Abstract
Objective
The impact of the atypical antipsychotics, olanzapine, quetiapine and risperidone on cognition in patients with Alzheimer’s disease is unclear. This report describes the effects of time and treatment on neuropsychological function during the Clinical Antipsychotic Trials of Intervention Effectiveness Alzheimer’s disease study (CATIE-AD).
Method
CATIE-AD included 421 Alzheimer’s disease outpatients with psychosis or agitated/aggressive behavior, randomized to masked, flexible-dose olanzapine, quetiapine, risperidone or placebo. Based on clinician’s judgment, patients could discontinue originally assigned medication and be randomized to another medication. They were followed for 36 weeks. Cognitive assessments were obtained at baseline, 12 weeks, 24 weeks and 36 weeks. Outcomes were compared among 357 patients with baseline and at least one follow-up cognitive measure obtained while on their prescribed medication or placebo for at least 2 weeks before cognitive testing.
Results
Overall, patients showed steady, significant declines over time in most cognitive areas, including Mini-mental State Examination (2.4 points over 36 weeks) and Alzheimer’s Disease Assessment Scale-cog (4.4 points). Patients on antipsychotics declined more than patients on placebo on multiple cognitive measures, including the MMSE (p=0.004), BPRS cognitive subscale (p=0.05), and a cognitive summary score summarizing change on 18 cognitive tests (p=0.004).
Conclusions
In CATIE-AD atypical antipsychotics were associated with worsening cognitive function at a magnitude consistent with one year’s deterioration compared with placebo. Further cognitive impairment is an additional risk of atypical antipsychotic treatment for Alzheimer’s disease patients that should be considered when considering treatment.
Introduction
Psychiatric and behavioral symptoms are common in patients with Alzheimer’s disease and contribute substantially to the morbidity of the illness (1–3). Delusions or hallucinations appear in 30–50% of patients with Alzheimer’s disease, and as many as 70% demonstrate agitated or aggressive behaviors. These symptoms contribute to patient and caregiver distress (4, 5), can compromise safety or promote institutionalization (6, 7).
Medications from several pharmacological classes have been used to treat psychosis and behavioral disturbances in Alzheimer’s disease. The majority of randomized, controlled trials examined the efficacy of atypical antipsychotic medications over 6 to 12 weeks. Some studies included outpatients, but most included patients with advanced Alzheimer’s disease residing in long-term care facilities. Several trials reported modest efficacy on behavior symptoms with individual atypical antipsychotic medications compared to placebo (8). However, efficacy is not seen in all trials or for all symptoms, and adverse events can occur, including further cognitive impairment.(8).
The effects of antipsychotic medication on cognition have largely been gleaned from studies of patients with schizophrenia. In that patient population, early optimism that second generation antipsychotic medications improved cognition (9, 10) was not confirmed by studies with designs that included randomized double-blind treatment conditions, acceptable dosing strategies (11), and consideration of practice effects (12).
The impact of these medications on cognition in Alzheimer’s disease is less certain. Clinical trials generally did not assess cognition beyond the use of the Mini-mental State Examination (8, 13).In the only two trials that report results there was an overall worsening on the Mini-mental State Examination with atypical antipsychotics compared to placebo of about 0.73 points over the 10 to 12 week lengths of the trials (8), and a worsening of about 4 points on the Alzheimer’s Disease Assessment Scale with olanzapine over 26 weeks in a trial of patients without behavioral problems (14).
The National Institute of Mental Health Clinical Antipsychotic Trials of Intervention Effectiveness – Alzheimer’s Disease (CATIE-AD) study was designed to compare the effectiveness of antipsychotics and placebo in patients with Alzheimer’s disease and psychosis or agitated/aggressive behavior (15), and by design included measures with which to investigate the cognitive effects of these medications. In contrast to many efficacy trials, CATIE-AD included outpatients in usual care settings, and assessed treatment effectiveness with a variety of outcomes over a nine-month intervention period. Initial CATIE-AD treatment (olanzapine, quetiapine, risperidone, or placebo) was randomized and double-blinded, yet the protocol allowed medication dose adjustments or switch to a different treatment based on the clinician’s judgment. The primary CATIE-AD outcome was the Phase 1 time to discontinuation of the initially-assigned medication for any reason (16), intended as an overall measure of effectiveness that incorporated the judgments of patients, caregivers, and clinicians reflecting therapeutic benefits in relation to undesirable effects.
This report describes the effects of time and of treatment on neuropsychological measures during the trial.
Methods
CATIE-AD study design
The rationale and design of CATIE-AD have been described (15, 16). Briefly, the 36-week study period occurred in up to 4 possible phases for each patient. Phase 1 began at baseline when 421 patients were randomized in a double-blind fashion to receive olanzapine, quetiapine, risperidone or placebo (randomized allocation 2:2:2:3). If discontinued from phase 1 medication, then the patient could enter phase 2 or open treatment (phase 4). In phase 2, if the patient had originally been assigned to an atypical antipsychotic, he/she was randomized in double-blind fashion to one of the other atypical antipsychotics or to the antidepressant citalopram (randomization allocation 3:3:2). If he/she had originally been assigned to placebo, he or she would be randomized to citalopram or an atypical antipsychotic (randomized allocation 3:1:1:1). Upon discontinuation of phase 2 medication, the patient could enter phase 3 and be randomly assigned to open label treatment with an atypical antipsychotic medication not previously assigned. At any time, the clinician could choose to enter the patient into phase 4, where data collection continued, but the physician prescribed medication.
Inclusion criteria
To participate in the trial, patients met the following criteria: DSM-IV criteria for dementia of the Alzheimer’s type (17) or NINCDS-ADRDA criteria for probable Alzheimer’s disease (18); ambulatory outpatients living at home or in an assisted-living facility; Mini-Mental State Examination (19) score of 5 to 26; delusions, hallucinations, agitation, or aggression must have occurred nearly every day over the previous week or intermittently over 4 weeks; symptom ratings were at least moderate in severity on the Brief Psychiatric Rating Scale conceptual disorganization, suspiciousness, or hallucinatory behavior item (20), or occurred at least weekly with moderate severity or greater on the delusion, hallucination, agitation, or aberrant motor behavior item of the Neuropsychiatric Inventory (21).
