Abstract
Resistance to anti-ErbB2 agents is a significant problem in the treatment of human ErbB2+ breast cancers. We show here that adhesion of human ErbB2+ breast cancer cells to basement membrane laminin-5 provides substantial resistance to trastuzumab and lapatinib, agents that respectively target the extracellular and kinase domains of ErbB2. Knockdown of laminin-binding integrins (α6β4, α3β1) or associated tetraspanin protein CD151 reversed laminin-5 resistance, and sensitized ErbB2+ cells to trastuzumab and lapatinib. CD151 knockdown, together with trastuzumab treatment, inhibited ErbB2 activation and downstream signaling through Akt, Erk1/2, and FAK. Hence, ErbB2 function in mammary tumor cells is promoted by integrin-mediated adhesion to laminin-5, with strong support by CD151, leading to signaling through FAK. Consequently, removal or inhibition of any of these components (laminin-5, integrin, CD151, FAK) markedly sensitizes cells to anti-ErbB2 agents. These new insights should be useful when devising strategies for overcoming drug resistance in ErbB2+ cancers.
Keywords: Laminin, Integrin, Trastuzumab, ErbB2, CD151, FAK
Introduction
ErbB2/HER2, an epidermal growth factor receptor family member, is a potent oncogenic receptor kinase driving progression, malignancy and metastasis of human breast cancer. ErbB2 activates via homodimerization or heterodimerization with other ErbB family members (1). Activated ErbB2 initiates signals through PI3K/Akt, Ras/MAPK, and other pathways, thus enhancing cell proliferation and survival (2). ErbB2 gene amplification, which occurs in 15–25% of human breast cancers, is associated with poor patient prognosis and survival (3). Anti-ErbB2 inhibitors trastuzumab and lapatinib are clinically effective in targeting ErbB2+ breast cancers. Trastuzumab (Herceptin), a HER2 specific humanized monoclonal antibody, inhibits ErbB2 signaling and triggers an anti-tumor antibody-dependent cellular cytotoxicity (ADCC) response (4). As a single agent, trastuzumab elicits objective tumor responses in 30% of patients with advanced ErbB2+ breast cancer and improves response rate and survival when added to chemotherapy in that patient population (5). Lapatinib, a small molecule inhibitor of ErbB2 and EGFR tyrosine kinase activities, induces apoptosis in ErbB2+ breast cancer cells, including those that are trastuzumab resistant (6). Consistent with this finding, lapatinib improves response rates and progression free survival when added to chemotherapy in patients with ErbB2+ breast cancer who had previously progressed on trastuzumab (7).
Unfortunately, more than 60% patients with ErbB2+ cancers do not respond to trastuzumab monotherapy, and most initial responders develop resistance within one year (8). Resistance may arise through constitutive activation of: the PI3K/Akt pathway, other ErbB family members, or alternative oncogenic pathways (4). Also, membrane associated glycoprotein MUC4 might cause resistance by masking the ErbB2 binding site for trastuzumab (4). Potential mechanisms of lapatinib resistance include ErbB2 kinase site mutations (9), PI3K/Akt pathway hyperactivation, and increased anti- to proapoptotic protein ratio (10).
Tumor-microenvironment interactions markedly affect anti-tumor drug responses. For example, extracellular matrix (ECM) proteins, including laminin-5, protect malignant mammary cells (11) and other cancer cells (12) from chemically induced apoptosis. In nearly all epithelial tissues laminin-5 regulates cell organization, gene expression, and survival (13). Although laminin-5 levels diminish upon malignant transformation of breast epithelium (14), it still can support mammary tumor survival (15) and tumor metastasis to lung (16), lymph node (17), and likely other tissues.
Integrins, at the tumor-ECM microenvironment interface, can promote tumor cell survival and protection from chemically induced apoptosis (18). The laminin-binding integrin α6β4 promotes breast tumor survival (11, 15). Furthermore, deletion of the β4 signaling domain sensitized ErbB2+ mouse mammary tumors to gefitinib/iressa (19), a tyrosine kinase domain inhibitor. Survival promotion by α6β4 sometimes may (20), or may not (21) involve activation of Akt, a key determinant of drug resistance (4). Laminin-binding integrins (α3β1, α6β1, α6β4) associate closely with CD151, a tetraspanin family member (22). CD151 minimally affects integrin-dependent cell adhesion to laminin, but rather influences adhesion strengthening, cell invasion and migration, and 3D cell morphology (22). CD151 expression correlates with poor prognosis in colon (23) and non-small cell lung cancers (24), and with invasiveness in mammary carcinoma cells (25). Ablation of CD151 protein affects tumor cell growth, invasion, migration, and EGF sensitivity in human basal-like breast cancer (26). Since α6β4 affects ErbB2+ breast tumor progression (19), and CD151 is elevated in 32% of ErbB2+ human tumors (26), we hypothesized that CD151 and/or α6β4 might influence sensitivity to ErbB2 targeted therapies.
Integrin-mediated cell adhesion typically results in integrins localizing into focal adhesion complexes, along with many cystoskeletal proteins and signaling molecules including focal adhesion kinase (FAK) (27). Integrin-mediated adhesion stimulates FAK activity (28), and in breast cancer FAK may control tumor initiation, proliferation, survival, invasion and metastasis (29). However, α6β4 does not localize into focal adhesions (30) and does not typically activate FAK (31). Tetraspanin CD151 also does not localize into focal adhesions (32), and CD151 ablation/expression may (26) or may not (33) affect FAK activation. Hence, it was unclear whether FAK would play a role in ErbB2 drug resistance, involving CD151 and laminin-binding integrins.
Here we show that trastuzumab and lapatinib resistance develops when ErbB2+ breast cancer cells use CD151-α6β4 (and α3β1) complexes to engage laminin-5, and activate FAK. Conversely, removal or inhibition of laminin-5, integrins, CD151, or FAK markedly enhances sensitivity to ErbB2 targeted drugs. These results are notable because i) neither CD151 nor other tetraspanins had been linked to tumor drug resistance, and ii) CD151 targeting could enhance drug sensitivity without radical disruption of laminin-binding integrins.
