Abstract
The Stroop color-naming task is one of the most widely studied tasks involving the inhibition of a prepotent response, regarded as an executive function. Several studies have examined performance on versions of the Stroop task under conditions of acute sleep deprivation. Though these studies revealed effects on Stroop performance, the results often do not differentiate between general effects of sleep deprivation on performance and effects specifically on interference in the Stroop task. To examine the effect of prolonged wakefulness on performance on the Stroop task, we studied participants in a 40-hour “constant routine” protocol during which they remained awake in constant conditions and performed a Stroop color-naming task every two hours. We found that reaction time was slowest when the color and word did not match (incongruent), fastest when the color and word did match (congruent), and intermediate when participants named the color of the non-word stimulus (neutral). Performance on all three trial types degraded significantly as a function of time awake. Extended wakefulness did not significantly change the additional time needed respond when the color and word did not match (Stroop interference), nor did it change the amount of facilitation when color and word matched. These results indicate that one night of sleep deprivation influences performance on the Stroop task by an overall increase in response time, but does not appear to impact the underlying processes of interference or facilitation. The results suggest that the degree to which an “executive function” is affected by sleep deprivation may depend on the particular executive function studied and the degree to which it is subserved by the prefrontal cortex.
Keywords: sleep deprivation, executive function, sleep loss, constant routine, performance
1. Introduction
Sleep loss can have profound effects on human performance. In the laboratory setting, a variety of performance task have been shown to be affected by sleep deprivation [e.g., (Lim & Dinges, 2008; Harrison & Horne, 2000; Killgore et al., 2007; Drummond, Paulus, & Tapert, 2006; Durmer & Dinges, 2005)]. One widely-used task to determine the effect of sleep loss on performance is the Psychomotor Vigilance Task (PVT), a test of simple reaction time which measures the ability to sustain attention (Dinges & Powell, 1985). Results from the PVT show that performance degrades with increasing wakefulness beyond the typical 16-hour day, measured as increases in reaction time (RT) and response errors (Lim & Dinges, 2008). Though the effects of sleep loss on the PVT are robust and consistent between experiments, it is less clear how sleep loss affects higher cognitive functions.
There is substantial evidence that the prefrontal cortex is particularly sensitive to sleep loss. Electroencephalographic (EEG) studies have demonstrated that increases in EEG theta activity associated with sleep deprivation are most evident in frontal brain regions (Finelli, Baumann, Borbély, & Achermann, 2000). PET neuroimaging studies corroborate these findings, with results indicating decreased metabolism in the pre-frontal cortex (PFC) with sleep deprivation (Thomas et al., 2000). The vulnerability of the PFC to sleep loss has led to the hypothesis that tasks involving the PFC, such as tasks of executive function, should be highly sensitive to sleep loss (Harrison, Horne, & Rothwell, 2000). Though results from many studies have supported this notion (Harrison & Horne, 1999; Harrison, Horne, & Rothwell, 2000; Harrison & Horne, 1998; Horne, 1988), many others have failed to demonstrate an influence of sleep loss on executive function (Tucker, Whitney, Belenky, Hinson, & Van Dongen, 2010; Sagaspe et al., 2006; Fallone, Acebo, Arnedt, Seifer, & Carskadon, 2001). The tasks used to measure executive function have been varied. (e.g., tests of reaction time, response inhibition, divergent thinking, decision making). Thus, it is likely that elements of these tasks are subserved by different neural systems, which may be differently affected by sleep loss.
The Stroop task (Stroop, 1935) is generally regarded as a task involving executive function, because correct responses on the task require the inhibition of a prepotent response. There are several versions of the Stroop task, with most versions having two trial types. In a “neutral” trial, participants must name the color of a control stimulus (e.g. XXXX). In an “incongruent” trial, participants are required to name the color of the stimulus, which is a color word different from the stimulus (e.g. the word “green” in blue ink/font). In the incongruent trial type, participants must inhibit a prepotent response of naming the written word in order to correctly respond by naming the color of the ink/font (Stroop interference). Other versions of the Stroop task include a “congruent” trial type (including the version used in the present study) in which some trials have the stimulus color and word matching. A benefit of including congruent trials among the incongruent and neutral trials is that they will decrease the tendency of subjects to use reading suppression strategy. With only incongruent and neutral trial types, avoidance of reading the word (e.g., by not focusing gaze) could lead to an apparent decrease in interference. By having congruent trials this strategy is avoided and tends to invoke the strategy of splitting attention over reading and color-naming dimensions, increasing interference on incongruent trials (MacLeod, 1991). Functional magnetic resonance imaging (fMRI) studies of Stroop interference have demonstrated activation of the dorsolateral prefrontal cortex (DLPFC) and the anterior cingulate cortex (ACC), structures involved in conflict monitoring and resolution (Banich et al., 2000; MacDonald, III, Cohen, Stenger, & Carter, 2000; Milham et al., 2001).