Patients could be taking cholinesterase inhibitor medication and were excluded if they were taking antidepressants or anticonvulsants for mood stabilization. The study was reviewed and approved, and the informed consent was documented and approved by the Institutional Review Board (IRB) at each of the 42 sites.
Current study
The current study assessed the weekly rate of change and the total change over 36 weeks in several measures of cognitive function. All trial participants who did not report sedation at baseline, who had data available for years of education (a model covariate) and who had baseline and at least one follow-up measure of cognitive function were included. Eight patients reported sedation at the 12-week visit, and their scores for that visit were excluded from these analyses. Changes in cognitive function were assessed for the total group and for subgroups defined by randomized medication.
Cognitive assessments
The following cognitive measures were collected at baseline, 12 weeks, 24 weeks and 36 weeks: Mini Mental State Examination (19); the Alzheimer’s Disease Assessment Scale (ADAS-Cog) (22): three additional subscales to the ADAS-Cog (23), Concentration/Distractibility, Number Cancellation, and Executive Function (mazes); Category Instances (semantic fluency, animal category) (24); Finger Tapping, preferred and non-preferred hand (25); Trails A (26): and the Working Memory Deficit determined by the difference in the 10-second delay and the no-delay Dot Tests(27).
Cognitive summary score
A two-step process was used to calculate the cognitive summary. First, the normalized z-scores for each of the component measures (after adjusting so that higher scores on each component test indicated higher function)were averaged. These averaged scores were then normalized. Z-scores were computed using baseline means and standard deviations for each component score among all subjects included in these analyses. The components of the summary score were: the 11 components of the ADAS-Cog, the three additional subscales to the ADAS-Cog (Concentration/Distractibility, Number Cancellation, and Executive Function), Category Instances, the mean of the scores for the preferred and the non-preferred hand on the Finger Tapping Test, Trails A, and the Working Memory Deficit. If a patient was missing more than four component scores, the cognitive summary was considered missing.
In addition, Clinical Global Impression of Change (CGIC) scores were collected. The CGIC is a seven-point scale of the clinician’s assessment of the patient’s change in mental status since study baseline, ranging from a sore of 1 indicating “very much improved,” to a score of 7 indicating “very much worse,” with 4 indicating no change (16). A physician-rated, cognitive dysfunction factor of the Brief Psychiatric Rating Scale (BPRS)(28)consisting of the conceptual disorganization and disorientation items was calculated, but was not included as part of the cognitive summary score.
Statistical Analysis
Mean cognitive scores at baseline were compared by categories of age, gender, years of education and pooled study site using t-tests or ANOVA as appropriate. The 7 sites with 18 or more patients were not pooled; the 35 sites with fewer than 18 patients were pooled according to a pre-determined algorithm into 8 pooled sites (16).
To accommodate longitudinal measures (multiple observations per patient over time) and the inherent within-patient correlations, mixed effects linear regression models were used. These models assessed the rate of change (slope) in cognition over the trial period for each of the cognitive measures and the cognitive summary score, adjusting for age, gender, education and pooled study site. Random effects were specified for the intercept and slope (time on-study in weeks).
In the first set of analyses study treatment was not considered. The dependent variable was the cognitive function score and the independent variables were the covariates and time in weeks since baseline. The regression coefficient for the time variable estimated the average weekly rate of change in the cognitive measure.
Further analyses assessed effect modification on weekly rate of change in cognition by baseline level of MMSE score (<19 or ≥19, more severe vs. mild impairment), baseline BPRS total score (≤27 or >27, median split on behavior severity), and study site size (<18 patients (pooled sites) or ≥18 patients (stand-alone sites)). Each cognitive measure was modeled as a function of the covariates, time (weeks) since baseline and an interaction term of time since baseline by baseline MMSE group, BPRS group, or study site size group. The interaction term tested if the rate of change (slope) in the cognitive score differed by baseline MMSE, BPRS or study site size.
The second set of mixed effects analyses assessed the effect of each treatment on the rate of change in cognitive function. Treatment was included, provided that the patient had been assigned to the treatment (olanzapine, quetiapine, risperidone or placebo) for at least 2 weeks immediately prior to the date of cognitive assessment. Follow-up cognitive assessments on dates when the subject was in the open-choice phase (phase 4) or had been on their study medication for less than two weeks were not included in these analyses. Separate models were fit for each cognitive variable. The independent variables included the covariates, treatment assignment, and number of weeks since baseline. An additional interaction term of time since baseline by treatment tested whether the rate of cognitive change differed among patients on a specific study medication compared to placebo patients.
The third set of mixed effects analyses was similar to the second except that all atypical antipsychotics were combined for comparison with placebo. This set includes more cognitive testing dates than the second set of analyses because a patient on a combination of atypical antipsychotic medications during the two weeks prior to cognitive testing would be included here, but excluded from the second set of models. In addition, we used the model estimates of weekly rates of change over the trial to estimate the change in cognitive function over the full, 36-week study duration by study group. Since the statistical tests are tests of slope over the full study period, whether changes are expressed per week, or over 36 weeks, makes no difference on the statistical significance.
Generalized estimating equations were used to estimate the average CGIC scores by treatment group, and test for differences from placebo, for patients on study medication for at least 2 weeks prior to cognitive testing.
All data were analyzed using SAS System for Windows, Version 9.1 (SAS Institute, Cary, NC). P-values are 2-sided.
Results
All 421 randomized patients had at least one of the cognitive measures at baseline. One reported sedation at baseline and 16 patients did not report years of education and were excluded from the analyses. In addition, 47 patients had no follow-up cognitive measures, leaving 357, 342 of whom had at least one follow-up cognitive measure at 12 weeks, 320 had at least one follow-up measure at 24 weeks, and 307 had at least one follow-up measure at 36 weeks. The study sample was 46% male, with mean age 77.6 years and mean education 12.3 years (Table 1); and 64% was taking cholinesterase inhibitors.