Materials and Methods
Cells and Antibodies
All ErbB2+ human mammary tumor cell lines BT474, ZR-75-1, SKBR3, and MD-MB-453 cells, from ATCC (Rockville, MD), were cultured in RPMI supplemented with 10% fetal bovine serum. Monoclonal antibodies to CD151 (5C11), CD9 (MM2/57), CD81 (M38), integrin α2 (IIE10), α5 (BIIG2), α3 (A3-X8), α6 (A6-ELE), and β1 (TS2/16) were referenced elsewhere (26). Monoclonal antibodies against α6 (GoH3) and β4 integrins were obtained from BD Bioscience. Antibodies against E-cadherin, FAK, phosph-FAK (Y397) were purchased from Santa Cruz Biotechnology, Inc. Claudin 3-specific antibody was from Invitrogen. Rabbit polyclonal antibodies against total and phosphorylated PTEN, MAPK, AKT, ErbB2 and EGFR were purchased from Cell Signaling Technology (Beverly, MA). Anti-β-actin was from Sigma (St. Louis, MO).
siRNA and shRNA targeting
For siRNA targeting, cells (~2–4 × 105 per well in 6-well plates) were pre-incubated with siRNA’s using RNAiMAX (Invitrogen), at days - 3 and -1 prior to assay. Prior to drug addition, siRNA-treated cells were essentially indistinguishable with respect to physical appearance and viability. All siRNA’s were purchased from Dharmacon. Sequences of siRNA’s targeting CD151, α6, α3 and CD9 were described (26). Independent siRNA’s for FAK are UAGUACAGCUCUUGCAUAU and GGACAUUAUUGGCCACUGU. PTEN was targeted using a 4 oligo pool (GAUCAGCAUACACAAAUUA, GACUUAGACUUGACCUAUA, GAUCUUGACCAAUGGCUAA, CGAUAGCAUUUGCAGUAUA). For stable knockdown of human CD151, ErbB2+ breast cancer cells were infected with Lentivirus as described (26). CD151-null cells were negatively selected by two color flow cytometry. Knockdown efficiency was evaluated by flow cytometry and immunoblotting.
Drug Sensitivity Assays
Matrix proteins collagen, fibronectin and Matrigel were from BD Bioscience. Laminin-5 was from human A431 cells. Plates (24-well) were precoated with ~ 5 ug/ml laminin-5 or Matrigel (overnight at 4°C), or with 20 ug/ml collagen I or 10 ug/ml fibronectin (1 h at 7°C), or left uncoated. Cells (~5 × 105 cells/well) were plated for 24 hours, then treated with trastuzumab (0–512 µg/ml) or lapatinib (0–500 nM). After another 48–72 hr, cells were quantitated using PicoGreen (Invitrogen). Each data point represents the mean of three separate experiments, each with triplicate measurements. Variability between experiments was typically less than 15%. Trastuzumab (herceptin) was from Genentech, lapatinib was from GlaxoSmithKline, and anti-FAK inhibitor TAE226 was from Novartis Co. Seven different ErbB2-negative cell lines showed no drug sensitivity, except at the highest doses of trastuzumab (256–512 µg/ml yielded mean decrease in cell number of 2–3%) and lapatinib (2.5–10 µM yielded a mean decrease in cell number of 3–10%).
Signaling assays
Control and CD151-deficient BT474 or ZR75 cells were detached using EDTA, washed, incubated at 37°C for 30 min, and then plated on laminin-5 ± trastuzumab. Cells were lysed in buffer containing 1 mM Na3VO4, 1% Triton X-100, 1% deoxycholate and 0.1% SDS and protease cocktail (Roche). After reducing electrophoresis, proteins were transferred to nitrocellulose and blotted for phosphorylated ErbB2 (Y1221/1222), Akt (S473), MAPK (T202/Y204, PTEN (T382/383) and FAK (Y397) and for total ErbB2, MAPK, Akt, PTEN, and FAK proteins. Anti-FAK and phospho-FAK (Y397) were from Santa Cruz Biotechnology, Inc. Rabbit antibodies to other proteins were from Cell Signaling Technology (Beverly, MA).
Results
Laminin-5, α3β1 and α6β4 integrins, and CD151 affect ErbB2 drug sensitivity
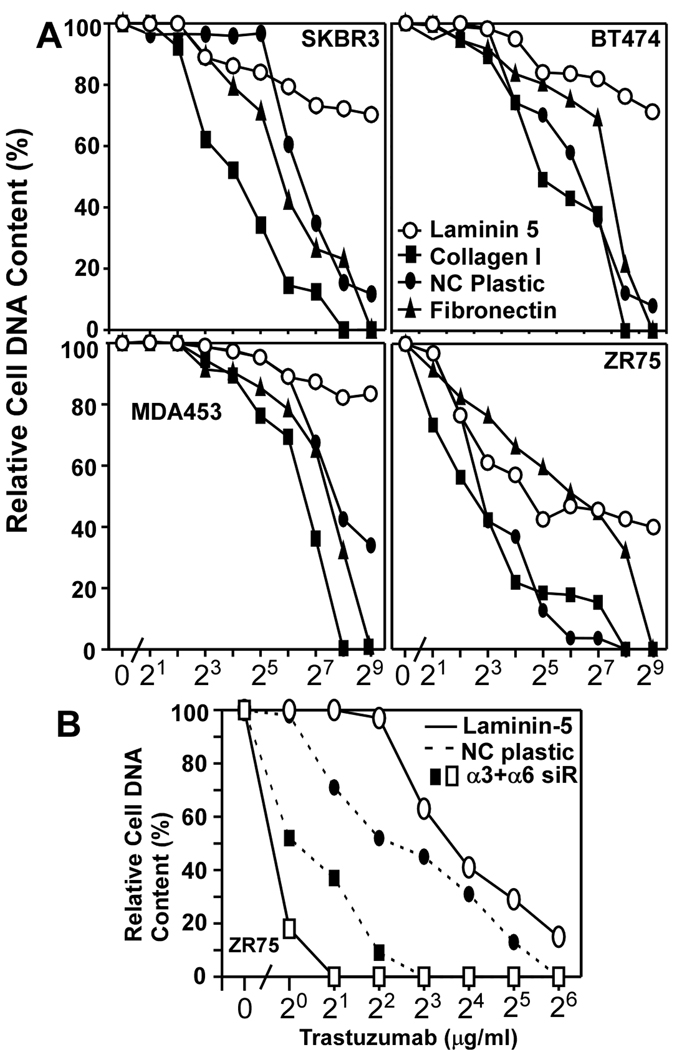
We hypothesized that laminin-5, a key epithelial cell microenvironment component (31), might affect sensitivity to ErbB2 targeted drugs. Indeed, four different ErbB2-positive mammary cell lines (SKBR3, BT474, MDA-MB-231, ZR75) were markedly more resistant to trastuzumab when grown on laminin-5, compared to fibronectin, collagen, non-coated plastic (Fig. 1A), or Matrigel (Supp. Fig. S1). These results suggest that drug resistance is supported by receptors for laminin-5 (integrins α6β4 and/or α3β1), but not by receptors for collagen (α2β1), fibronectin (α5β1) or Matrigel (α6β1). These integrins are variably present in the ErbB2+ cells (Suppl. Figs. S2, S3B). Consistent with the laminin-5 results, simultaneous ablation of integrin α3 and α6 subunits (by 78–95%, Suppl. Figs. S3A, S3B) markedly sensitized ZR75 cells to trastuzumab (Fig. 1B), as the IC50 decreased by a factor of ~16 (~12.1 → 0.76 µg/ml). By contrast, for ZR75 cells on non-coated plastic, the IC50 decreased by a factor of only ~4.5 (4.9 → 1.1 µg/ml; Fig. 1B).