Performance on the Stroop task has been reported to be affected by sleep deprivation, although in many of those studies it is not clear which aspect of Stroop performance is affected by sleep deprivation. Several studies have found slower reaction times and increased errors after sleep loss (Lingenfelser et al., 1994; McCarthy & Waters, 1997; Stenuit & Kerkhofs, 2008). Such performance decrements may not be due to the effects of sleep loss on executive function, but rather due to effects on reaction time and attention, as seen in the PVT. As such, these studies do not address the influence of sleep loss on Stroop interference. One recent study examined the effects of sleep loss on Stroop performance while distinguishing between executive and non-executive aspects of performance. Using a version of the Stroop task with incongruent and neutral trials, Sagaspe et al. (2006) found an effect of time awake on reaction time, but no interaction between time awake and trial type, indicating that sleep loss did not affect trials involving executive control (inhibition of the prepotent response in the incongruent trials) differently than the neutral trials. Though this may indicate that the cognitive process underlying interference in the Stroop task does not change with sleep loss, the study included only twelve participants, and may not have had the statistical power to detect the effects of sleep loss on Stroop interference, given inter-individual differences in performance.
In the present study, we extended the findings of Sagaspe et al. by examining performance on a Stroop task in a larger group of 30 participants across an extended wake episode in constant conditions. We included both men and women in our study and scheduled each participant at his/her own habitual sleep-wake times. We administered a computerized version of the Stroop test every 2 hours, and the test included neutral, incongruent, and congruent trial types. The inclusion of congruent trials is an important difference between the current study and that of Sagaspe et al. as versions using only an incongruent and neutral trial types promote the use of a reading suppression response, which would diminish the influence of executive function on performance. This version of the Stroop test was designed to allow us to better understand whether sleep loss-related decrements in performance on the Stroop are mainly due to slowed reaction times, or whether executive function is also affected by sleep deprivation. Using a mixed-model analysis to account for individual differences in performance, we analyzed Stroop performance across 38 hours of wakefulness, including examination of the processes of interference and facilitation in the incongruent and congruent trial types. Based on the findings of Sagaspe et al., we hypothesized that sleep deprivation would affect reaction time and error rate, but not interference in the Stroop task. Such a finding would indicate that attention and vigilance may be more sensitive to sleep loss than the executive functions involved in Stroop interference.
2. Methods
2.1 Participants
Thirty healthy participants (19 men, 11 women; mean ± SD age 23.13 ± 3.18) were studied in a 21-day laboratory protocol in the Intensive Physiological Monitoring Unit of the Center for Clinical Investigation at the Brigham and Women’s Hospital. Participants were medically and psychologically healthy as determined through pre-study screening tests and exams (medical history, physical examination, EKG, blood and urine chemistries, the Minnesota Multiphasic Personality Inventory, the Beck Depression Inventory, and an interview with a clinical psychologist). Participants were excluded if they reported behavior that could lead to circadian misalignment (regular night work within the past year, crossing more than one time zone in the previous three months), and were further instructed to maintain a regular 8-hour self-selected sleep-wake schedule for at least two weeks at home immediately prior to the study. Participants were asked to refrain from using alcohol, nicotine, prescription and nonprescription drugs, dietary supplements, and caffeine both prior to and throughout the course of the study; urinary toxicological analysis was performed to ensure that participants were drug-free upon admission to the laboratory. One of the 30 participants spoke English as a second language. This subject was fluent in English and their performance was similar to that from the other participants, so their data were included in the analysis. Each subject gave written informed consent before starting the study protocol, which was approved by the Human Research Committee of the Partners HealthCare System, and was conducted in accordance with the Declaration of Helsinki.
2.2 Protocol
Throughout the study, each participant lived in a private study room in an environment free from time cues, with social contact limited to trained laboratory personnel who were present 24 hours a day. All studies began with three 24-hour baseline days consisting of 16 hours of wakefulness and 8 hours of bed rest in the dark, scheduled at the times reported from the previous week of screening.