Table 1.
Baseline values for demographic, cognitive, and functional variables.
| N | Mean | SD | Minimum | Maximum | |
|---|---|---|---|---|---|
| Demographic and clinical variables | |||||
| Age | 357 | 77.6 | 7.4 | 51 | 103 |
| Education (years) | 357 | 12.3 | 3.4 | 2 | 21 |
| Male | 163 (46%) | ||||
| Cholinesterase inhibitor drug use | 249 (64%) | ||||
| BPRS total score | 355 | 27.5 | 12.1 | 4 | 66 |
| Neuropsychiatric Rating Scale | 354 | 36.6 | 18.2 | 3 | 104 |
| ADCS-ADL scale score | 353 | 39.7 | 16.7 | 6 | 76 |
| Cognitive variables | |||||
| MMSE Score | 355 | 15.2 | 5.7 | 4 | 28 |
| BPRS cognitive factor | 355 | 5.3 | 2.4 | 0 | 12 |
| ADAS-Cog | 328 | 34.4 | 13.3 | 8 | 67 |
| ADAS concention/distraction | 333 | 1.8 | 1.5 | 0 | 5 |
| ADAS number cancellation | 306 | 10.5 | 7.9 | 0 | 38 |
| ADAS executive function (maze) | 291 | 68.1 | 84.2 | 3 | 240 |
| Category Instances | 317 | 6.4 | 4.2 | 0 | 21 |
| Finger Tapping preferred hand | 290 | 28.9 | 14.5 | 0 | 75 |
| Finger Tapping non-preferred hand | 289 | 26.8 | 13.4 | 0 | 75 |
| Trails A (time, seconds) | 245 | 111.5 | 97.1 | 20 | 300 |
| Working memory deficita | 72 | 1.0 | 0.9 | 0 | 3.0 |
| Cognitive summaryb | 301 | 0 | 1.0 | −2.7 | 1.8 |
The working memory deficit is the difference between the 10-second-delay score and the no-delay score (deficit scores were considered to be missing in patients with no-delay scores above 4.0).
The cognitive summary was the normalized average of the sign-adjusted, normalized, baseline z-scores for each of the 11 components of the ADAS-Cog, ADAS Concentration/Distractibility, ADAS Number Cancellation, ADAS Executive Function, Category Instances, the mean of the scores for the preferred and the non-preferred hand on the Finger Tapping Test, Trails A, and the working memory deficit.
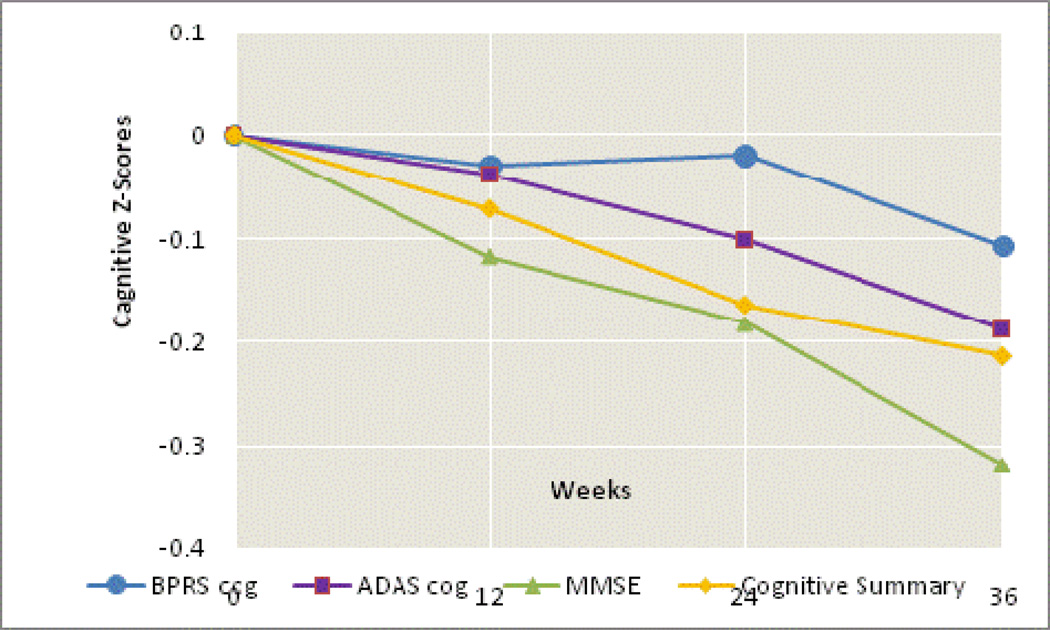
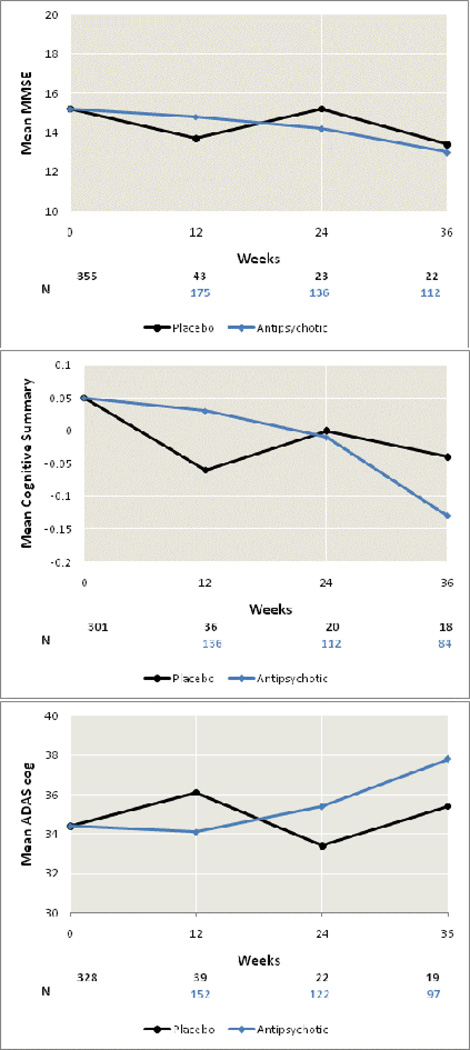
Over the 36-week follow-up period, the study sample significantly declined on several measures of cognitive function (MMSE, ADAS-Cog, ADAS Concentration/Distractibility, ADAS Number Cancellation, Category Instances, both finger tapping tests, Trails A, and the cognitive summary), and on the BPRS cognitive factor(Table 2). The models in Table 2 can be used to predict test scores changes for a patient with specified covariate values in this sample. For a male age 77.6 years (the sample mean), with 12.3 years of education (the sample mean) in the study site that pooled all of the sites with 5 or fewer patients, the model-estimated declines over 36 weeks were as follows: the MMSE score from 15.6 to 13.2, the ADAS-Cog score worsened from 34.2 to 38.6, the cognitive summary decreased from −0.06 to −0.46, and the BPRS cognitive factor score worsened from 4.6 to 5.0.Figure 1 includes both patients randomized to atypical antipsychotics and placebo and shows that the declines in z-scores for these tests over the 36-week study period are linear. This figure also shows that the normalized change in scores over time is more pronounced for the ADAS-cog, the MMSE and the cognitive summary than in the BPRS-Cog which is more behaviorally related.Figure 2 shows the changes in raw MMSE, ADAS-Cog, and the cognitive summary scores over time for the full study population.