Figure 1. Laminin-5 promotes trastuzumab resistance.
A) Semi-confluent human ErbB2+ breast cancer cells were plated on indicated ECM proteins or on non-coated (NC) plastic. After treatment with trastuzumab for 3 days, cell numbers were assessed. B) ZR75 cells were transfected with siRNA’s, plated on non-coated plastic or on laminin-5, treated with trastuzumab for 3 days, and then cell numbers were assessed. Efficiency of α3 and α6 knockdown was evaluated by immunoblotting (Supplemental Fig. S3A) and/or by flow cytometry (Supplemental Fig. S3B).
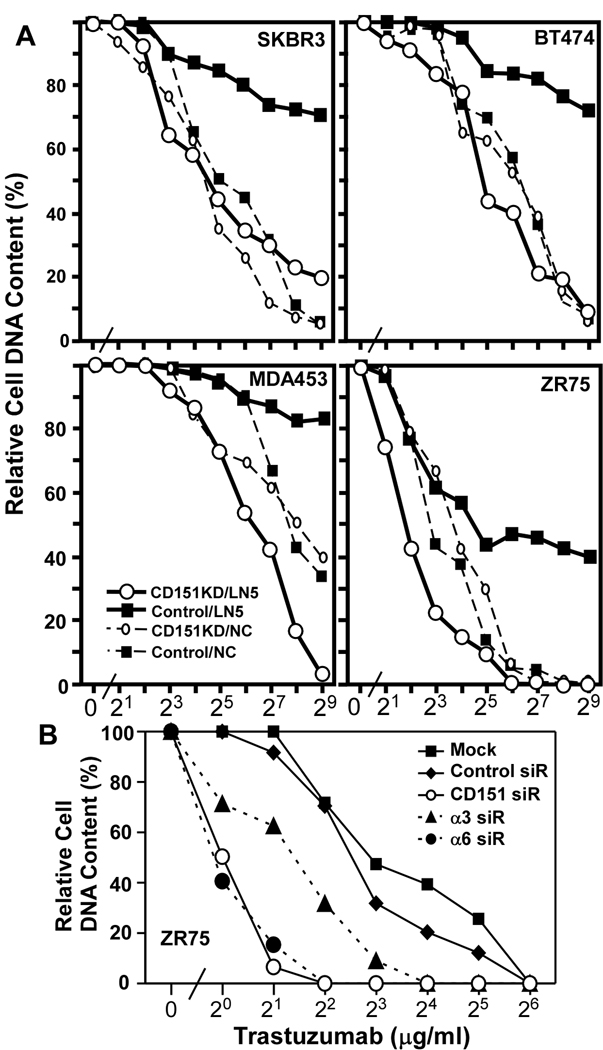
The α3 and α6 integrins associate closely with tetraspanin CD151 whenever it is co-expressed. As an alternative to ablating α3 and α6 integrins, which disrupts adhesion to laminin-5, we stably knocked down CD151 (by >90%, Suppl. Fig. S3C), which minimally affects cell adhesion. Nonetheless, stable CD151 knockdown markedly enhanced trastuzumab sensitivity of SKBR3, BT474, MDA-MB-453, and ZR75 cells on laminin-5 (Fig. 2A). By contrast, CD151 knockdown minimally affected sensitivity for cells on non-coated tissue culture plastic (Fig. 2A), Matrigel (Suppl. Fig. S1) or collagen (Suppl. Fig. S1). For representative ZR75 cells on laminin-5, trastuzumab sensitivity was enhanced similarly by siRNA knockdown of either CD151 or α6 integrin (IC50 6–8 →0.9–1.0 µg/ml; Fig. 2B). Knockdown of α3 integrin increased trastuzumab sensitivity to a lesser extent (IC50 = 2.5 µg/ml; Fig. 2B). Knockdown of CD151 did not affect surface levels of α3, α6, β1, or β4 integrins (Suppl. Fig. S3B).
Figure 2. Ablation of CD151 restores trastuzumab sensitivity to cells on laminin-5.
A) Human ErbB2+ cells stably expressing control or CD151-specific shRNA’s were plated on laminin-5 (LN5) or non-coated (NC) plastic. Note that in each panel, the two control curves (without CD151 knockdown) are the same as those shown in Fig. 1A, and are included again here to allow comparison of CD151 knockdown effects. B) ZR75 cells were transfected with two rounds of the indicated siRNA’s, and then plated on laminin-5. All cells were then treated with trastuzumab 3 days, and viability was assessed using PICOGreen. Efficiency of CD151, α3 and α6 knockdowns was evaluated as in Supplemental Figs. S3A and S3B.
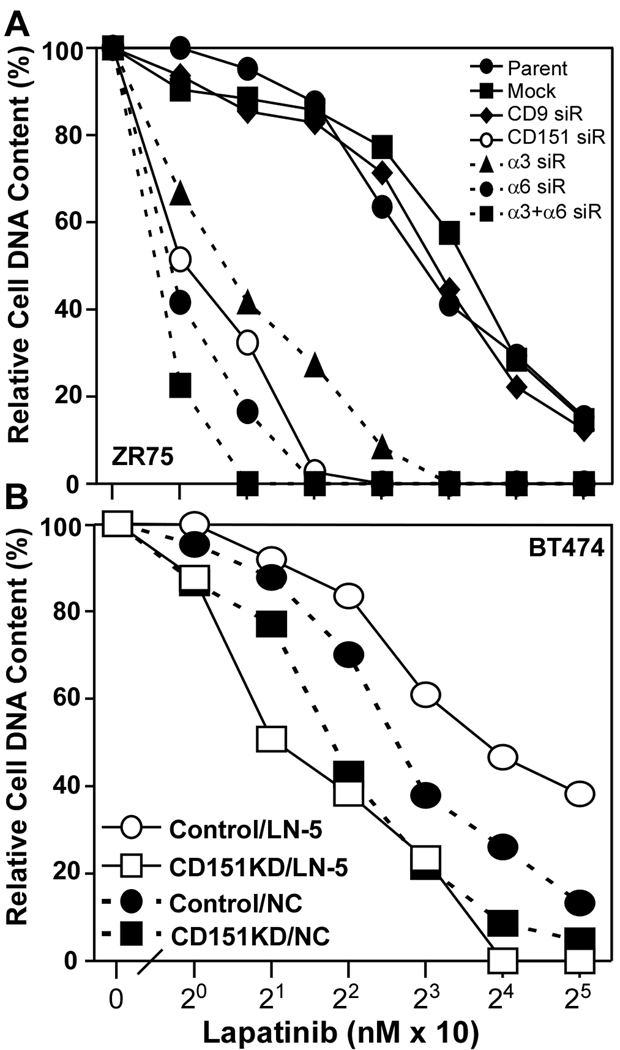
We also tested breast cancer cell sensitivity to lapatinib, a membrane permeable ErbB2 kinase inhibitor (34). Again, control IC50’s (120–190 nM) were markedly diminished upon knockdown of α6 or CD151 (9–11 nM), or α3 (~16 nM) or both integrin subunits together (~8 nM) in ZR75 cells (Fig. 3A). Knockdown of tetraspanin protein CD9 had negligible effect (IC50 = nM (Fig. 3A). In BT474 cells, CD151 knockdown (by >90%, Suppl. Fig. S4) again had more impact for cells on laminin-5 (IC50 decreased from ~14 down to 2 nM), compared to tissue culture plastic (6.5 down to 3.5 nM; Fig. 3B).
