Abstract
Excessive synaptic loss is thought to be one of the earliest events in Alzheimer’s disease. Amyloid beta (Aβ), a peptide secreted in an activity-modulated manner by neurons, has been implicated in the pathogenesis of Alzheimer’s disease by removing dendritic spines, sites of excitatory synaptic transmission. However, issues regarding the subcellular source of Aβ, as well as the mechanisms of its production and actions that lead to synaptic loss, remain poorly understood. In rat organotypic slices, we found that acute overproduction of either axonal or dendritic Aβ reduced spine density and plasticity at nearby (~5–10 µm) dendrites. The production of Aβ and its effects on spines were sensitive to blockade of action potentials or nicotinic receptors; the effects of Aβ (but not its production) were sensitive to NMDA receptor blockade. Notably, only 30–60 min blockade of Aβ overproduction permitted induction of plasticity. Our results indicate that continuous overproduction of Aβ at dendrites or axons acts locally to reduce the number and plasticity of synapses.
The early stages of Alzheimer’s disease pathogenesis are thought to occur at the synapse, since synapse loss is the best correlate with memory dysfunction1. Considerable evidence suggests that Aβ, a secreted proteolytic derivative of amyloid precursor protein (APP), is important for the early ‘synaptic failure’ that is seen in Alzheimer’s disease pathogenesis (reviewed in ref. 2). Aβ oligomers bind to synaptic sites3 and reduce the density of spines in organotypic hippocampal slice cultures4–6, dissociated cultured neurons7–9 and transgenic mouse models10–12. Consistent with these structural abnormalities, neurons treated with Aβ or that overexpress APP show depressed glutamatergic transmission4,7,13–16.
Given the memory deficits observed in Alzheimer’s disease, it is notable that soluble Aβ oligomers impair long-term potentiation (LTP, a synaptic model of memory)11,17,18 and memory11,19,20, which can be ameliorated by treatment with antibody to Aβ or small molecules that inhibit Aβ aggregation21–24. Despite the well-studied effect of Aβ on electrophysiological LTP, its effect on spine structural plasticity, which occurs during LTP25–28, has not been examined. For instance, it is not known if increased production of Aβ in one neuron will affect structural plasticity in a nearby neuron.
Although Aβ perturbs synaptic transmission and plasticity, such Aβ-mediated processes are subject to activity-dependent modulation. The level of Aβ secretion is controlled by neural activity in brain slices13 and in vivo29. In humans, regions of the brain with high resting activity are positively correlated with Aβ plaque load30. In addition to Aβ production, the effects of Aβ may also depend on neural activity. For instance, NMDA receptor activation is required for Aβ-mediated spine loss5 and synaptic depression13.
To understand the interaction between Aβ and synaptic function, we sought to identify the subcellular sites from which Aβ acts. In particular, it has not been clearly established whether the Aβ that produces synaptic deficits is generated in pre- or postsynaptic compartments. APP and its derivatives, as well as components of the APP-processing enzymes β-secretase and γ-secretase, have been detected in axons and dendrites by biochemical, immunostaining and electron-microscopy studies31–37. However, detecting effects produced from axonally or dendritically produced Aβ has been challenging. We isolated the sites of increased Aβ production by selectively expressing APP in pre- or postsynaptic neurons. We used two-photon laser-scanning imaging to monitor the synaptic deficits caused by such dendritic or axonal Aβ. We found that either dendritic or axonal Aβ overproduction was sufficient to cause local spine loss and compromise plasticity in the nearby dendrites of neurons that did not overexpress Aβ. Furthermore, Aβ-mediated synaptic dysfunction could be pharmacologically ameliorated by blockade of neural activity, NMDA receptors or nicotinic acetylcholine receptors. Our findings indicate that local levels and effects of dendritic and axonal Aβ can be controlled by activity-modulated mechanisms.