Table 2.
Weekly rates of change in cognitive function in total sample and by treatment group
|
Weekly Rate of Change in Total Sample |
Weekly Rate of Change in Placebo |
Mean Difference from Placebo in Change per Week among Patients on the Same Medication or Placebo for at Least 2 Weeks prior to Cognitive Testingh |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Olanzapine vs. Placebo |
Quetiapine vs. Placebo |
Risperidone vs. Placebo |
|||||||||||
| Cognitive variables | Favorable direction a |
Change b | p-value c | DF g | Change b | p-value c | DF g | Change d | p-value e | Change d | p-value e | Change d | p-value e |
| MMSE Score | ↑ | −.067 | <0.001 | 343 | −.007 | .81 | 104 | −.080 | 0.05 | −.045 | 0.26 | −.055 | 0.19 |
| BPRS cognitive factor | ↓ | .010 | 0.003 | 356 | −.010 | .47 | 118 | .014 | 0.46 | .036 | 0.05 | .008 | 0.68 |
| ADAS-Cog | ↓ | .123 | <0.001 | 308 | .050 | .46 | 82 | .073 | 0.41 | .073 | 0.40 | .141 | 0.13 |
| ADAS Concentration/Distractibility | ↓ | .006 | 0.01 | 317 | .001 | .92 | 89 | .014 | 0.32 | .006 | 0.67 | .008 | 0.60 |
| ADAS Number Cancellation | ↑ | −.054 | <0.001 | 281 | −.002 | .97 | 70 | −.061 | 0.30 | −.033 | 0.57 | −.114 | 0.07 |
| ADAS Executive Function (maze) | ↓ | .174 | 0.21 | 268 | .62 | .39 | 69 | −.785 | 0.40 | −.192 | 0.84 | −.100 | 0.92 |
| Category Instances | ↑ | −.041 | <0.001 | 290 | −.024 | .34 | 73 | −.003 | 0.92 | −.019 | 0.55 | −.052 | 0.14 |
| Finger Tapping Preferred hand | ↑ | −.057 | 0.001 | 262 | .083 | .41 | 71 | −.174 | 0.18 | −.100 | 0.44 | −.166 | 0.17 |
| Finger Tapping Non-preferred hand | ↑ | −.047 | 0.04 | 260 | .074 | .46 | 70 | −.136 | 0.30 | −.112 | 0.38 | −.270 | 0.06 |
| Trails A Time to complete test (in seconds) | ↓ | .866 | <0.001 | 234 | −.513 | .50 | 52 | 1.60 | 0.12 | 1.66 | 0.10 | 1.51 | 0.15 |
| Working memory deficit | ↓ | −.003 | 0.51 | 58 | −.020 | .42 | 8 | .007 | 0.82 | .039 | 0.23 | .027 | 0.47 |
| Cognitive summary f | ↑ | −.011 | <0.001 | 277 | −.001 | .89 | 71 | −.013 | 0.04 | −.011 | 0.08 | −.018 | 0.001 |
↑ an increase in score indicates improved function, ↓ a decrease in score indicates improved function.
Mixed effects regression model β in time in weeks (i.e., the weekly change in cognitive variables), adjusted for age, gender, education and pooled study site.
P-value for weekly change rate significantly different from 0, adjusted for age, gender, education and pooled study site.
Mixed effects regression model β in time in weeks, compared to placebo, (i.e., by atypical antipsychotic, the weekly change in cognitive variables in excess of that observed in placebo patients), adjusted for age, gender, education and pooled study site.
P-value for weekly change rate compared to placebo, adjusted for age, gender, education and pooled study site.
The cognitive summary was the normalized average of the sign-adjusted, normalized, baseline z-scores for each of the 11 components of the ADAS-Cog, ADAS Concentration/Distractibility, ADAS Number Cancellation, ADAS Executive Function, Category Instances, the mean of the scores for the preferred and the non-preferred hand on the Finger Tapping Test, Trails A, and the working memory deficit.
Degrees of freedom
Number of patients on the same treatment for at least 2 weeks prior to 12-week, 24-week and 36-week cognitive testing: placebo: 48, 27, 25; olanzapine: 58, 41, 42; quetiapine: 64, 55, 44; risperidone: 60, 51, 35.
Figure 1.
