours (n=7-9, max ΔF/F +/- SEM; 0H starvation= -1.0 +/- 0.8; 24H starvation= -0.5+/- 0.6; t-test NS). These results argue that TH-VUM is not part of the primary taste pathway from taste detection to proboscis extension. As it does not respond to taste compounds, it is also unlikely to report the reward value of a taste compound.
An alternative possibility is that the dopaminergic neuron modulates proboscis extension more indirectly and on a different time scale than taste activation. Our behavioral studies suggest that dopaminergic activity might adjust the range of proboscis extension, with increased activity promoting extension. To test this, the basal activity of TH-VUM was monitored under different satiety conditions, when extension probability varied. Mosaic flies were generated that expressed dTRPA1 and CD8-GFP in subpopulations of TH-Gal4 cells. Flies that extended the proboscis to heat were selected for electrophysiology. Loose-patch recordings were performed on live flies with cuticle removed to expose the subesophageal ganglion (Root et al., 2007; Wilson et al., 2004). Brains were stained with anti-GFP after recording to ensure that the neuron recorded was TH-VUM.
TH-VUM showed tonic firing rates that correlated with satiety state. The lowest average tonic firing rate (1 Hz) was found in flies that had recently been fed, whereas the highest rate (25 Hz) was found in flies that had been food-deprived for 24 hours (Figure 6). Thus, firing rate is low under conditions where the probability of proboscis extension is low and increases under conditions where extension probability is high. Monitoring the activity of the three other dopaminergic neurons in the ventral SOG did not reveal a change in firing rate based on starvation time (Figure S3). These electrophysiological experiments are consistent with the notion that the activity of TH-VUM modulates the probability of proboscis extension, serving to increase proboscis extension in animals that are food-deprived.
Figure 6. The activity of TH-VUM is modulated by satiety state.
A. Sample electrophysiological recordings of TH-VUM in live animals that have been food-deprived for 0H, 12H or 24H.
B. Summary plot of spike rate in TH-VUM in animals with 0H, 12H or 24H food-deprivation. Each dot is the average spike rate of a single TH-VUM neuron. Spike rate was determined over the interval of 0-30 seconds. 5 animals per condition. (**P<0.01; ***P<0.001; t-test to 0H).
C. Raster plots showing spike patterns in each animal. The interval of 0-15 sec is shown. See also Figure S3 for electrophysiology of non-TH-VUM neurons in the SOG.
Discussion
Invertebrate models with less complex nervous systems and robust sensory-motor behaviors may illuminate simple neural modules that regulate behavior. In this study, we examine flexibility in a gustatory-driven behavior and find that a dopaminergic neuron is a critical modulator. Our loss-of-function and gain-of-function studies argue that increased dopaminergic activity promotes proboscis extension to sucrose and decreased dopaminergic activity inhibits it. Our studies show that a single dopaminergic neuron in the SOG, TH-VUM, can drive proboscis extension. TH-VUM does not respond to sugars, arguing that it is not directly in the pathway from taste detection to behavior, but instead acts over a longer time scale or in response to other cues to modulate proboscis extension to sucrose. Consistent with this idea, satiety state influences TH-VUM activity, promoting activity when the animal is food-deprived and the probability of proboscis extension is increased. Our studies suggest that dopaminergic activity regulates the probability of extension according to an animal's nutritional needs.
The finding that dopamine neural activity affects proboscis extension to sucrose but not water argues that dopamine regulation occurs upstream of shared motor neurons involved in proboscis extension. The pathway selectivity also argues that different molecular mechanisms modulate food and water intake independently in the fly, with parallels to hunger and thirst drives in mammals. Where dopamine acts in the sugar pathway is not known. Experiments to test for proximity between sugar sensory neurons and TH-VUM using the GRASP approach (Gordon and Scott, 2009) suggested that a few fibers are in close proximity (data not shown), but the significance is unclear. The broad arborizations of TH-VUM suggest it may have many targets.