RESULTS
Dendritic Aβ reduces spine density at nearby dendrites
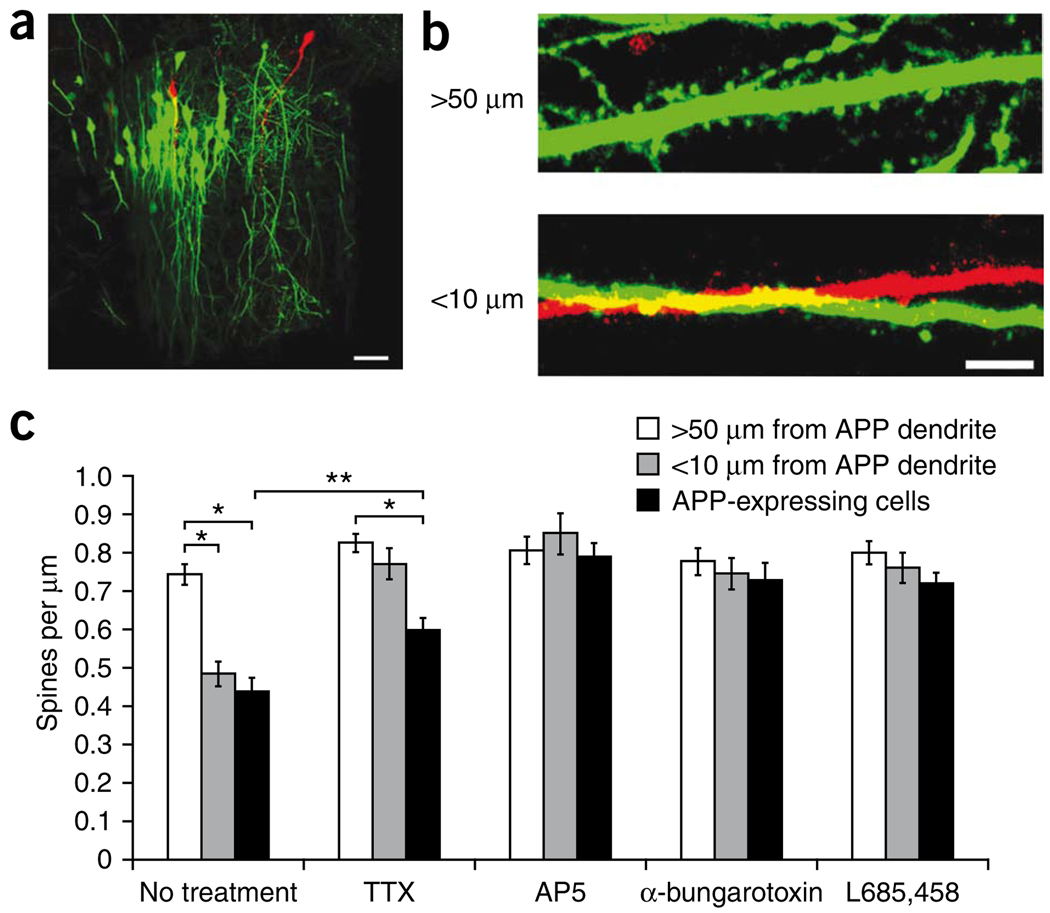
Previous studies have shown that overexpression of APP leads to Aβ secretion and a loss of spines on neurons overproducing APP4,10 that phenocopies quantitatively the effects of exogenous addition of oligomeric Aβ5,6,9,38 at concentrations reached in the brains of individuals with Alzheimer’s disease36. This robust effect of Aβ on spine density allowed us to use an imaging assay to monitor the effects of local Aβ. We reasoned that if Aβ overproduction could cause spine loss in APP-overexpressing neurons, then neighboring neurons close to Aβ-containing structures may also be exposed to higher levels of secreted Aβ and therefore lose their spines. We first tested whether Aβ from dendrites of APP-overexpressing neurons can affect neighboring dendrites that do not overexpress APP. The CA1 region of a hippocampal slice culture was infected with two Sindbis viruses: an enhanced green fluorescent protein (EGFP) virus that did not express APP at a moderate titer to label multiple neurons and a double promoter APP/tomato virus at a very low titer so that only one or a few cells were infected and expressed APP (Fig. 1a). Two-photon laser-scanning microscopy was used to image 50–100-µm segments of CA1 pyramidal neuron apical dendrites located 150–250 µm from the cell body 1 d after APP overexpression. EGFP-labeled CA1 dendrites that did not overexpress APP were divided into two groups: a group of dendrites running parallel and staying within 10 µm of an APP-overexpressing dendrite and a control group consisting of dendrites from the same infected slices, but which were more than 50 µm away from any APP-overexpressing structures (Fig. 1b). The spine density of EGFP-labeled dendrites more than 50 µm from APP-overexpressing dendrites was not significantly different from the spine density in slices with no APP overexpression (P = 0.2, data not shown). However, both EGFP-labeled dendrites within 10 µm of APP-overexpressing dendrites and APP-overexpressing dendrites showed a reduction in spine density (Fig. 1c). The reduction in spine density was blocked by a γ-secretase inhibitor (L685,458; Fig. 1c), indicating that Aβ from dendrites of APP-overexpressing cells caused spine loss in a population of nearby dendrites. Similar effects were observed when we overexpressed APP in hippocampal neurons in vivo; dendrites expressing only EGFP that were within 10 µm of a dendrite from an APP-overexpressing neuron showed a reduction in spine density (Supplementary Fig. 1).