Average cognitive summary score and Z-scores for MMSE, BPRS-Cog, and ADAS-Cogby study period for the full study population (including placebo).A one-unit change in Z-scores for MMSE, BPRS-Cog and ADAS-Cog represents a decline of one standard deviation of mean baseline score for the variable, with BPRS-Cog and ADAS-Cog Z-scores being further adjusted so that declines in the Z-score indicate declines in cognitive function. The cognitive summary is the normalized average of normalized cognitive scores (see Table 2). Mean raw scores at baseline, week 12, week 24, and week 36, respectively, were: MMSE 15.2, 14.5, 14.1, 13.3; BPRS-Cog: 5.3, 5.4, 5.4, 5.6; ADAS-Cog: 34.4, 35.0, 35.8, 36.9; cognitive summary: 0, −0.08, −0.14, −0.20.
Figure 2.
Average MMSE, ADAS-Cog, and Cognitive Summary scores by study period and by antipsychotic treatment vs. placebo. Decreases in MMSE and the Cognitive Summary, and increases in ADAS-Cog are indicative of cognitive decline. Mean raw scores at baseline, week 12, week 24, and week 36, respectively, were: MMSE placebo: 15.2, 13.7, 15.2, 13.4; MMSE antipsychotic: 15.2, 14.8, 14.2, 13.0; Cognitive Summary placebo: .05, −.06, 0, −.04; Cognitive Summary antipsychotic: .05, .03, −.01, −.13; ADAS-Cog placebo: 34.4, 36.1, 33.4, 35.4; ADAS-Cog antipsychotic: 34.4, 34.1, 35.4, 37.8.
The rates of change in cognitive function did not significantly differ by baseline MMSE score (<19 or ≥19), BPRS total score (≤27 or >27) and study site size (<18 patients or ≥18 patients) (data not shown).
No significant differences were obtained in the rates of change in most cognitive function measures between individual medication groups and placebo (Table 2). However, on the cognitive summary measure, patients on olanzapine or risperidone for at least 2 weeks prior to follow-up cognitive testing had significantly greater rates of decline than patients on placebo for at least 2 weeks prior to testing. Compared with patients on placebo, significantly greater rates of cognitive decline were observed on the MMSE in patients on olanzapine, on the BPRS cognitive factor in patients on quetiapine, and on the cognitive summary in patients on olanzapine and risperidone.
Patients on any atypical antipsychotic for at least 2 weeks prior to assessment had significantly greater rates of decline in cognitive function measured by MMSE, Category Instances, the cognitive summary, and the BPRS cognitive factor than patients on placebo(Table 3). Although not necessarily statistically significant, on all cognitive measures, patients on atypical antipsychotics declined more than patients on placebo. The association between cognitive decline and atypical antipsychotic vs. placebo did not vary by baseline MMSE or BPRS score or by study site (data not shown), indicating that there was little or no effect modification by these variables.
Table 3.
Changes in cognitive function over 36 weeks among patients on olanzapine, quetiapine, or risperidone compared to patients on placebo for at least two weeks prior to the date of follow-up cognitive testing
| Model-estimated change over 36 weeks (95% CI) a | |||||||
|---|---|---|---|---|---|---|---|
| Cognitive variables, units | Favorable Direction b |
Patients in model (n) |
Test visits in model (n) |
df | Olanzapine, quetiapine or risperidone |
Placebo | P-value c |
| MMSE Total Score, points | ↑ | 262 | 925 | 305 | −2.67 (−3.52,1.82) | −0.21 (−1.89,1.46) | 0.004 |
| BPRS-Cog, points | ↓ | 275 | 963 | 330 | 0.24 (−0.07,0.54) | −0.53 (−1.19,0.13) | 0.05 |
| ADAS-Cog, points | ↓ | 240 | 834 | 257 | 5.21 (3.41,7.01) | 2.46 (−0.96,5.88) | 0.11 |
| ADAS Concentration/Distractibility | ↓ | 245 | 854 | 267 | 0.23 (0.02,0.44) | −0.01 (−0.48,0.47) | 0.38 |
| ADAS Number Cancellation, points | ↑ | 220 | 756 | 222 | −2.30 (−3.36,−1.25) | −1.38 (−3.53,0.78) | 0.43 |
| ADAS Executive Function (maze) | ↓ | 211 | 730 | 219 | 13.11 (−0.33,26.55) | −7.05 (−37.72,23.63) | 0.26 |
| Category Instances | ↑ | 229 | 789 | 236 | −1.72 (−2.26,−1.18) | −0.14 (−1.23,0.95) | 0.01 |
| Finger Tapping Preferred hand | ↑ | 206 | 723 | 217 | −1.24 (−3.36,0.87) | 3.29 (−1.22,7.80) | 0.07 |
| Finger Tapping Non-preferred hand | ↑ | 203 | 716 | 214 | −1.88 (−4.11,0.36) | 2.43 (−2.32,7.18) | 0.10 |
| Trails A Time to complete test (in seconds) | ↓ | 183 | 624 | 177 | 41.83 (23.91,59.74) | 5.36 (−31.68,42.41) | 0.07 |
| Working memory deficit | ↓ | 68 | 177 | 30 | −0.06 (−0.38,0.26) | −0.29 (−0.95,0.37) | 0.59 |
| Cognitive summary, z score units | ↑ | 218 | 756 | 228 | −0.51 (−0.66,−0.36) | −0.16 (−0.41,0.10) | 0.004 |
Mixed effects regression model, adjusted for age, gender, education and pooled study site. The model change is the predicted change in cognitive function test score from baseline to 36 weeks for a patient on atypical antipsychotic or on placebo for at least 2 weeks prior to cognitive testing, adjusted for age, gender, education and pooled study site. 95% CI is the 95% confidence interval for the predicted change in cognitive function
↑ an increase in score indicates improved function, ↓ a decrease in score indicates improved function.
P-value for atypical antipsychotic change rate compared to placebo, adjusted for age, gender, education and pooled study site.
The average CGIC for patients on placebo was 3.13, indicating minimal improvement. The average CGIC scores for patients on atypical antipsychotic also indicated minimal improvement (3.11, 2.83, and 2.81 for olanzapine, quetiapine and risperidone, respectively) and these changes did not differ significantly from that found in patients on placebo (p=0.93, 0.23, and 0.19, respectively).