Figure 3. Sensitization to lapatinib.
A) ZR75 cells were transfected with siRNA’s, plated on laminin-5, and then treated with lapatinib for 3 days. B) BT474 cells expressing control or CD151-specific shRNA’s were seeded onto laminin-5 or non-coated plastic, and treated as in A. Note that increased efficiency of CD151 knockdown by siRNA (panel A), compared to shRNA (panel B) results in more dramatic lapatinib sensitization in panel A. Knockdown efficiencies are demonstrated in Supplemental Figs. S3A, S3B (for panel A) and in Supplemental Fig. S3C (for panel B).
ErbB2 and CD151 signaling
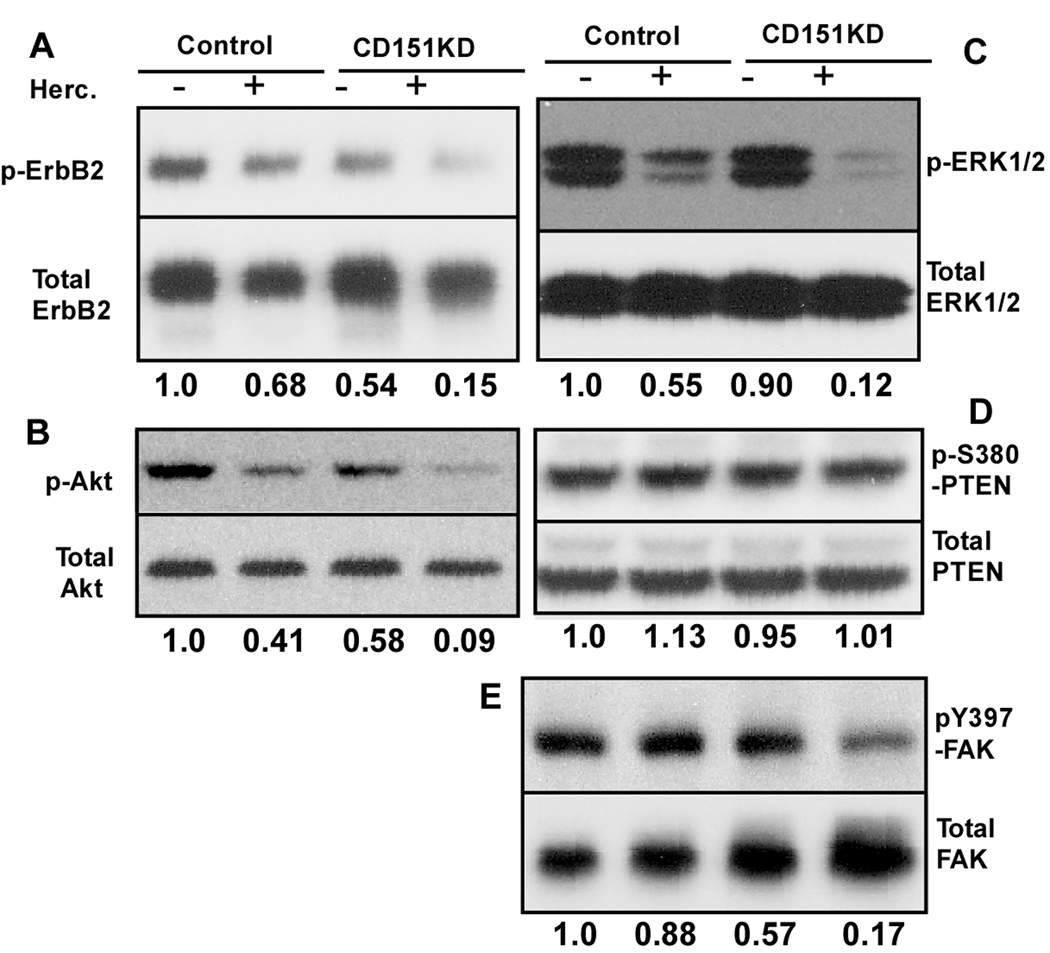
CD151 ablation did not affect ErbB2 oligomerization in BT474 cells, as seen by chemical crosslinking (Suppl. Fig. S5). For BT474 cells on laminin-5, in the presence of 1% serum, ErbB2 phosphorylation was reduced upon adding trastuzumab (by~32%) or ablation of CD151 (by ~46%), while both together had a roughly additive effect (~85% reduction, Fig. 4A). Trastuzumab treatment and CD151 ablation similarly diminished AKT phosphorylation in an additive manner (trastuzumab by 59%; CD151 by 42%; both by 91%). For Erk1/2, another signaling molecule downstream of ErbB2, inhibition was synergistic. Erk1/2 phosphorylation was diminished by trastuzumab (45%) and CD151 ablation (10%), with both together yielding 88% reduction (Fig. 4C). Interference with PTEN, an upstream phosphatase that suppresses PI3K and AKT, can enhance trastuzumab resistance (35). However, for BT474 cells already resistant due to plating on laminin-5, knockdown of PTEN yielded little or no further increase in resistance to trastuzumab or lapatinib (Suppl. Figure S6). Furthermore, trastuzumab treatment and/or CD151 ablation minimally affected phosphorylation of PTEN at S380 (Fig. 4D).
Figure 4. Trastuzumab and CD151 effects on signaling.
BT474 cells expressing control or CD151-shRNA’s were plated on laminin-5 (in 1% FBS, +/- 10 µg/ml trastuzumab for 20 hour), lysed and blotted for indicated molecules. Protein density ratios were determined (phosphorylated/total) and normalized relative to control conditions (lane 1 = 1.0).