Dopamine is a potent modulator of a variety of behaviors in mammals and flies. In mammals, functions of dopamine include motor control, reward, arousal, motivation and saliency (Bromberg-Martin et al., 2010; Graybiel et al., 1994). Dopamine also critically regulates feeding behavior. Mice mutant for tyrosine hydroxylase fail to initiate feeding although they distinguish sucrose concentrations and have the motor ability to consume (Szczypka et al., 1999). Dopamine pathways that regulate feeding are complex, with the tuberoinfundibular, nigrostriatal and mesolimbic/mesocortical pathways implicated in different aspects of feeding regulation (Vucetic and Reyes, 2010). Although several studies show that dopamine promotes positive aspects of feeding, there is debate over whether dopamine is involved in pleasure (‘liking’), motivation/salience (‘wanting’), associative learning or sensorimotor activation (Berridge, 2007). With 20,000-30,000 TH-positive neurons in mice and 400,000-600,000 in humans (Bjorklund and Dunnett, 2007), the complexity of dopaminergic regulation makes it difficult to parse the function of different neurons.
In Drosophila, as in mammals, dopamine participates in conditioning and arousal (Nitz et al., 2002; Schwaerzel et al., 2003; Tempel et al., 1984) and our work highlights a shared role in feeding regulation. There are only a few hundred TH-positive neurons in Drosophila (Friggi-Grelin et al., 2003; Nassel and Elekes, 1992) and recent studies have begun to elucidate the function of different dopaminergic neural subsets (Aso et al., 2010; Kong et al., 2010; Krashes et al., 2009; Lebestky et al., 2009; Mao and Davis, 2009). Our work demonstrates that a single dopaminergic neuron in the SOG potently modulates proboscis extension behavior. Other dopaminergic neurons have cell bodies near TH-VUM and extensive projections in the SOG, yet activation of these neurons is not associated with proboscis extension. It is possible that additional dopaminergic neurons regulate other aspects of taste behavior but they are insufficient to drive proboscis extension.
In mammals, dopamine levels in the nucleus accumbens, the target of the mesolimbic pathway, increase upon sugar detection in the absence of consumption (Hajnal et al., 2004) or upon nutrient consumption in the absence of detection (de Araujo et al., 2008), suggesting that dopamine encodes multiple rewarding aspects of sugar: intensity on the tongue and nutritional value. Recent studies in Drosophila also show that they sense nutritional content independent of taste detection and this influences ingestion (Burke and Waddell, 2011; Dus et al., 2011; Fujita and Tanimura, 2011). It will be interesting to determine if dopamine plays a role in sensing internal nutritional state and regulates other aspects of ingestion in addition to its role in proboscis extension.
The anatomical location of the dopaminergic interneuron highlights the central role of the SOG in taste processing and suggests that local SOG circuits may control proboscis extension behavior. Future studies identifying the downstream targets of TH-VUM will ultimately enable a deeper understanding of how dopamine achieves spatial and temporal modulation of extension probability. Our current study identifies an essential role for dopamine in gain control of proboscis extension to sucrose and underscores the exquisite specificity of single neurons as thin threads to behavior.
Supplementary Material
Acknowledgements
The Scott lab provided comments on the experiments and manuscript. Wendi Neckameyer generously provided anti-TH antisera. Michael Gordon provided images of E49 motor neurons and Gr5a sensory neurons. Pavel Masek provided assistance with laser activation experiments. This research was supported by a Scholars Award from the John Merck Fund and a grant from the NIDCD 1R01DC009470 to K.S. K.S. is an Early Career Scientist of the Howard Hughes Medical Institute.