Discussion
Overall, Alzheimer’s disease patients with behavioral disturbances show a steady decline over 36 weeks in most cognitive areas, regardless of antipsychotic treatment or placebo. Over the 36-week study period, these declines were not only statistically significant, but also clinically meaningful. The estimated rate of decline among placebo patients on the ADAS-Cog was similar to that seen in Alzheimer’s patients without behavioral disturbances in other trials(14, 29). Moreover, the rates of decline did not vary with initial level of cognitive impairment as determined by baseline MMSE score. Our method of analysis used all data points from patients who had baseline and at least one follow-up testing. However, at the later durations, there were fewer test scores mainly due to patients’ inability to perform the tests or dropping out of the study, meaning that data cannot be assumed to be missing at random. Therefore, the cognitive decline over time is likely to be greater than what we have documented (30).
We evaluated the effect of treatment with atypical antipsychotics on cognitive function by comparing the weekly change (i.e., the slope of the change in cognitive function over time) among patients who had been on their current medication (or placebo) for at least two weeks prior to the date of cognitive testing. For most cognitive tests, the rate of cognitive change did not significantly differ by atypical antipsychotic. However, when the treatment groups were pooled, the group of patients on olanzapine, quetiapine, or risperidone had greater declines in cognitive function than patients on placebo on all tests except the ADAS Executive Function. As the cognitive effect of each of the medications was similar, all medications were combined and compared as a class to placebo. Combining the active treatment groups provided greater statistical power and many of the tests of cognitive functions showed significantly greater rates of decline in patients on one of the three atypical antipsychotic medications compared to patients on placebo. Over the 36 week trial period, patients on any antipsychotic had an average 2.46 point greater decline on the MMSE than placebo patients, a difference both statistically significant (p=.004) and clinically relevant.
In our comparisons of the study medications to placebo, we limited our analysis to patients who had been on the same drug for at least 2 weeks prior to the date of cognitive testing, essentially testing for a short-term effect. Alternatively, analyses could have been based on total exposure or exposure over some longer or lagged time-period. However, basing exposure on the sum over the trial would have mixed recent and distant exposures, possibly obscuring the short-term cognitive effect. Using a continuous exposure of longer than two weeks would have substantially reduced the number of patients available for analysis since many patients switched medications after relatively short exposure periods. Decline in cognitive function may be one reason patients switch medication.
This study only measured APD-effects on cognition in patients who were on medications for relatively short periods of time. We did not measure the differences in the rates of cognitive decline among patients over longer exposure periods. Therefore we cannot address the speculation whether these drugs would accelerate cognitive decline permanently or merely impair cognition during acute administration. It is also possible that this worsening of patients on antipsychotic medications would attenuate over time. It is unknown whether the greater decline in cognitive function in patients on these medications is a worsening of Alzheimer’s disease pathology or an independent effect. One possible explanation for decreased cognitive function among patients on these drugs would be sedation. However, we excluded all test dates at which the subject’s caregiver reported sedation. It is well known that antipsychotic medications degrade cognition in most non-psychotic patient groups, when used, for example, for dyskinesia control in Tourette’s and have been shown to impair aspects of cognition in schizophrenia.
Although there is strong evidence for a detrimental effect of this class of drugs on cognitive function (8, 14), it is not clear if this effect is equally strong in the different cognitive domains. The most significant effect that we found when comparing individual drugs to placebo was in the cognitive summary score. Since this variable combines many of the other tests, it is less subject to random fluctuations allowing differences to be recognized. If significance was not found within a given cognitive domain, however, it may not be due to absence of effect, but rather to insensitivity of the test. Patients on atypical antipsychotic improved overall clinically as evidenced by CGIC scores; however, the improvements were not statistically different from improvement seen in the placebo group.
In addition to testing a variety of cognitive domains, this study has the strength of reflecting prescribing practices with the most commonly prescribed atypical antipsychotic medications for Alzheimer’s disease. The relatively small sample size, however, with patients spread over 3 active and one placebo arm did not provide the ability to evaluate differences between the three drugs. Nevertheless, we note that the differences between the active treatments tend to be smaller than the differences between active treatment and placebo.
Some early studies and meta-analyses conducted in non-demented schizophrenic patients indicated that cognitive function may improve with the use of atypical antipsychotics compared to conventional antipsychotics (9).Data from the CATIE schizophrenia trial, however, indicated that these improvements were small and not different from conventional antipsychotic treatment, leading the authors to conclude that these effects were likely due to the effects of expectation or practice (31). Similarly, improvements in cognitive function reported for 104 patients with schizophrenia randomized to either olanzapine or risperidone were consistent with the improvement due to practice effects seen in 84 healthy subjects without schizophrenia (12). The fact that the Alzheimer’s disease patients in this study did not improve cognitively with treatment may be due to their overall declining cognitive function (as seen in the full study population), vulnerability to the deleterious cognitive effects of these medications, as well as their inability to benefit from the practice improvement found in non-demented patients.
Individual trials in Alzheimer’s disease patients generally report null effects of atypical antipsychotics on MMSE scores, for the most part the only cognitive measure assessed (8). Meta-analysis of these trials comparing olanzapine, quetiapine, risperidone, haloperidol, and aripiprazole to placebo over 6 weeks to 26 weeks (8), in particular, including 863 patients using olanzapine, quetiapine or risperidone compared to 314 placebo patients reported a weighted mean difference for drug vs. placebo on the MMSE of 0.73 (p<.0001) with poorer scores in drug group. The additional decline in MMSE in risperidone in our study compared to placebo was statistically significant, while the declines for olanzapine and quetiapine patients were not.