Figure 1.
Dendritic Aβ reduces local spine density in an activity-dependent manner. (a) Viral-infected CA1 region of a hippocampal slice culture showing many EGFP-expressing and a few APP/tomato-expressing neurons. Scale bar represents 50 µm. (b) Apical dendrites of CA1 pyramidal neurons expressing EGFP or APP/tomato as in a. Top, an EGFP-labeled dendritic segment >50 µm away from APP-labeled structures. Bottom, an EGFP-labeled dendritic segment that is within 10 µm of an APP-labeled dendrite. Scale bar represents 5 µm. (c) Bar graph of spine density in EGFP neurons near or far from APP dendrites, and APP-expressing neurons in normal medium or medium containing TTX, AP5, α-bungarotoxin or L685,458 (no treatment (14 slices): 21, 14 and 17 dendrites for >50 µm from APP dendrite, <10 µm from APP dendrites and APP-expressing cells, respectively; TTX (13 slices): 18, 14 and 25; AP5 (10 slices): 15, 13 and 18; α-bungarotoxin (15 slices): 13, 15 and 19; L685,458 (13 slices): 19, 13 and 11). Error bars represent s.e.m. Two-way ANOVA showed significant drug × APP interaction (F = 4.38, P < 0.001; t test, * P < 0.0001, ** P = 0.002).
Neural activity has been shown to be involved in the modulation of Aβ production and effects5,13,29,30. We examined how activity influenced spine loss from dendritic Aβ production. Slices were infected with the EGFP virus and the APP virus and cultured with either the sodium channel antagonist tetrodotoxin (TTX), the NMDA receptor antagonist d(−)-2-amino-5-phosphonovaleric acid (AP5), α-bungarotoxin, an antagonist of the α7 subunit–containing nicotinic acetylcholine receptor (nAChR) or no drugs. We imaged EGFP-labeled and APP-overexpressing dendrites 1 d after APP overexpression and measured spine density. All of the drugs had a protective effect on spine loss mediated by dendritic Aβ production (Fig. 1c). These results indicate that spine loss resulting from 1 d of dendritic Aβ overproduction can be reduced by blockade of action potentials, nAChRs or NMDA receptors.