Methods
Drosophila Stocks and Genetics
w1118 flies were used as control wild type flies. The following Gal4 lines were used: Akh-Gal4 (Lee and Park, 2004), dilp3-Gal4 (Buch et al., 2008), tdc2-gal4 (Cole et al., 2005), hugin-Gal4 (Melcher and Pankratz, 2005), TH-Gal4 (Friggi-Grelin et al., 2003), hs-flp, MKRS (Bloomington stock collection), Npf-Gal4 (Wu et al., 2003), UAS-Kir2.1 (Baines et al., 2001); tub-Gal80ts (McGuire et al., 2004), ptub-FRT-Gal80-FRT and Gr5a-lexA (Gordon and Scott, 2009), UAS-mCD8::GFP (Lee and Luo, 1999), UAS-dTRPA1 (Hamada et al., 2008). DopR mutants (f02676) and D2R mutants (f06521) were obtained from the Exelixis collection (Bellen et al., 2004; Thibault et al., 2004). Flies were grown on standard fly food.
Behavioral Experiments
Measurement of PER was performed as described using females (Wang et al., 2004) except that flies were glued to glass slides using nail polish. Flies were stimulated with water on their tarsi and allowed to drink ad libitum. For concentration curves, tarsi were stimulated with increasing concentrations of sucrose (10mM - 1M sucrose) and washed with water in between stimulations. Flies that responded to any of three trials of a given stimulus were recorded as extenders. For conditional inactivation experiments using UAS-Kir2.1 and tub-Gal80ts, flies were grown at room temp (~22°C) for 6-9 days and then moved to 30°C for 2-3 days to inactivate Gal80. Flies were fasted for different time periods on water. For inducible activation experiments, flies were grown at room temperature. They were immobilized and then moved to a heating pad at 30°C. Flies were observed for proboscis extension after 2 min on the pad. For demonstration purposes, dTRPA1 was also heat activated using a 2 sec infrared laser pulse and the behavior of flies recorded using a digital camera, as described (Masek and Scott, 2010). Drug experiments involved feeding flies for 3 days on food containing a mixture of 1% agarose, 1% sucrose, plus either 1% methyl-tyrosine or 1% iodo-tyrosine. 0.5% dihydroxyphenylalanine was added in addition to the inhibitors in rescue experiments.
Immunohistochemistry
Antibody staining and imaging was carried out as described (Wang et al., 2004). The following antibodies were used - rabbit anti-GFP (Invitrogen, 1:1000), mouse anti-GFP (Sigma, 1:100), rabbit anti-TH (1:500) (Neckameyer et al., 2000). Brightness/contrast of single channels were adjusted for the entire image using ImageJ software.
Genetic mosaics
Genetic mosaics were generated as described (Gordon and Scott, 2009), except that the flies of genotype tub>Gal80>; UAS-dTRPA1/UAS-mCD8::GFP; MKRS, hs-FLP/TH-GAL4 were grown at room temperature and subjected to a heat shock of 37°C for 30-60 min during late larval to pupal stages. This paradigm produced labeling in a small subset of TH-Gal4 neurons.
G-CamP imaging
Responses were monitored as previously described (Marella et al., 2006).
Electrophysiology
Flies used for recording were 3-10 day-old females. Flies were anesthetized using CO2 and their legs were removed using scissors. Flies were then placed into a small slit on a plastic mount at the cervix such that the head was in a different compartment than the rest of the body. The head was then immobilized using nail polish. The head cuticle was dissected in ice-cold AHL lacking calcium and magnesium (Wang et al., 2003). The antennae, proboscis and surrounding cuticle were gently removed using fine forceps, exposing the SOG. The perineural sheath was also removed on the lateral side of the SOG. Before recording, the dissecting AHL was replaced with AHL containing calcium and magnesium.
Electrodes (5-7MOhm) containing AHL were used to carry out extracellular recording in a loose patch configuration with a resistances ranging from 50 -500MOhm. VUM or other TH positive neurons were identified by the presence of GFP. Spikes were recorded in voltage-clamp mode using a multiclamp 700B recorder at 20kHz and low-pass filtered at 5kHz. Recordings were then bandpass filtered between 100 and 3000Hz using a butterworth type filter. Spikes were identified by threshold detection, typically between 5-10pA, using a custom Python script. The average spike rate for a 30 second window was calculated for each recording. Statistical analysis was performed using a two-tailed student's t-test.