Following this meta-analysis, other trials assessed cognitive change in Alzheimer’s disease patients using atypical antipsychotics. A randomized double-blind placebo-controlled trial of 80 patients found greater declines in cognitive function (measured by the Severe Impairment Battery) among those randomized to quetiapine than in those randomized to placebo (13). Another randomized double-blind placebo-controlled trial of 268 Alzheimer’s disease patients who did not have significant behavioral problems reported greater declines on both the MMSE and ADAS-Cog among patients randomized to olanzapine than those randomized to placebo (14). Further, the difference in ADAS-Cog scores was only significant in patients with lower baseline MMSE scores. We did not find differences in cognitive decline or treatment effect when stratified by baseline MMSE score or baseline BPRS score.
In contrast, a retrospective chart review of 58 Alzheimer’s disease patients prescribed risperidone, olanzapine or quetiapine found no decline in MMSE scores in any of the drug groups (32). The patients in this study, however, tended to be younger and have higher baseline MMSE scores than the patients in CATIE-AD. Moreover, this chart review required patients to continue medications for 6 months and thus many patients who experienced negative cognitive effects would likely not have been included.
Our results provide additional and broad evidence that atypical antipsychoticsas compared to placebo are associated with greater rates of decline in cognitive function in Alzheimer’s disease patients with psychotic or aggressive behavior and that the magnitude of the additional declines are clinically relevant, at least as great a magnitude of the effect of cholinesterase inhibitors but in the negative direction (29). Furthermore, these results suggest that the declines in cognitive function span a range of cognitive domains, but given our relatively limited sample size we were not able to precisely determine the difference in effect by cognitive domain. Although the sample size was not sufficient to determine if the rates of decline vary by the particular atypical antipsychotic used, the declines were evident for all three medications compared to placebo. Despite the evidence for worsening cognitive function and other adverse events with antipsychotics, improvement in psychotic and aggressive behavior may still warrant their use in individual cases (16, 33). To aid in choosing the best medication for a given patient, the relative adverse effects on cognitive function within this class of medication needs to be addressed in future studies, this might include assessment of attention, psychomotor, and executive function as appropriate for the level of impairment.
Acknowledgments
Supported in part by NIMH research contract N01 MH-9001, USC Alzheimer’s Disease Research Center NIH P50 AG05142, and by the U.S. Department of Veterans Affairs Medications for this study were provided by AstraZeneca Pharmaceuticals, Forest Pharmaceuticals, Janssen Pharmaceutica, and Eli Lilly.
References
- 1.Lyketsos CG, Steinberg M, Tschanz JT, Norton MC, Steffens DC, Breitner JC. Mental and behavioral disturbances in dementia: findings from the Cache County study on memory in aging. Am J Psychiatry. 2000;157(5):708–714. doi: 10.1176/appi.ajp.157.5.708. [DOI] [PubMed] [Google Scholar]
- 2.Ropacki SA, Jeste DV. Epidemiology of and risk factors for psychosis of Alzheimer's Disease: a review of 55 studies published from 1990–2003. Am J Psychiatry. 2005;162:2022–2030. doi: 10.1176/appi.ajp.162.11.2022. [DOI] [PubMed] [Google Scholar]
- 3.Lopez OL, Becker JT, Sweet RA, Klunk W, Kaufer DI, Saxton J, et al. Psychiatric symptoms vary with the severity of dementia in probable Alzheimer's disease. J Neuropsychiatry Clin Neurosci. 2003;15:346–353. doi: 10.1176/jnp.15.3.346. [DOI] [PubMed] [Google Scholar]
- 4.Craig D, Mirakhur A, Hart DJ, McIlroy SP, Passmore AP. A cross sectional study of neuropsychiatric symptoms in 435 patients with Alzheimer's disease. Am J Geriatr Psychiatry. 2005;13:460–468. doi: 10.1176/appi.ajgp.13.6.460. [DOI] [PubMed] [Google Scholar]
- 5.Schulz R, O'Brien AT, Bookwala J, Fleissner K. Psychiatric and physical morbidity effects of dementia caregiving: prevalence, correlates and causes. The Gerontologist. 1995;35:771–791. doi: 10.1093/geront/35.6.771. [DOI] [PubMed] [Google Scholar]
- 6.Magni E, Binetti G, Bianchetti A, Trabucchi M. Risk of mortality and institutionalization in demented patients with delusions. J Geriatr Psychiatry Neurol. 1996;9:123–126. doi: 10.1177/089198879600900303. [DOI] [PubMed] [Google Scholar]
- 7.Stern Y, Tang M-X, Albert MS, Brandt J, Jacobs DM, Bell K, et al. Predicting time to nursing home care and death in individuals with Alzheimer disease. JAMA. 1997;227(10):806–812. [PubMed] [Google Scholar]
- 8.Schneider LS, Dagerman K, Insel PS. Efficacy and adverse effects of atypical antipsychotics for dementia: meta-analysis of randomized, placebo-controlled trials. American Journal of Geriatric Psychiatry. 2006;14(3):191–210. doi: 10.1097/01.JGP.0000200589.01396.6d. [DOI] [PubMed] [Google Scholar]
- 9.Keefe RS, Silva SG, Perkins DO, Lieberman JA. The effects of atypical antipsychotic drugs on neurocognitive impairment in schizophrenia: a review and meta-analysis. Schizophrenia Bulletin. 1999;25(2):201–222. doi: 10.1093/oxfordjournals.schbul.a033374. [DOI] [PubMed] [Google Scholar]
- 10.Woodward ND, Purdon SE, Meltzer HY, Zald DH. A meta-analysis of neuropsychological change to clozapine, olanzapine, quetiapine, and risperidone in schizophrenia. International Journal of Neuropsychopharmacology. 2005 Sep;8(3):457–472. doi: 10.1017/S146114570500516X. [DOI] [PubMed] [Google Scholar]