The three baseline days were followed by a 40 hour constant routine (CR) (Mills, Minors, & Waterhouse, 1978; Duffy & Dijk, 2002) which began upon awakening after the third baseline night. During the CR, participants remained in a semi-recumbent posture (in bed with the head of the bed elevated to approximately 45°), in dim light [approximately 0.0048 W/m2 (~1.8 lux) in the middle of the room], and were fed equicaloric snacks each hour. A staff member was always with the participant to help maintain wakefulness and to ensure that all protocol requirements were met.
Following the CR, the participants were scheduled to live on a non-24-hour sleep-wake cycle, designed to determine the period of their circadian timing system. Results from that segment of the study, which did not involve sleep deprivation, will be reported elsewhere.
2.3 Stroop Task
A computerized version of the Stroop color-naming task was used in the study. This version included three trials types: congruent, incongruent and neutral. In all cases, the word was presented against a black background. In the congruent trials, the font color and word were the same. In the incongruent trials, font color and word were different. In the neutral trials, a row of four “Xs” were used. The neutral trial was used to provide a measure of the time taken to name a color in the absence of interfering or facilitating words. For all trial types, participants were instructed to respond by naming the color (red, green, blue, or yellow) of the font by typing the first letter of the color on a keyboard (R, G, B, or Y). Each Stroop session consisted of 200 trials, with 60% incongruent, 20% congruent, and 20% neutral trials. As interference was the primary outcome of interest, we chose to have a high number of incongruent trials. Each test began with 40 neutral practice trials which were not included in the analysis. The test was given every two hours on the baseline days 2 and 3 (up to 13 tests during baseline) and throughout the CR (up to 19 tests during the CR), with the first test scheduled approximately two hours after waking.
2.4 Data Analysis
Mean reaction time and error rates for congruent, incongruent, and neutral trial types were calculated for each Stroop test. Difference scores were calculated and used as indices of interference and facilitation. For the index of interference, mean reaction time of the neutral trials was subtracted from mean reaction time of the incongruent trials within a single test session. This index reflects the additional time needed to respond when reading the word interferes with responding to the color. For the index of facilitation, mean reaction time of the congruent trials was subtracted from mean reaction time of the neutral trials within a single test session. This index reflects the increased speed of reaction when color and word matched.
Statistical analyses were performed using mixed model regression analysis (PROC MIXED; SAS 9.1; SAS Institute, Cary, NC) on raw data, incorporating into the model a random intercept statement allowing for means to vary between participants. For statistical analyses, mean reaction time (RT) was transformed (reciprocal transformation) in order to better approximate a normal distribution.
Mean RT and error rate were first assessed by testing for the main effects of TIME AWAKE (number of hours since awakening at the beginning of the constant routine; treated as a categorical rather than continuous variable) and TRIAL TYPE (congruent, incongruent, or neutral trial type). We next tested the two-way interaction between TIME AWAKE and TRIAL TYPE for each measure, and proceeded to drop all non-significant two-way interactions from further analysis. The main effect of TIME AWAKE was tested on the indices of facilitation and on interference.
Paired student’s t tests were performed for reaction time and error rates. For these tests, incongruent and neutral results were compared at each time point, as were congruent and neutral results. Due to the increased family-wise error of the 19 comparisons (for both reaction time and error rates), a Bonferroni correction was used (α = 0.0026).
3. Results
For all but four participants, all 13 Stroop tests scheduled during baseline days 2 and 3 were taken. For all but five participants, all 19 scheduled Stroop tests during the CR were taken. Most of these scheduled tests were missed due to equipment problems or scheduling issues related to other aspects of the protocol. For two participants, single tests were omitted from analysis (for 1 participant due to incorrect posture during the test, and for 1 participant due to having an error rate greater than 50% in that test; the latter criterion was determined to be the threshold for eliminating a test a priori, as it indicated possible non-compliance with the test).
For performance during the CR, there was a significant main effect of TIME AWAKE on RT (F(18, 1642) = 58.82, P < 0.0001), such that reaction time slowed with increasing time awake (Figure 1a). There was a significant main effect of TRIAL TYPE on RT (F(2, 1642) = 103.85, P < 0.0001). Incongruent trials showed the slowest RT, congruent trials showed the fastest RT, and neutral trials had intermediate RT. There was no significant interaction between the main effects of TIME AWAKE and TRIAL TYPE on RT (F(36, 1635) = 0.19, P = 1.00).
Figure 1.