To ensure that TH-VUM was the neuron recorded, mosaic animals were generated that expressed UAS-dTRPA1 and UAS-CD8-GFP in TH-Gal4 subsets. Animals were screened for PER to heat to select animals with TH-VUM labeled. Animals that extended were selected for electrophysiology and GFP-positive neurons in the ventral SOG were used for recording. Brains were stained with GFP antisera after recording to ensure that TH-VUM was labeled and other ventral SOG neurons were not.
Statistical Analysis
Proboscis extension data was analyzed with Fisher's exact test, and mean and 95% confidence intervals (CI) were reported, appropriate for testing the relation of two categorical variables (two conditions).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
S.M. initiated the project and performed the majority of experiments. K.M. carried out electrophysiology as well as behavioral experiments with NaChBac. K.S. wrote the manuscript and generated most figures with critical input from S.M. and K.M.
References
- Aso Y, Siwanowicz I, Bracker L, Ito K, Kitamoto T, Tanimoto H. Specific dopaminergic neurons for the formation of labile aversive memory. Curr Biol. 2010;20:1445–1451. doi: 10.1016/j.cub.2010.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines RA, Uhler JP, Thompson A, Sweeney ST, Bate M. Altered electrical properties in Drosophila neurons developing without synaptic transmission. J Neurosci. 2001;21:1523–1531. doi: 10.1523/JNEUROSCI.21-05-01523.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellen HJ, Levis RW, Liao G, He Y, Carlson JW, Tsang G, Evans-Holm M, Hiesinger PR, Schulze KL, Rubin GM, et al. The BDGP gene disruption project: single transposon insertions associated with 40% of Drosophila genes. Genetics. 2004;167:761–781. doi: 10.1534/genetics.104.026427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology. 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Bjorklund A, Dunnett SB. Dopamine neuron systems in the brain: an update. Trends in neurosciences. 2007;30:194–202. doi: 10.1016/j.tins.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 2010;68:815–834. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buch S, Melcher C, Bauer M, Katzenberger J, Pankratz MJ. Opposing effects of dietary protein and sugar regulate a transcriptional target of Drosophila insulin-like peptide signaling. Cell metabolism. 2008;7:321–332. doi: 10.1016/j.cmet.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Burke CJ, Waddell S. Remembering nutrient quality of sugar in Drosophila. Curr Biol. 2011;21:746–750. doi: 10.1016/j.cub.2011.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron P, Hiroi M, Ngai J, Scott K. The molecular basis for water taste in Drosophila. Nature. 2010;465:91–95. doi: 10.1038/nature09011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Wang Q, Wang Z. The amiloride-sensitive epithelial Na+ channel PPK28 is essential for drosophila gustatory water reception. J Neurosci. 2010;30:6247–6252. doi: 10.1523/JNEUROSCI.0627-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SH, Carney GE, McClung CA, Willard SS, Taylor BJ, Hirsh J. Two functional but noncomplementing Drosophila tyrosine decarboxylase genes: distinct roles for neural tyramine and octopamine in female fertility. The Journal of biological chemistry. 2005;280:14948–14955. doi: 10.1074/jbc.M414197200. [DOI] [PubMed] [Google Scholar]
- de Araujo IE, Oliveira-Maia AJ, Sotnikova TD, Gainetdinov RR, Caron MG, Nicolelis MA, Simon SA. Food reward in the absence of taste receptor signaling. Neuron. 2008;57:930–941. doi: 10.1016/j.neuron.2008.01.032. [DOI] [PubMed] [Google Scholar]