A central role for focal adhesion kinase (FAK)
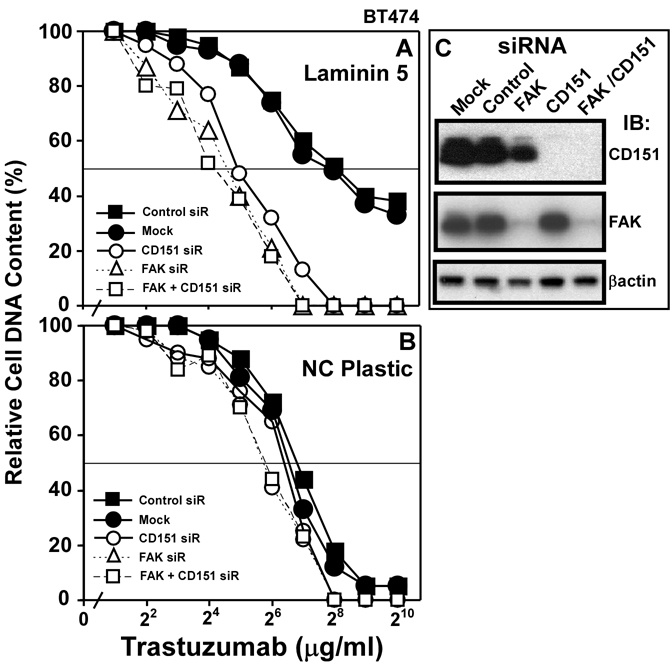
For BT474 cells on laminin-5, FAK activation (Y397 phosphorylation) was slightly diminished by trastuzumab treatment (12%), and CD151 knockdown had a modest effect (43% reduction, Fig. 4E). However, both together reduced FAK activation by 83% (Fig. 4E). Consistent with ErbB2 signaling through FAK, knockdown of FAK (>90%, Fig. 5C) markedly increased trastuzumab sensitivity of BT474 cells on laminin-5 (IC50 = ~210 → 13–16 µg/ml; Fig. 5A). Knockdown of CD151 or FAK, alone or together, similarly enhanced trastuzumab sensitivity (Fig. 5A). By contrast, BT474 cells on tissue culture plastic did not show resistance to trastuzumab, and were relatively unaffected by FAK knockdown (Fig. 5B). Hence, laminin-5 and CD151 may promote trastuzumab resistance entirely by acting through FAK.
Figure 5. Ablation of FAK affects trastuzumab sensitivity.
A) BT474 cells, transfected with siRNA’s, were plated on A) laminin-5 (1 × 104 cells/well) or B) non-coated plastic, treated with trastuzumab for 2 days, and cell numbers were assessed. C) Efficiency of FAK and CD151 knockdown was determined by immunoblotting.
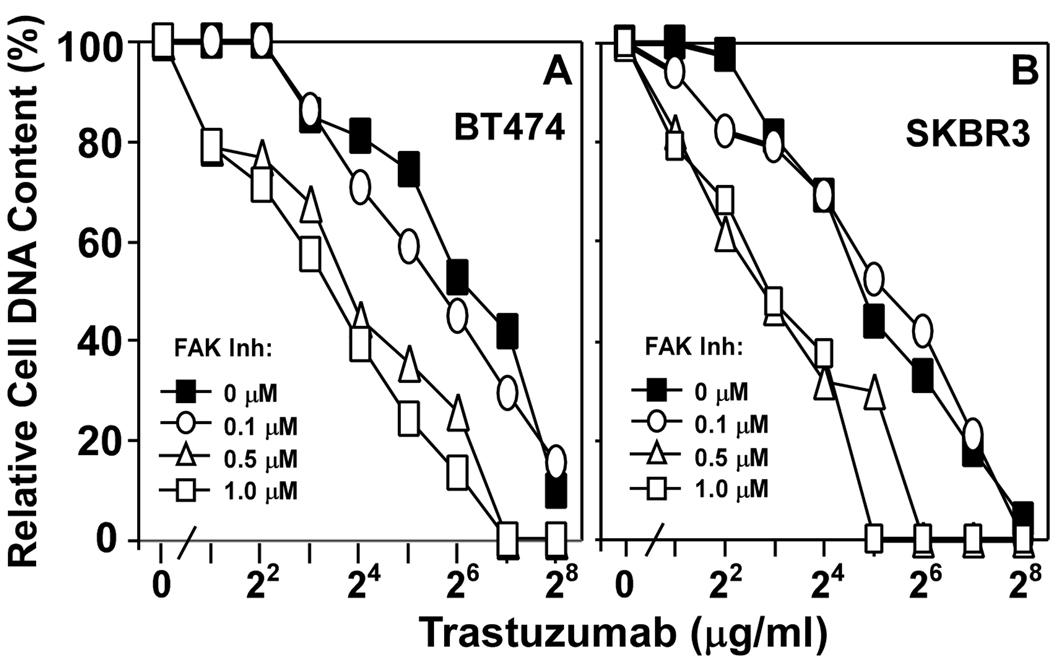
A small molecule inhibitor of FAK, TAE226, inhibits multiple tumor cell types (36, 37). Titration of TAE226 up to 1.0 µM caused only slight (27–29%) inhibition of BT474, SKBR3, ZR75, and MDA-MB-453 cell growth during a 48 hr assay (not shown). However when combined with trastuzumab, the effects of TAE226 were more dramatic. At 0.5–1.0 µM, TAE226 markedly increased trastuzumab sensitivity of BT474 (Fig. 6A) and SKBR3 (Fig. 6B) as IC50’s shifted downwards by a factor of ~4–7. FAK ablation (by siRNA) and inhibition (by TAE226) yielded similar sensitization to trastuzumab. Hence, the secondary ability of TAE226 to inhibit IGF-1R (38) appears not to be a factor in these experiments.
Figure 6. Inhibition of FAK affects trastuzumab sensitivity.
A) BT474 and B) SKBR3 cells were plated on laminin-5, and then treated with trastuzumab, together with the FAK inhibitor TAE226 at the indicated concentrations.
Discussion
Targeting of ErbB2 by trastuzumab (extracellularly) and by lapatinib (intracellularly) can effectively inhibit ErbB2+ breast cancer. Removal, replacement or inhibition of laminin-5, integrin α6β4 (or α3β1), CD151, or FAK sensitized cells to trastuzumab and lapatinib and diminished ErbB2 signaling. Hence, there is a functional collaboration between ErbB2 and laminin-5−integrin−CD151 complexes, with FAK playing a key downstream role. Our cells were sensitized to respond to trastuzumab at doses (~4–128 µg/ml) comparable to steady state levels (~80 µg/ml) obtained in human patients.