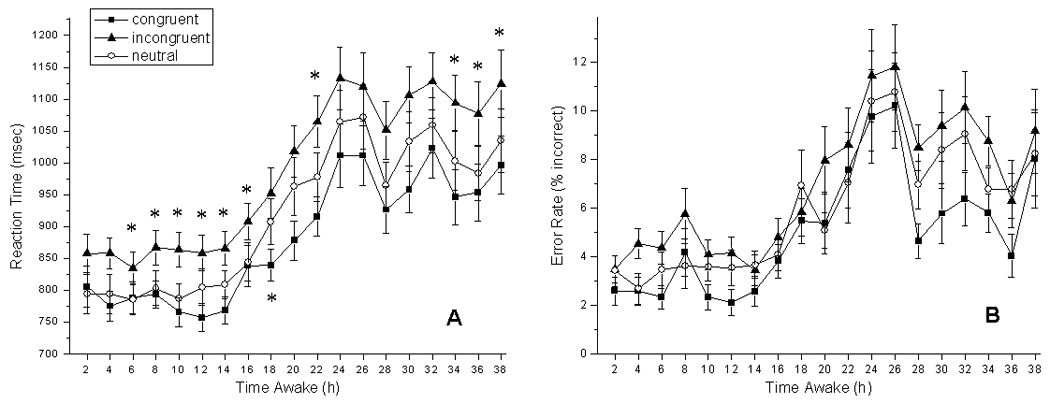
Mean reaction time (± standard error) (A) and mean error rates (B) for incongruent (triangles), congruent (squares), and neutral (circles) trial types across 38 hours awake. Asterisks indicate significant pairwise Bonferroni-corrected comparisons (α=0.0026). Asterisks above the lines indicate significant comparisons between incongruant and neutral. The asterisk below the lines indicates a significant comparison between congruent and neutral.
There was a significant main effect of TIME AWAKE on the error rate of responses (F(18, 1642) = 20.31, P < 0.0001), with more errors with increasing time awake (Figure 1b). There was a significant main effect of TRIAL TYPE on error rate (F(2, 1642) = 19.90, P < 0.0001). Participants made the most errors in the incongruent trials, fewest errors in the congruent trials, and an intermediate number of errors in the neutral trials (Figure 1b). There was no significant interaction between the main effects of TIME AWAKE and TRIAL TYPE on the error rate of responses (F(36, 1635) = 0.37, P = 0.99).
There was no significant main effect of TIME AWAKE on indexes of interference (F(18, 516) = 0.76, P = 0.75) or facilitation (F(18, 516) = 1.17, P = 0.28; Figure 2).
Figure 2.
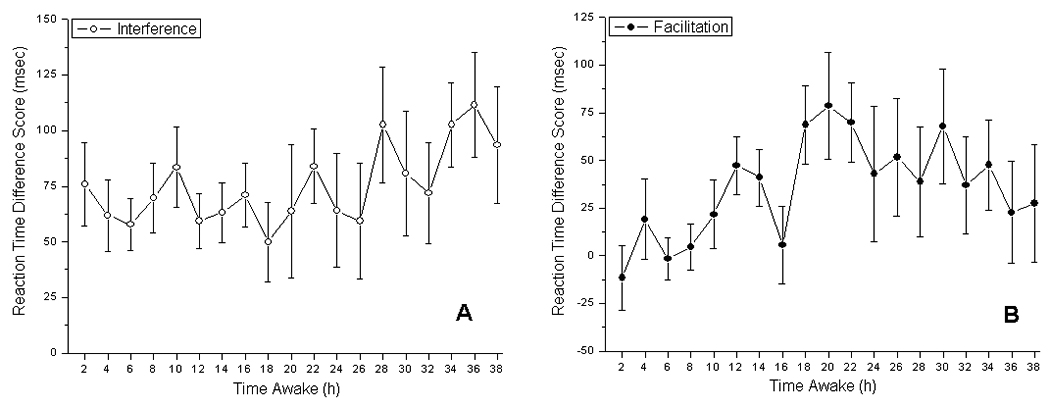
Performance on indexes of interference (A) and facilitation (B) across 38 hours awake. Left panel: performance on the index of interference, calculated as the mean difference score of neutral and incongruent trial types. Right panel: performance on the index of facilitation, calculated as the mean difference score for neutral and congruent trial types. Error bars indicate standard error.
For the multiple paired Student’s t tests performed on reaction time and error rates, the Bonferroni-corrected α value was p=0.0026. For reaction time, significant differences between incongruent and neutral trials were observed at 6 (p=0.0025), 8 (p=0.0003), 12 (p=0.0008), 14 (p=0.0004), 16 (p=0.0007), 22 (p=0.000006), 34 (p=0.00005), 36 (p=0.0005), and 38 (p=0.0021) hours awake. For reaction time, significant differences between congruent and neutral trials were observed at 18 hours awake (p=0.0025). For error rates, no significant differences between incongruent and neutral trials, or congruent and neutral trials were seen at any time point with the Bonferroni-corrected α value.