- Dethier VG. The Hungry Fly. Harvard University Press; Cambridge, MA: 1976. [Google Scholar]
- Dus M, Min S, Keene AC, Lee GY, Suh GS. Taste-independent detection of the caloric content of sugar in Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:11644–11649. doi: 10.1073/pnas.1017096108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes PE, Ho G, Narayanan R, Ramaswami M. Synaptic localization and restricted diffusion of a Drosophila neuronal synaptobrevin - green fluorescent protein chimera in vivo. J. Neurogenetics. 2000;13:233–255. doi: 10.3109/01677060009084496. [DOI] [PubMed] [Google Scholar]
- Friggi-Grelin F, Coulom H, Meller M, Gomez D, Hirsh J, Birman S. Targeted gene expression in Drosophila dopaminergic cells using regulatory sequences from tyrosine hydroxylase. Journal of neurobiology. 2003;54:618–627. doi: 10.1002/neu.10185. [DOI] [PubMed] [Google Scholar]
- Fujita M, Tanimura T. Drosophila evaluates and learns the nutritional value of sugars. Curr Biol. 2011;21:751–755. doi: 10.1016/j.cub.2011.03.058. [DOI] [PubMed] [Google Scholar]
- Gordon MD, Scott K. Motor control in a Drosophila taste circuit. Neuron. 2009;61:373–384. doi: 10.1016/j.neuron.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotzes F, Balfanz S, Baumann A. Primary structure and functional characterization of a Drosophila dopamine receptor with high homology to human D1/5 receptors. Receptors & channels. 1994;2:131–141. [PubMed] [Google Scholar]
- Graybiel AM, Aosaki T, Flaherty AW, Kimura M. The basal ganglia and adaptive motor control. Science (New York, N.Y. 1994;265:1826–1831. doi: 10.1126/science.8091209. [DOI] [PubMed] [Google Scholar]
- Hajnal A, Smith GP, Norgren R. Oral sucrose stimulation increases accumbens dopamine in the rat. American journal of physiology. 2004;286:R31–37. doi: 10.1152/ajpregu.00282.2003. [DOI] [PubMed] [Google Scholar]
- Hamada FN, Rosenzweig M, Kang K, Pulver SR, Ghezzi A, Jegla TJ, Garrity PA. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454:217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroi M, Marion-Poll F, Tanimura T. Differentiated response to sugars among labellar chemosensilla in Drosophila. Zoolog Sci. 2002;19:1009–1018. doi: 10.2108/zsj.19.1009. [DOI] [PubMed] [Google Scholar]
- Kong EC, Woo K, Li H, Lebestky T, Mayer N, Sniffen MR, Heberlein U, Bainton RJ, Hirsh J, Wolf FW. A pair of dopamine neurons target the D1-like dopamine receptor DopR in the central complex to promote ethanol-stimulated locomotion in Drosophila. PloS one. 2010;5:e9954. doi: 10.1371/journal.pone.0009954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, DasGupta S, Vreede A, White B, Armstrong JD, Waddell S. A neural circuit mechanism integrating motivational state with memory expression in Drosophila. Cell. 2009;139:416–427. doi: 10.1016/j.cell.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebestky T, Chang JS, Dankert H, Zelnik L, Kim YC, Han KA, Wolf FW, Perona P, Anderson DJ. Two different forms of arousal in Drosophila are oppositely regulated by the dopamine D1 receptor ortholog DopR via distinct neural circuits. Neuron. 2009;64:522–536. doi: 10.1016/j.neuron.2009.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G, Park JH. Hemolymph sugar homeostasis and starvation-induced hyperactivity affected by genetic manipulations of the adipokinetic hormone-encoding gene in Drosophila melanogaster. Genetics. 2004;167:311–323. doi: 10.1534/genetics.167.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T, Luo L. Mosaic analysis with a repressible neurotechnique cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- Leopold P, Perrimon N. Drosophila and the genetics of the internal milieu. Nature. 2007;450:186–188. doi: 10.1038/nature06286. [DOI] [PubMed] [Google Scholar]