The tumor microenvironment can dramatically affect drug sensitivity (39) and integrin-dependent adhesion generally supports tumor cell drug resistance (40). Indeed, plating on laminin-5 enhanced ErbB2+ breast cancer cell resistance to trastuzumab and lapatinib. However, ErbB2+ cell resistance was not enhanced by collagen I, Matrigel, fibronectin, or non-coated plastic. There are few reports of “matrix-specific” drug resistance. Adhesion to laminin-1 promoted small cell lung cancer drug resistance (41), contrasting with our results, in which Matrigel/laminin-1 was not protective. Elsewhere, laminin-5, but not other ECM proteins, was modestly protective when added to already adherent hepatocellular carcinoma cells treated with Iressa/gefitinib (42), an EGFR tyrosine kinase inhibitor. Ours may be the first study to show a specific protective effect for laminin-5 adhesion on tumor cell drug resistance in general, and drugs targeting ErbB2 in particular. Although laminin-5 (laminin 332) may be lost as breast cancers become malignant (14), it has been suggested that laminin-5 present in normal breast tissue may support the initial transition to invasive cancer (43). In addition, laminin-5 can appear at the invasive front in some breast carcinomas (44), and its appearance may correlate with decreased breast cancer patient survival (45).
While this study focused on laminin-5, the results are relevant to other laminins. For example, laminin-10 (laminin 511), which contributes to breast cancer progression (46) and is elevated in ~35% of ErbB2+ human breast cancer samples, also supports trastuzumab resistance (not shown). Other notable similarities between laminin 10 and laminin 5 include i) recognition by integrins α3β1 and α6β4, ii) regulatory involvement of CD151, and iii) signaling through FAK (47).
Consistent with the role of laminin-5, major laminin receptors (integrins α6β4 and α3β1) also contribute to trastuzumab resistance. In a prior study, the β4 integrin cytoplasmic tail enhanced murine HER2/ErbB2+ cell resistance to gefitinib (19). However our results point not only to α6β4, but also α3β1, acting together with laminin-5 and tetraspanin CD151 to enhance ErbB2+ cell resistance to both extracellular (trastuzumab) and intracellular (lapatinib) agents. Matrigel, containing laminin-1, did not support drug resistance, most likely because its receptor (α6β1) was minimally present on our ErbB2+ cells.
Ablation of CD151 (but not tetraspanin CD9) was nearly as effective as α6 and α3 ablation, with respect to drug sensitization. Removal of CD151 does not affect cell surface integrin levels, and minimally affects cell adhesion to laminin. Although we did not obtain evidence for a direct association between ErbB2, integrins, and/or FAK, we suspect that the presence or absence of CD151 may nonetheless affect drug resistance by influencing the distributions of ErbB2, laminin-binding integrins and/or FAK relative to one another. In this regard, CD151 does indeed affect the distribution of laminin-binding integrins (26). Furthermore, CD151 recruits laminin-binding integrins into tetraspanin-enriched microdomains (TEMs), containing a variety of tetraspanins and other proteins (22). Through one or more of these proteins, CD151-integrin complexes could indirectly affect ErbB2 functional efficiency. Indeed, CD151 ablation did partly diminish ErbB2 phosphorylation in cells on laminin-5. Consistent with CD151 support of ErbB2 functional efficiency (and therefore drug resistance), mice lacking CD151 showed a marked delay in the spontaneous appearance of ErbB2-driven mammary tumors (manuscript in preparation). Hence, the integrin-CD151-ErbB2 collaboration supporting trastuzumab resistance in vitro is likely to be also relevant in vivo.
Although others suggested direct association between ErbB2 and laminin-binding integrins (48, 49), we failed to co-immunoprecipitate ErbB2 with α3β1 or α6β4, either in the presence or absence of CD151. Elsewhere, we showed that CD151 supports α6 integrin-dependent functions and promotes EGFR efficiency in human basal-like breast cancer cells (26). We suspect that support of ErbB2 and EGFR functions by laminin-5−integrin−CD151 complexes will be mechanistically similar.
FAK plays a key role in breast cancer initiation and progression (29) but was not known to affect resistance to ErbB2 (or EGFR) targeting agents. Here we show that FAK plays a key role downstream of both CD151-integrin complexes and ErbB2. CD151 ablation plus ErbB2 inhibition synergized to prevent FAK activation, suggesting that both independently activate the same FAK. CD151 ablation plus ErbB2 inhibition also synergized to prevent activation of ERK1/2, a key regulator of cell proliferation, downstream of FAK. Furthermore, FAK and CD151 knockdowns yielded similar trastuzumab sensitization (without additive effects), and drug sensitization was seen on laminin-5, but not other substrates. Hence, drug resistance promoted by CD151-integrin-laminin complexes appears to be entirely FAK-dependent. Although ablation of FAK can sensitize cells to various other anti-cancer agents (36, 50), we provide the first evidence for sensitization to ErbB2-targeting agents. Although others saw activated FAK physically associate with ErbB2 and α6β4, but not α3β1 (48), we did not see FAK coimmunoprecipitation with either α6β4 or α3β1.
The AKT kinase cascade typically regulates drug resistant cancer cell survival (35). For cells on laminin-5, trastuzumab treatment or CD151 ablation each partly diminished AKT activation, consistent with diminished cell survival. Activation of PTEN, a phosphatase that suppresses the AKT pathway, can enhance trastuzumab sensitivity, whereas PTEN loss is associated with resistance (35). However, for ErbB2+ cells on laminin-5, PTEN ablation minimally affected trastuzumab resistance. Also, neither trastuzumab nor CD151 ablation activated PTEN, despite increasing trastuzumab sensitivity. Hence, the role of PTEN is minimized for cells on laminin-5.
Inhibition of laminin-5, α3β1, α6β4, CD151 or FAK should enhance breast cancer sensitivity to drugs targeting ErbB2. Indeed, we show that an inhibitor of FAK (TAE226) can enhance trastuzumab sensitivity. Elsewhere, the same FAK inhibitor reduced cell survival in imatinib-resistant gastrointestinal stromal tumor cells (36) and enhanced ovarian cancer inhibition by the chemotherapeutic agent docetaxel (50). Our new insights into CD151 effects on drug sensitivity add to the emergence of CD151 and other tetraspanins as potential targets in cancer, infectious disease, and other pathologies (51). Targeting of CD151 offers potential advantages. First, it should provide drug sensitization while minimally disrupting vital integrin-mediated cell adhesion and signaling processes. Second, it affects functions of multiple laminin binding integrins at once. Third, it could affect other growth factor receptors, such as EGFR, which may be supported by CD151 (26). In conclusion, we have identified novel regulators of ErbB2 drug sensitivity, which may provide a new approach to sensitizing cells to drugs targeting ErbB2 and possibly other receptor tyrosine kinases.