Axonal Aβ reduces spine density at nearby dendrites
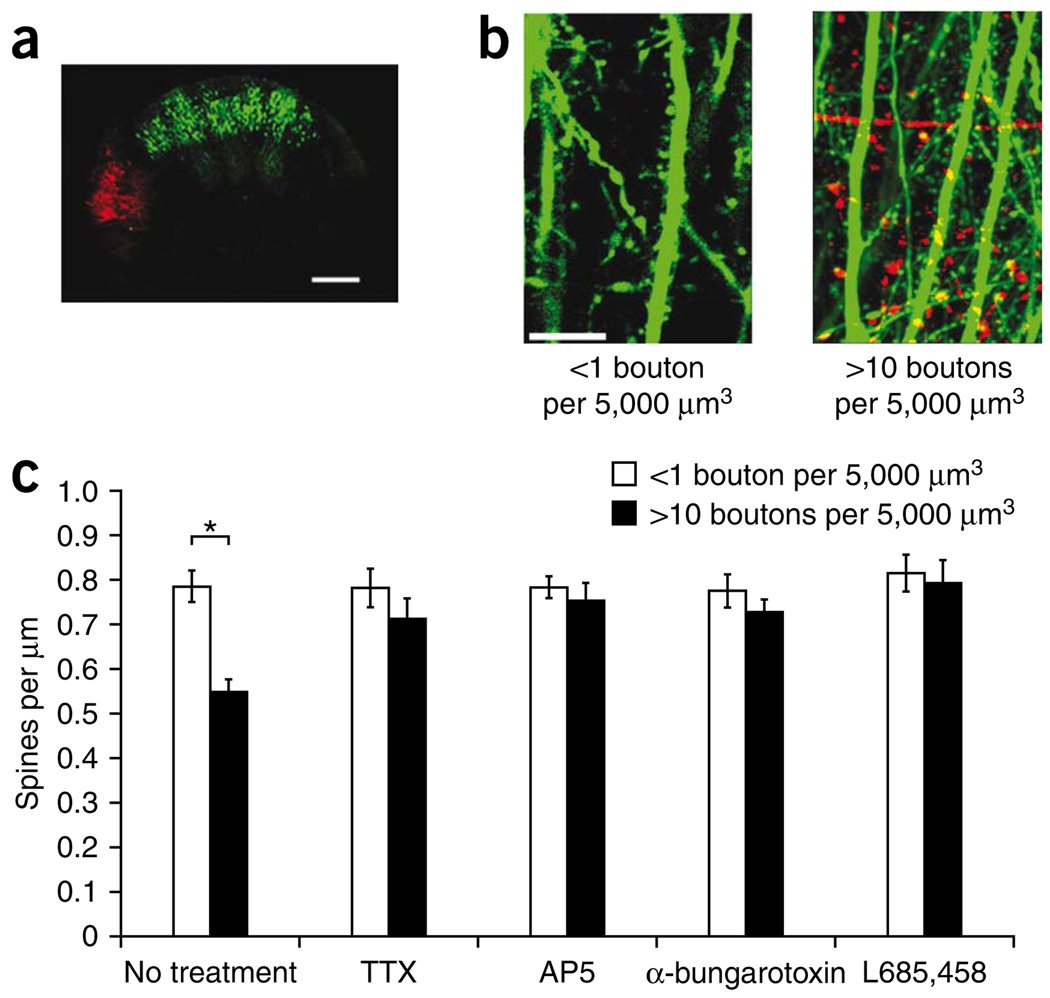
We next examined whether the axonal compartment can also be a source of Aβ. To specifically examine the effect of axons, we infected CA3 neurons with the APP/tomato virus and CA1 neurons with the EGFP virus (Fig. 2a). We examined CA1 regions far (>200 µm) from the CA3 dendritic region that contained axonal projections from CA3 pyramidal neurons. Thus, axons from APP virus–infected CA3 neurons were the only APP-overexpressing structures in the CA1 regions that we examined. In each slice, we were able to find areas in the CA1 stratum radiatum containing many APP-overexpressing axons (more than ten APP-overexpressing boutons per 5,000 µm3, near APP axons) as well as areas containing very few or no APP-overexpressing axons (less than one APP-overexpressing bouton per 5,000 µm3, far from APP axons; Fig. 2b). After 2 d of infection, the spine density of EGFP dendrites in APP virus–infected slices, but far from APP-labeled axons was not significantly different from the spine density of EGFP dendrites in slices with no APP overexpression (P = 0.8, data not shown). However, EGFP-labeled dendrites near APP axons showed reduced spine density compared with the EGFP group far from APP axons (Fig. 2c). The spine reduction by axonally expressed APP was blocked by γ-secretase inhibition, indicating that Aβ from axons reduced local spine density.