- Mao Z, Davis RL. Eight different types of dopaminergic neurons innervate the Drosophila mushroom body neuropil: anatomical and physiological heterogeneity. Frontiers in neural circuits. 2009;3:5. doi: 10.3389/neuro.04.005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marella S, Fischler W, Kong P, Asgarian S, Rueckert E, Scott K. Imaging taste responses in the fly brain reveals a functional map of taste category and behavior. Neuron. 2006;49:285–295. doi: 10.1016/j.neuron.2005.11.037. [DOI] [PubMed] [Google Scholar]
- Masek P, Scott K. Limited taste discrimination in Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:14833–14838. doi: 10.1073/pnas.1009318107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire SE, Mao Z, Davis RL. Spatiotemporal gene expression targeting with the TARGET and gene-switch systems in Drosophila. Sci STKE. 2004;2004:l6. doi: 10.1126/stke.2202004pl6. [DOI] [PubMed] [Google Scholar]
- Melcher C, Bader R, Pankratz MJ. Amino acids, taste circuits, and feeding behavior in Drosophila: towards understanding the psychology of feeding in flies and man. The Journal of endocrinology. 2007;192:467–472. doi: 10.1677/JOE-06-0066. [DOI] [PubMed] [Google Scholar]
- Melcher C, Pankratz MJ. Candidate gustatory interneurons modulating feeding behavior in the Drosophila brain. PLoS biology. 2005;3:e305. doi: 10.1371/journal.pbio.0030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier N, Marion-Poll F, Lansky P, Rospars JP. Estimation of the individual firing frequencies of two neurons recorded with a single electrode. Chem Senses. 2003;28:671–679. doi: 10.1093/chemse/bjg059. [DOI] [PubMed] [Google Scholar]
- Nassel DR, Elekes K. Aminergic neurons in the brain of blowflies and Drosophila: dopamine- and tyrosine hydroxylase-immunoreactive neurons and their relationship with putative histaminergic neurons. Cell and tissue research. 1992;267:147–167. doi: 10.1007/BF00318701. [DOI] [PubMed] [Google Scholar]
- Neckameyer WS, Woodrome S, Holt B, Mayer A. Dopamine and senescence in Drosophila melanogaster. Neurobiology of aging. 2000;21:145–152. doi: 10.1016/s0197-4580(99)00109-8. [DOI] [PubMed] [Google Scholar]
- Nitabach MN, Wu Y, Sheeba V, Lemon WC, Strumbos J, Zelensky PK, White BH, Holmes TC. Electrical hyperexcitation of lateral ventral pacemaker neurons desynchronizes downstream circadian oscillators in the fly circadian circuit and induces multiple behavioral periods. J Neurosci. 2006;26:479–489. doi: 10.1523/JNEUROSCI.3915-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitz DA, van Swinderen B, Tononi G, Greenspan RJ. Electrophysiological correlates of rest and activity in Drosophila melanogaster. Curr Biol. 2002;12:1934–1940. doi: 10.1016/s0960-9822(02)01300-3. [DOI] [PubMed] [Google Scholar]
- Ramos EJ, Meguid MM, Campos AC, Coelho JC. Neuropeptide Y, alpha-melanocyte-stimulating hormone, and monoamines in food intake regulation. Nutrition (Burbank, Los Angeles County, Calif. 2005;21:269–279. doi: 10.1016/j.nut.2004.06.021. [DOI] [PubMed] [Google Scholar]