Supplementary Material
Acknowledgments
This work was supported by NIH grant CA42368 (to M.E.H.), an S.G. Komen Career Catalyst Award, and a DOD W81XWH-06-BCRP-CA Concept Award (to X.H.Y.). Also we acknowledge the Dana-Farber Cancer Institute Center for Clinical and Translational Research.
References
- 1.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 2.Pinkas-Kramarski R, Soussan L, Waterman H, et al. Diversification of Neu differentiation factor and epidermal growth factor signaling by combinatorial receptor interactions. EMBO J. 1996;15:2452–2467. [PMC free article] [PubMed] [Google Scholar]
- 3.Esteva FJ, Cheli CD, Fritsche H, et al. Clinical utility of serum HER2/neu in monitoring and prediction of progression-free survival in metastatic breast cancer patients treated with trastuzumab-based therapies. Breast Cancer Res. 2005;7:R436–R443. doi: 10.1186/bcr1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nahta R, Esteva FJ. HER2 therapy: molecular mechanisms of trastuzumab resistance. Breast Cancer Res. 2006;8:215. doi: 10.1186/bcr1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 6.Nahta R, Yuan LX, Du Y, Esteva FJ. Lapatinib induces apoptosis in trastuzumab-resistant breast cancer cells: effects on insulin-like growth factor I signaling. Mol Cancer Ther. 2007;6:667–674. doi: 10.1158/1535-7163.MCT-06-0423. [DOI] [PubMed] [Google Scholar]
- 7.Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 8.Esteva FJ, Valero V, Booser D, et al. Phase II study of weekly docetaxel and trastuzumab for patients with HER-2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:1800–1808. doi: 10.1200/JCO.2002.07.058. [DOI] [PubMed] [Google Scholar]
- 9.Trowe T, Boukouvala S, Calkins K, et al. EXEL-7647 inhibits mutant forms of ErbB2 associated with lapatinib resistance and neoplastic transformation. Clin Cancer Res. 2008;14:2465–2475. doi: 10.1158/1078-0432.CCR-07-4367. [DOI] [PubMed] [Google Scholar]
- 10.Martin AP, Miller A, Emad L, et al. Lapatinib resistance in HCT116 cells is mediated by elevated MCL-1 expression and decreased BAK activation and not by ERBB receptor kinase mutation. Mol Pharmacol. 2008;74:807–822. doi: 10.1124/mol.108.047365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weaver VM, Lelievre S, Lakins JN, et al. beta4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell. 2002;2:205–216. doi: 10.1016/s1535-6108(02)00125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sethi T, Rintoul RC, Moore SM, et al. Extracellular matrix proteins protect small cell lung cancer cells against apoptosis: a mechanism for small cell lung cancer growth and drug resistance in vivo. Nat Med. 1999;5:662–668. doi: 10.1038/9511. [DOI] [PubMed] [Google Scholar]
- 13.Ryan MC, Lee K, Miyashita Y, Carter WG. Targeted disruption of the LAMA3 gene in mice reveals abnormalities in survival and late stage differentiation of epithelial cells. J Cell Biol. 1999;145:1309–1323. doi: 10.1083/jcb.145.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henning K, Berndt A, Katenkamp D, Kosmehl H. Loss of laminin-5 in the epithelium-stroma interface: an immunohistochemical marker of malignancy in epithelial lesions of the breast. Histopathology. 1999;34:305–309. doi: 10.1046/j.1365-2559.1999.00634.x. [DOI] [PubMed] [Google Scholar]
- 15.Zahir N, Lakins JN, Russell A, et al. Autocrine laminin-5 ligates alpha6beta4 integrin and activates RAC and NFkappaB to mediate anchorage-independent survival of mammary tumors. J Cell Biol. 2003;163:1397–1407. doi: 10.1083/jcb.200302023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang H, Fu W, Im JH, et al. Tumor cell alpha3beta1 integrin and vascular laminin-5 mediate pulmonary arrest and metastasis. J Cell Biol. 2004;164:935–941. doi: 10.1083/jcb.200309112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawano K, Yanagisawa S. Predictive value of laminin-5 and membrane type 1-matrix metalloproteinase expression for cervical lymph node metastasis in T1 and T2 squamous cell carcinomas of the tongue and floor of the mouth. Head Neck. 2006;28:525–533. doi: 10.1002/hed.20349. [DOI] [PubMed] [Google Scholar]
- 18.Zutter MM. Integrin-mediated adhesion: tipping the balance between chemosensitivity and chemoresistance. Adv Exp Med Biol. 2007;608:87–100. doi: 10.1007/978-0-387-74039-3_6. [DOI] [PubMed] [Google Scholar]
- 19.Guo W, Pylayeva Y, Pepe A, et al. Beta 4 integrin amplifies ErbB2 signaling to promote mammary tumorigenesis. Cell. 2006;126:489–502. doi: 10.1016/j.cell.2006.05.047. [DOI] [PubMed] [Google Scholar]
- 20.Bachelder RE, Ribick MJ, Marchetti A, et al. p53 inhibits alpha 6 beta 4 integrin survival signaling by promoting the caspase 3-dependent cleavage of AKT/PKB. J Cell Biol. 1999;147:1063–1072. doi: 10.1083/jcb.147.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedland JC, Lakins JN, Kazanietz MG, et al. alpha6beta4 integrin activates Rac-dependent p21-activated kinase 1 to drive NF-kappaB-dependent resistance to apoptosis in 3D mammary acini. J Cell Sci. 2007;120:3700–3712. doi: 10.1242/jcs.03484. [DOI] [PubMed] [Google Scholar]
- 22.Hemler ME. Tetraspanin proteins mediate cellular penetration, invasion and fusion events, and define a novel type of membrane microdomain. Ann Rev Cell Dev Biol. 2003;19:397–422. doi: 10.1146/annurev.cellbio.19.111301.153609. [DOI] [PubMed] [Google Scholar]
- 23.Hashida H, Takabayashi A, Tokuhara T, et al. Clinical significance of transmembrane 4 superfamily in colon cancer. Br J Cancer. 2003;89:158–167. doi: 10.1038/sj.bjc.6601015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tokuhara T, Hasegawa H, Hattori N, et al. Clinical significance of CD151 gene expression in non-small cell lung cancer. Clin Cancer Res. 2001;7:4109–4114. [PubMed] [Google Scholar]
- 25.Sauer G, Kurzeder C, Grundmann R, et al. Expression of tetraspanin adaptor proteins below defined threshold values is associated with in vitro invasiveness of mammary carcinoma cells. Oncol Rep. 2003;10:405–410. [PubMed] [Google Scholar]