- 11.Harvey PD, Keefe RS. Studies of cognitive change in patients with schizophrenia following novel antipsychotic treatment. American Journal of Psychiatry. 2001 Feb;158(2):176–184. doi: 10.1176/appi.ajp.158.2.176. [DOI] [PubMed] [Google Scholar]
- 12.Goldberg TE, Goldman RS, Burdick KE, Malhotra AK, Lencz T, Patel RC, et al. Cognitive improvement after treatment with second-generation antipsychotic medications in first-episode schizophrenia: is it a practice effect? Archives of General Psychiatry. 2007 Oct;64(10):1115–1122. doi: 10.1001/archpsyc.64.10.1115. [DOI] [PubMed] [Google Scholar]
- 13.Ballard C, Margallo-Lana M, Juszczak E, Douglas S, Swann A, Thomas A, et al. Quetiapine and rivastigmine and cognitive decline in Alzheimer's disease: randomised double blind placebo controlled trial. Bmj. 2005;330(7496):874. doi: 10.1136/bmj.38369.459988.8F. [see comment]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kennedy J, Deberdt W, Siegal A, Micca J, Degenhardt E, Ahl J, et al. Olanzapine does not enhance cognition in non-agitated and non-psychotic patients with mild to moderate Alzheimer's dementia. International Journal of Geriatric Psychiatry. 2005;20(11):1020–1027. doi: 10.1002/gps.1397. [DOI] [PubMed] [Google Scholar]
- 15.Schneider LS, Ismail MS, Dagerman K, Davis S, Olin J, McManus D, et al. Clinical antipsychotic trials of intervention effectiveness (CATIE): Alzheimer's disease trial. Schizophrenia Bulletin. 2003;29(1):57–72. doi: 10.1093/oxfordjournals.schbul.a006991. [DOI] [PubMed] [Google Scholar]
- 16.Schneider LS, Tariot PN, Dagerman KS, Davis SM, Hsiao JK, Ismail S, et al. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer's disease. New England Journal of Medicine. 2006;355:1525–1538. doi: 10.1056/NEJMoa061240. [DOI] [PubMed] [Google Scholar]
- 17.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, D.C: American Psychiatric Association Press; 1994. [Google Scholar]
- 18.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of the Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 19.Folstein M, Folstein S, McHugh P. "Mini-Mental State": A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 20.Overall JE, Gorham DR. The Brief Psychiatric Rating Scale (BPRS): recent developments in ascertainment and scaling. Psychopharmacol Bull. 1988;24:97–99. [PubMed] [Google Scholar]
- 21.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 22.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. American Journal of Psychiatry. 1984;141(11):1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 23.Mohs RC, Knopman D, Petersen RC, Ferris SH, Ernesto C, Grundman M, et al. Development of cognitive instruments for use in clinical trials of antidementia drugs: additions to the Alzheimer's Disease Assessment Scale (ADAS) that broaden its scope. Alzheimer's Disease and Associated Disorders. 1997;11 Supplement 2:S13–S21. [PubMed] [Google Scholar]
- 24.Benton AL. Development of a multilingual aphasia battery. Progress and problems. 1969 Jul–Aug;:39–48. doi: 10.1016/0022-510x(69)90057-4. [DOI] [PubMed] [Google Scholar]
- 25.Doody RS, Vacca JL, Massman PJ, Liao TY. The influence of handedness on the clinical presentation and neuropsychology of Alzheimer disease. Archives of Neurology. 1999 Sep;56(9):1133–1137. doi: 10.1001/archneur.56.9.1133. [DOI] [PubMed] [Google Scholar]
- 26.Amieva H, Lafont S, Auriacombe S, Rainville C, Orgogozo JM, Dartigues JF, et al. Analysis of error types in the trial making test evidences an inhibitory deficit in dementia of the Alzheimer type. Official Journal of the International Neuropsychological Society. 1998 Apr;20(2):280–285. doi: 10.1076/jcen.20.2.280.1161. [DOI] [PubMed] [Google Scholar]
- 27.Keefe RS, Roitman SE, Harvey PD, Blum CS, DuPre RL, Prieto DM, et al. A pen-and-paper human analogue of a monkey prefrontal cortex activation task: spatial working memory in patients with schizophrenia. Schizophrenia Research. 1995 Sep;17(1):25–33. doi: 10.1016/0920-9964(95)00027-j. [DOI] [PubMed] [Google Scholar]
- 28.Beller SA, Overall JE. The Brief Psychiatric Rating Scale (BPRS) in geropsychiatric research. II. Representative profile patterns. Journal of Gerontology. 1984;39:194–200. doi: 10.1093/geronj/39.2.194. [DOI] [PubMed] [Google Scholar]
- 29.Birks J. Cholinesterase inhibitors for Alzheimer's disease. Cochrane Database of Systematic Reviews. 2006:CD005593. doi: 10.1002/14651858.CD005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomas RG, Berg JD, Sano M, Thal L. Analysis of longitudinal data in an Alzheimer's disease clinical trial. Stat Med. 2000;19(11–12):1433–1440. doi: 10.1002/(sici)1097-0258(20000615/30)19:11/12<1433::aid-sim435>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 31.Keefe RS, Bilder RM, Davis SM, Harvey PD, Palmer BW, Gold JM, et al. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE Trial. Archives of General Psychiatry. 2007 Jun;64(6):633–647. doi: 10.1001/archpsyc.64.6.633. [DOI] [PubMed] [Google Scholar]
- 32.Rocca P, Marino F, Montemagni C, Perrone D, Bogetto F. Risperidone, olanzapine and quetiapine in the treatment of behavioral and psychological symptoms in patients with Alzheimer's disease: preliminary findings from a naturalistic, retrospective study. Psychiatry & Clinical Neurosciences. 2007;61(6):622–629. doi: 10.1111/j.1440-1819.2007.01729.x. [DOI] [PubMed] [Google Scholar]
- 33.Sultzer DL, Davis SM, Tariot PN, Dagerman KS, Lebowitz BD, Lyketsos CG, et al. Clinical Symptom Responses to Atypical Antipsychotic Medications in Alzheimer's Disease: Phase 1 Outcomes From the CATIE-AD Effectiveness Trial. American Journal of Psychiatry. 2008;165(7):844–854. doi: 10.1176/appi.ajp.2008.07111779. [DOI] [PMC free article] [PubMed] [Google Scholar]