To determine learning effects of baseline trials on interference and facilitation, we examined performance across the 13 baseline trials. For the baseline data, there was no significant main effect of TRIAL (trials 1–13) on facilitation (F(12, 341) = 1.0, P = 0.45), or interference (F(12, 341) = 1.73, P = 0.06). We also examined both interference and facilitation by study day (baseline day 2 vs. 3) by averaging performance across each day for each participant. There was no significant main effect of baseline DAY on facilitation (F(1, 352) = 3.3, P = 0.07), but there was a significant effect of baseline DAY on interference (F(1, 352) = 9.16, P < 0.003), with less interference on day 3 than day 2, indicating that subjects had learned to decrease interference with practice.
4. Discussion
Consistent with standard Stroop effects (MacLeod, 1991), we found that reaction times were slowest for incongruent trials, fastest for congruent trials, and intermediate for neutral trials. This same pattern was also seen for the error rates for each trial type. Participants made the most errors in the incongruent trials, fewest errors in the congruent trials, with errors being intermediate in the neutral trials. Though the number of “errors” were fewest in the congruent trials, errors resulting from responding to the word rather than the color in congruent trials are not possible to detect, and would be registered as correct, even though the participant made a response error.
Performance on all three trial types was stable across the first 14–16 hours of the CR, corresponding to the usual waking hours for the participants. Following that, both reaction time and error rates showed increases over the next 10–12 hours (corresponding to the usual sleep time of the participants), suggesting no apparent speed/accuracy tradeoff. Reaction time on all three trial types stabilized after about 24 hours awake, remaining at a similar level and showing no further significant decline through the remainder of the 38-hour test session. This time (from 24–38 hours awake) corresponds to the usual waking hours of the participants. The pattern of performance rebound in the morning after a night awake is similar to what has been observed in many other types of cognitive performance, reflecting an underlying circadian rhythm in sleep-wake propensity (Blatter & Cajochen, 2007). The error rates on all three trial types not only stabilized after 24 hours awake, but rebounded slightly between hours 28 and 36. Overall, the participants made the most errors at the time of day when they were also slowest, so increases in reaction time do not appear to have been due to attempts to maintain accuracy (speed/accuracy tradeoff).
One concern about repeating a Stroop task multiple times is that participants may develop a “reading supression response” (Dulaney & Rogers, 1994). Had the participants adopted such a strategy, we would expect that reaction times in the incongruent and congruent trials would approach the reaction time of the neutral trials. Instead, we found that reaction time in both the incongruent and congruent trials continued to differ from the neutral trials, indicating that, in general, these participants did not adopt such a strategy. Performance on the Stroop task, therefore, continued to require the inhibition of responding to the word throughout the extended wake episode.
Under constant environmental and behavioral conditions, we found that performance on the Stroop task was negatively affected by sleep deprivation. After approximately 16 hours of wakefulness, reaction times for all trial types increased, and did so in a similar manner. The index of interference that we used (difference between neutral and incongruent trials) was not significantly affected by sleep deprivation. Consistent with prior reports (Sagaspe et al., 2006), response interference, indexed by the extra time needed to inhibit the prepotent response of responding to the word, was unaffected by one night of sleep loss. This executive function, therefore, did not appear to be very sensitive to one night of sleep loss. Similarly, the index of facilitation that we used did not show a significant influence of one night of sleep loss. Though these results suggest that the processes underlying interference and facilitation in this task are insensitive to sleep deprivation, it may be that one night of sleep loss is not sufficient to affect these processes. It should also be noted that the participants in these studies were young, healthy, and fairly well-rested prior to the sleep deprivation. It is possible that individuals with disrupted sleep, or those who chronically restrict their sleep (like a majority of adults (National Sleep Foundation, 2005) may show greater interference effects from one night of sleep loss. Future studies with longer episodes of sleep deprivation, or in individuals who have more typical sleep durations prior to study may reveal an influence of sleep loss on interference and/or facilitation.