- Root CM, Semmelhack JL, Wong AM, Flores J, Wang JW. Propagation of olfactory information in Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:11826–11831. doi: 10.1073/pnas.0704523104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaerzel M, Monastirioti M, Scholz H, Friggi-Grelin F, Birman S, Heisenberg M. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J Neurosci. 2003;23:10495–10502. doi: 10.1523/JNEUROSCI.23-33-10495.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitaraman D, Zars M, Laferriere H, Chen YC, Sable-Smith A, Kitamoto T, Rottinghaus GE, Zars T. Serotonin is necessary for place memory in Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:5579–5584. doi: 10.1073/pnas.0710168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S, Sadegh L, Elle IC, Christensen AG, Faergeman NJ, Ashrafi K. Serotonin regulates C. elegans fat and feeding through independent molecular mechanisms. Cell metabolism. 2008;7:533–544. doi: 10.1016/j.cmet.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker RF. The organization of the chemosensory system in Drosophila melanogaster: a review. Cell Tissue. Res. 1994;275:3–26. doi: 10.1007/BF00305372. [DOI] [PubMed] [Google Scholar]
- Sugamori KS, Demchyshyn LL, McConkey F, Forte MA, Niznik HB. A primordial dopamine D1-like adenylyl cyclase-linked receptor from Drosophila melanogaster displaying poor affinity for benzazepines. FEBS letters. 1995;362:131–138. doi: 10.1016/0014-5793(95)00224-w. [DOI] [PubMed] [Google Scholar]
- Szczypka MS, Mandel RJ, Donahue BA, Snyder RO, Leff SE, Palmiter RD. Viral gene delivery selectively restores feeding and prevents lethality of dopamine-deficient mice. Neuron. 1999;22:167–178. doi: 10.1016/s0896-6273(00)80688-1. [DOI] [PubMed] [Google Scholar]
- Tempel BL, Livingstone MS, Quinn WG. Mutations in the dopa decarboxylase gene affect learning in Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 1984;81:3577–3581. doi: 10.1073/pnas.81.11.3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault ST, Singer MA, Miyazaki WY, Milash B, Dompe NA, Singh CM, Buchholz R, Demsky M, Fawcett R, Francis-Lang HL, et al. A complementary transposon tool kit for Drosophila melanogaster using P and piggyBac. Nature genetics. 2004;36:283–287. doi: 10.1038/ng1314. [DOI] [PubMed] [Google Scholar]
- Thorne N, Chromey C, Bray S, Amrein H. Taste perception and coding in Drosophila. Curr Biol. 2004;14:1065–1079. doi: 10.1016/j.cub.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Van Swinderen B, Andretic R. Dopamine in Drosophila: setting arousal thresholds in a miniature brain. Proceedings of the Royal Society B:Biology. 2011;278:906–913. doi: 10.1098/rspb.2010.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucetic Z, Reyes TM. Central dopaminergic circuitry controlling food intake and reward: implications for the regulation of obesity. Wiley interdisciplinary reviews. 2010;2:577–593. doi: 10.1002/wsbm.77. [DOI] [PubMed] [Google Scholar]
- Waddell S. Dopamine reveals neural circuit mechanisms of fly memory. Trends in neurosciences. 2010;33:457–464. doi: 10.1016/j.tins.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JW, Wong AM, Flores J, Vosshall LB, Axel R. Two-photon calcium imaging reveals an odor-evoked map of activity in the fly brain. Cell. 2003;112:271–282. doi: 10.1016/s0092-8674(03)00004-7. [DOI] [PubMed] [Google Scholar]
- Wang Z, Singhvi A, Kong P, Scott K. Taste representations in the Drosophila brain. Cell. 2004;117:981–991. doi: 10.1016/j.cell.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Turner GC, Laurent G. Transformation of olfactory representations in the Drosophila antennal lobe. Science (New York, N.Y. 2004;303:366–370. doi: 10.1126/science.1090782. [DOI] [PubMed] [Google Scholar]
- Wu Q, Wen T, Lee G, Park JH, Cai HN, Shen P. Developmental control of foraging and social behavior by the Drosophila neuropeptide Y-like system. Neuron. 2003;39:147–161. doi: 10.1016/s0896-6273(03)00396-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.