- 26.Yang XH, Richardson AL, Torres-Arzayus MI, et al. CD151 accelerates breast cancer by regulating α6 integrin functions, signaling, and molecular organization. Cancer Research. 2008;68:3204–3213. doi: 10.1158/0008-5472.CAN-07-2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 28.Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci. 2003;116:1409–1416. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- 29.Mitra SK, Lim ST, Chi A, Schlaepfer DD. Intrinsic focal adhesion kinase activity controls orthotopic breast carcinoma metastasis via the regulation of urokinase plasminogen activator expression in a syngeneic tumor model. Oncogene. 2006;25:4429–4440. doi: 10.1038/sj.onc.1209482. [DOI] [PubMed] [Google Scholar]
- 30.Carter WG, Kaur P, Gil SG, Gahr PJ, Wayner EA. Distinct functions for integrins alpha3\beta1 in focal adhesions and alpha6beta4/bullous pemphigoid antigen in a new stable anchoring contact (SAC) of keratinocytes: relation to hemidesmosome. J Cell Biol. 1990;111:3141–3154. doi: 10.1083/jcb.111.6.3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nguyen BP, Gil SG, Carter WG. Deposition of laminin 5 by keratinocytes regulates integrin adhesion and signaling. J Biol Chem. 2000;275:31896–31907. doi: 10.1074/jbc.M006379200. [DOI] [PubMed] [Google Scholar]
- 32.Berditchevski F, Odintsova E. Characterization of integrin-tetraspanin adhesion complexes: role of tetraspanins in integrin signaling. J Cell Biol. 1999;146:477–492. doi: 10.1083/jcb.146.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takeda Y, Kazarov AR, Butterfield CE, et al. Deletion of tetraspanin Cd151 results in decreased pathologic angiogenesis in vivo and in vitro. Blood. 2007;109:1524–1532. doi: 10.1182/blood-2006-08-041970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Medina PJ, Goodin S. Lapatinib: a dual inhibitor of human epidermal growth factor receptor tyrosine kinases. Clin Ther. 2008;30:1426–1447. doi: 10.1016/j.clinthera.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 35.Nagata Y, Lan KH, Zhou X, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6:117–127. doi: 10.1016/j.ccr.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 36.Sakurama K, Noma K, Takaoka M, et al. Inhibition of focal adhesion kinase as a potential therapeutic strategy for imatinib-resistant gastrointestinal stromal tumor. Mol Cancer Ther. 2009;8:127–134. doi: 10.1158/1535-7163.MCT-08-0884. [DOI] [PubMed] [Google Scholar]
- 37.Halder J, Lin YG, Merritt WM, et al. Therapeutic efficacy of a novel focal adhesion kinase inhibitor TAE226 in ovarian carcinoma. Cancer Res. 2007;67:10976–10983. doi: 10.1158/0008-5472.CAN-07-2667. [DOI] [PubMed] [Google Scholar]
- 38.Liu TJ, LaFortune T, Honda T, et al. Inhibition of both focal adhesion kinase and insulin-like growth factor-I receptor kinase suppresses glioma proliferation in vitro and in vivo. Mol Cancer Ther. 2007;6:1357–1367. doi: 10.1158/1535-7163.MCT-06-0476. [DOI] [PubMed] [Google Scholar]
- 39.Meads MB, Hazlehurst LA, Dalton WS. The bone marrow microenvironment as a tumor sanctuary and contributor to drug resistance. Clin Cancer Res. 2008;14:2519–2526. doi: 10.1158/1078-0432.CCR-07-2223. [DOI] [PubMed] [Google Scholar]
- 40.Damiano JS. Integrins as novel drug targets for overcoming innate drug resistance. Curr Cancer Drug Targets. 2002;2:37–43. doi: 10.2174/1568009023334033. [DOI] [PubMed] [Google Scholar]
- 41.Tsurutani J, West KA, Sayyah J, Gills JJ, Dennis PA. Inhibition of the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin pathway but not the MEK/ERK pathway attenuates laminin-mediated small cell lung cancer cellular survival and resistance to imatinib mesylate or chemotherapy. Cancer Res. 2005;65:8423–8432. doi: 10.1158/0008-5472.CAN-05-0058. [DOI] [PubMed] [Google Scholar]
- 42.Giannelli G, Azzariti A, Fransvea E, et al. Laminin-5 offsets the efficacy of gefitinib ('ressa') in hepatocellular carcinoma cells. Br J Cancer. 2004;91:1964–1969. doi: 10.1038/sj.bjc.6602231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carpenter PM, Dao AV, Arain ZS, et al. Motility induction in breast carcinoma by mammary epithelial laminin 332 (laminin 5) Mol Cancer Res. 2009;7:462–475. doi: 10.1158/1541-7786.MCR-08-0148. [DOI] [PubMed] [Google Scholar]
- 44.Pyke C, Romer J, Kallunki P, et al. The gamma 2 chain of kalinin/laminin 5 is preferentially expressed in invading malignant cells in human cancers. Am J Pathol. 1994;145:782–791. [PMC free article] [PubMed] [Google Scholar]
- 45.Faverly DR, Burgers L, Bult P, Holland R. Three dimensional imaging of mammary ductal carcinoma in situ: clinical implications. Semin Diagn Pathol. 1994;11:193–198. [PubMed] [Google Scholar]
- 46.Chia J, Kusuma N, Anderson R, et al. Evidence for a role of tumor-derived laminin-511 in the metastatic progression of breast cancer. Am J Pathol. 2007;170:2135–2148. doi: 10.2353/ajpath.2007.060709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamada M, Sumida Y, Fujibayashi A, et al. The tetraspanin CD151 regulates cell morphology and intracellular signaling on laminin-511. FEBS J. 2008;275:3335–3351. doi: 10.1111/j.1742-4658.2008.06481.x. [DOI] [PubMed] [Google Scholar]
- 48.Wang SE, Xiang B, Zent R, et al. Transforming growth factor beta induces clustering of HER2 and integrins by activating Src-focal adhesion kinase and receptor association to the cytoskeleton. Cancer Res. 2009;69:475–482. doi: 10.1158/0008-5472.CAN-08-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hintermann E, Bilban M, Sharabi A, Quaranta V. Inhibitory role of alpha 6 beta 4-associated erbB-2 and phosphoinositide 3-kinase in keratinocyte haptotactic migration dependent on alpha 3 beta 1 integrin. J Cell Biol. 2001;153:465–478. doi: 10.1083/jcb.153.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Halder J, Landen CN, Jr, Lutgendorf SK, et al. Focal adhesion kinase silencing augments docetaxel-mediated apoptosis in ovarian cancer cells. Clin Cancer Res. 2005;11:8829–8836. doi: 10.1158/1078-0432.CCR-05-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hemler ME. Targeting of tetraspanin proteins - potential benefits and strategies. Nature Drug Discovery. 2008;7:747–758. doi: 10.1038/nrd2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.