Our finding that there was little or no effect of one night of sleep loss on an executive function is consistent with some studies (Sagaspe, Charles, Taillard, Bioulac, & Philip, 2003; Binks, Waters, & Hurry, 1999), although inconsistent with others (Venkatraman, Chuah, Huettel, & Chee, 2007; Drummond, Paulus, & Tapert, 2006). Recently, Tucker et al. (2010) posited that inconsistent findings on the effects of sleep deprivation on executive function may arrise from “task impurity” problems. Though a particular task may involve an executive function, there are typically non-executive components which also influence performance. Sleep loss may affect overall performance on the task via either one or more of these components. Using a modified Sternberg Task, Tucker et al. (2010) demonstrated that 51 hours of sleep deprivation did not affect executive components of the task (working memory scanning efficiency and resistance to proactive interference), but did affect the non-executive component (reaction time intercept). Their results are similar to our present findings for the Stroop task. We found a strong effect of time awake on reaction time, but not on interference. Together, these findings challenge the notion that executive functions, in general, are especially sensitive to sleep deprivation.
The idea that executive function tasks are particularly sensitive to sleep deprivation stems from the finding that the frontal lobe is highly responsive to sleep loss. Though the term “executive function” is often used synonymously with “frontal lobe function”, the neural systems underlying executive functions are not localized to the PFC, but are distributed (Andres, 2003). Neuroimaging studies have shown that several cortical regions are activated during performance of the Stroop task (Andres, 2003). Much of the focus of neuroimaging studies during the Stroop task has been on activation of the dorsolateral prefrontal cortex (DLPFC) and anterior cingulate cortex (ACC) (MacLeod & MacDonald, 2000). These brain regions have been associated with different aspects of the task demands. While the DLPFC appears to be involved in maintaining attentional set toward task-relevant information (ink color identification) (MacDonald, III, Cohen, Stenger, & Carter, 2000; Banich et al., 2000), the ACC appears to be involved in conflict resolution when there are competing responses (responding to the color or word) (MacDonald, III, Cohen, Stenger, & Carter, 2000; Carter et al., 2000). The present results may reflect a differential effect of sleep deprivation on these different functions. We did not find an effect of sleep loss on interference, suggesting that the ACC may not be negatively affected in responding to conflict resolution on the Stroop task by this level of sleep loss. Supporting this notion, the activity of the ACC has been found to increase following sleep deprivation in a variety of tasks (Drummond, Brown, Salamat, & Gillin, 2004; Drummond & Brown, 2001; Habeck et al., 2004). These findings exemplify the problem in equating executive tasks with the PFC. It may be that executive tasks can be sensitive to sleep loss, but it is likely to be only when the executive component is subserved by the PFC.
4.1 Limitations and conclusion
One night of sleep loss slows down reaction time and increases error in Stroop performance, but appears to have no significant impact on Stroop interference or facilitation in healthy, young, well-rested individuals. Two limitations of this study may have contributed to these findings. First, the level of sleep loss we tested (one night of lost sleep) may not be sufficient to affect intereference and facilitation. Second, subjects in our study performed up to 13 Stroop tests prior to beginning the sleep loss portion of the study. We found a significant effect of study day (baseline day 2 vs. 3) on the index of intereference. By the third baseline day, interference reached a level that was similar to that seen at the beginning of the CR and similar to practice effects seen in another study (MacLeod, 1998), indicating that subjects were well-practiced. Additional studies are needed to determine if longer amounts of acute sleep deprivation affect Stroop interference and facilitation. Further, studies with less practice prior to sleep deprivation may reveal that Stroop interference and facilitation are more sensitive to sleep loss than we found in the present study.
Acknowledgements
We thank the study participants; A. O’Malley and D. Klements for coordinating the participant recruitment and screening; the technical, dietary, and nursing staff of the Center for Clinical Investigation at BWH where the studies were conducted; C. Dennison and the technical staff of the Chronobiology Core for monitoring the participants throughout the constant routines; Dr. W. Wang for statistical advice; Dr. K.P. Wright, Jr. for his efforts to implement the Stroop task in our laboratory; and Dr. C.A. Czeisler for overall support. This study was supported by a grant from the National Heart Lung and Blood Institute (R01 HL080978) awarded to JFD, and was conducted in the Brigham and Women’s Hospital Center for Clinical Investigation (part of the Harvard Clinical and Translational Science Center) supported by M01 RR02635 and RR025758. SWC was supported in part by a fellowship from the Natural Sciences and Engineering Research Council of Canada. Additional support for the analyses was provided by NIH grant P01 AG09975. This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (NIH Award #UL1 RR 025758 and financial contributions from Harvard University and its affiliated academic health care centers). The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, the National Center for Research Resources, or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Andres P. Frontal cortex as the central executive of working memory: time to revise our view. Cortex. 2003;39:871–895. doi: 10.1016/s0010-9452(08)70868-2. [DOI] [PubMed] [Google Scholar]
- Banich MT, Milham MP, Atchley R, Cohen NJ, Webb A, Wszalek T, Kramer AF, Liang ZP, Wright A, Shenker J, Magin R. fMri studies of Stroop tasks reveal unique roles of anterior and posterior brain systems in attentional selection. Journal of Cognitive Neuroscience. 2000;12:988–1000. doi: 10.1162/08989290051137521. [DOI] [PubMed] [Google Scholar]
- Binks PG, Waters WF, Hurry M. Short-term total sleep deprivations does not selectively impair higher cortical functioning. Sleep. 1999;22:328–334. doi: 10.1093/sleep/22.3.328. [DOI] [PubMed] [Google Scholar]
- Blatter K, Cajochen C. Circadian rhythms in cognitive performance: methodological constraints, protocols, theoretical underpinnings. Physiol Behav. 2007;90:196–208. doi: 10.1016/j.physbeh.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Carter CS, Macdonald AM, Botvinick M, Ross LL, Stenger VA, Noll D, Cohen JD. Parsing executive processes: strategic vs. evaluative functions of the anterior cingulate cortex. Proc Natl Acad Sci U S A. 2000;97:1944–1948. doi: 10.1073/pnas.97.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinges DF, Powell JW. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behavior Research Methods, Instruments & Computers. 1985;17:652–655. [Google Scholar]
- Drummond SP, Brown GG, Salamat JS, Gillin JC. Increasing task difficulty facilitates the cerebral compensatory response to total sleep deprivation. Sleep. 2004;27:445–451. [PubMed] [Google Scholar]
- Drummond SPA, Brown GG. The effects of total sleep deprivation on cerebral responses to cognitive performance. Neuropsychopharmacology. 2001;25:s68–s73. doi: 10.1016/S0893-133X(01)00325-6. [DOI] [PubMed] [Google Scholar]
- Drummond SPA, Paulus MP, Tapert SF. Effects of two nights sleep deprivation and two nights recovery sleep on response inhibition. Journal of Sleep Research. 2006;15:261–265. doi: 10.1111/j.1365-2869.2006.00535.x. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Dijk DJ. Getting through to circadian oscillators: why use constant routines? Journal of Biological Rhythms. 2002;17:4–13. doi: 10.1177/074873002129002294. [DOI] [PubMed] [Google Scholar]
- Dulaney CL, Rogers WA. Mechanisms underlying reduction in Stroop interference with practice for young and old adults. J Exp Psychol Learn.Mem.Cogn. 1994;20:470–484. doi: 10.1037//0278-7393.20.2.470. [DOI] [PubMed] [Google Scholar]
- Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin.Neurol. 2005;25:117–129. doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
- Fallone G, Acebo C, Arnedt JT, Seifer R, Carskadon MA. Effects of acute sleep restriction on behavior, sustained attention, and response inhibition in children. Percept Mot Skills. 2001;93:213–229. doi: 10.2466/pms.2001.93.1.213. [DOI] [PubMed] [Google Scholar]
- Finelli LA, Baumann H, Borbély AA, Achermann P. Dual electroencephalogram markers of human sleep homeostasis: correlation between theta activity in waking and slow-wave activity in sleep. Neuroscience. 2000;101:523–529. doi: 10.1016/s0306-4522(00)00409-7. [DOI] [PubMed] [Google Scholar]
- Habeck C, Rakitin BC, Moeller J, Scarmeas N, Zarahn E, Brown T, Stern Y. An event-related fMRI study of the neurobehavioral impact of sleep deprivation on performance of a delayed-match-to-sample task. Brain Res Cogn Brain Res. 2004;18:306–321. doi: 10.1016/j.cogbrainres.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Harrison Y, Horne JA. Sleep loss impairs short and novel language tasks having a prefrontal focus. Journal of Sleep Research. 1998;7:95–100. doi: 10.1046/j.1365-2869.1998.00104.x. [DOI] [PubMed] [Google Scholar]
- Harrison Y, Horne JA. One Night of Sleep Loss Impairs Innovative Thinking and Flexible Decision Making. Organ Behav.Hum.Decis.Process. 1999;78:128–145. doi: 10.1006/obhd.1999.2827. [DOI] [PubMed] [Google Scholar]
- Harrison Y, Horne JA. The impact of sleep deprivation on decision making: A review. Journal of Experimental Psychology. 2000;6:236–249. doi: 10.1037//1076-898x.6.3.236. [DOI] [PubMed] [Google Scholar]
- Harrison Y, Horne JA, Rothwell A. Prefrontal neuropsychological effects of sleep deprivation in young adults- a model for healthy aging? Sleep. 2000;23:1067–1073. [PubMed] [Google Scholar]
- Horne JA. Sleep loss and "divergent" thinking ability. Sleep. 1988;11:528–536. doi: 10.1093/sleep/11.6.528. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Killgore DB, Day LM, Li C, Kamimori GH, Balkin TJ. The effects of 53 hours of sleep deprivation on moral judgment. Sleep. 2007;30:345–352. doi: 10.1093/sleep/30.3.345. [DOI] [PubMed] [Google Scholar]
- Lim J, Dinges DF. Sleep deprivation and vigilant attention. Ann N Y Acad Sci. 2008;1129:305–322. doi: 10.1196/annals.1417.002. [DOI] [PubMed] [Google Scholar]
- Lingenfelser T, Kaschel R, Weber A, Zaiser-Kaschel H, Jakober B, Kuper J. Young hospital doctors after night duty: Their task-specific cognitive status and emotional condition. Medical Education. 1994;28:566–572. doi: 10.1111/j.1365-2923.1994.tb02737.x. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, III, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- MacLeod CM. Half a century of research on the Stroop effect: an integrative review. Psychological Bulletin. 1991;109:163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- MacLeod CM. Training on integrated versus separated Stroop tasks: the progression of interference and facilitation. Memory and Cognition. 1998;26:201–211. doi: 10.3758/bf03201133. [DOI] [PubMed] [Google Scholar]
- MacLeod CM, MacDonald PA. Interdimensional interference in the Stroop effect: uncovering the cognitive and neural anatomy of attention. Trends Cogn Sci. 2000;4:383–391. doi: 10.1016/s1364-6613(00)01530-8. [DOI] [PubMed] [Google Scholar]
- McCarthy ME, Waters WF. Decreased attentional responsivity during sleep deprivation: Orienting response latency, amplitude, and habituation. Sleep. 1997;20:115–123. doi: 10.1093/sleep/20.2.115. [DOI] [PubMed] [Google Scholar]
- Milham MP, Banich MT, Webb A, Barad V, Cohen NJ, Wszalek T, Kramer AF. The relative involvement of anterior cingulate and prefrontal cortex in attentional control depends on nature of conflict. Brain Res Cogn Brain Res. 2001;12:467–473. doi: 10.1016/s0926-6410(01)00076-3. [DOI] [PubMed] [Google Scholar]
- Mills JN, Minors DS, Waterhouse JM. Adaptation to abrupt time shifts of the oscillator[s] controlling human circadian rhythms. Journal of Physiology (London) 1978;285:455–470. doi: 10.1113/jphysiol.1978.sp012582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Sleep Foundation. Executive summary of the 2005 "Sleep in America" poll. 2005 Ref Type: Report.
- Sagaspe P, Charles A, Taillard J, Bioulac B, Philip P. [Inhibition and working memory: effect of acute sleep deprivation on a random letter generation task] Can J Exp Psychol. 2003;57:265–273. [PubMed] [Google Scholar]
- Sagaspe P, Sanchez-Ortuno M, Charles A, Taillard J, Valtat C, Bioulac B, Philip P. Effects of sleep deprivation on Color-Word, Emotional, and Specific Stroop interference and on self-reported anxiety. Brain Cogn. 2006;60:76–87. doi: 10.1016/j.bandc.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Stenuit P, Kerkhofs M. Effects of sleep restriction on cognition in women. Biol Psychol. 2008;77:81–88. doi: 10.1016/j.biopsycho.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychology. 1935;18:643–662. [Google Scholar]
- Thomas M, Sing H, Belenky G, Holcomb H, Mayberg H, Dannals R, Wagner H, Thorne D, Popp K, Rowland L, Welsh A, Balwinski S, Redmond D. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. Journal of Sleep Research. 2000;9:335–352. doi: 10.1046/j.1365-2869.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- Tucker AM, Whitney P, Belenky G, Hinson JM, Van Dongen HPA. Effects of sleep deprivation on dissociated components of executive functioning. Sleep. 2010;33:47–57. doi: 10.1093/sleep/33.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatraman V, Chuah YM, Huettel SA, Chee MW. Sleep deprivation elevates expectation of gains and attenuates response to losses following risky decisions. Sleep. 2007;30:603–609. doi: 10.1093/sleep/30.5.603. [DOI] [PubMed] [Google Scholar]