Figure 2.
Axonal Aβ reduces local spine density in an activity-dependent manner. (a) Tiled two-photon laser-scanning microscopy images of a hippocampal slice culture infected with EGFP virus in the CA1 region and APP/tomato virus in the CA3 region. Scale bar represents 300 µm. (b) Images from CA1 stratum radiatum region showing a control region containing EGFP-labeled apical dendrites and <1 APP bouton per 5,000 µm3 (left) or a region containing EGFP-labeled apical dendrites and >10 APP boutons per 5,000 µm3 (right). Scale bar represents 10 µm. (c) Bar graph of spine density of EGFP neurons in regions containing <1 APP bouton per 5,000 µm3 and in regions containing >10 APP boutons per 5,000 µm3. Slices were maintained in normal medium or in medium containing TTX, AP5, α-bungarotoxin or L685,458, as indicated (no treatment (21 slices): 21 and 20 dendrites for <1 APP bouton per 5,000 µm3 and >10 APP boutons per 5,000 µm3; TTX (10 slices): 20 and 15; AP5 (12 slices): 24 and 17; α-bungarotoxin (10 slices): 20 and 39; L685,458 (10 slices): 15 and 11). Two-way ANOVA showed significant drug × APP interaction (F = 2.77, P = 0.005; t test, *P < 0.0001). Similar effects, although less robust, were seen after 1 d of APP overexpression (data not shown). Error bars represent s.e.m.
To examine whether neural activity can modulate spine loss from axonal Aβ, we infected slices with the EGFP and APP/tomato viruses and maintained them in TTX, AP5 or α-bungarotoxin. All three drugs prevented axonal Aβ–mediated spine loss in EGFP-labeled dendrites near APP axons (Fig. 2c). These results indicate that spine loss from axonal Aβ is subject to activity-dependent modulation.
Pharmacological control of production and effects of Aβ
Although blockade of action potentials, nAChR or NMDA receptors affected Aβ-mediated spine loss, it is not clear whether these treatments regulate Aβ production or the effects of Aβ on dendritic spines. We wished to determine whether AP5, TTX or α-bungarotoxin had an effect on Aβ production. The CA1 region of hippocampal slice cultures was infected with APP virus and incubated in normal medium or in medium containing AP5, TTX, α-bungarotoxin or L685,458 for 24 h. Both the infected slices and slice culture media were harvested at the end of the drug treatment. Aβ was measured in the media by ELISA and full-length APP was measured in slices by western blotting. Secreted Aβ levels were compared across samples by normalizing secreted Aβ by levels of APP expression. Treatment of slices with TTX, α-bungarotoxin or L685,458 led to reduced Aβ levels in the medium of APP virus–infected slices (Fig. 3a,b). Notably, AP5 had no effect on secretion of Aβ.
Figure 3.
Aβ secretion is sensitive to blockade of action potentials or nAChRs, but not to blockade of NMDA receptors. (a) The CA1 region of hippocampal slice cultures was infected with APP and incubated in normal media (control) or in media containing AP5, TTX, α-bungarotoxin or L685,458. Secreted Aβ (1–42) levels were normalized to that of the control group (control, 8 membranes; AP5, 8 membranes; TTX, 4 membranes; α-bungarotoxin, 5 membranes; L685,458, 5 membranes; ANOVA, F = 4.2, P = 0.009; t test, * P < 0.002). (b) Secreted Aβ (1–40) levels were analyzed as in a (control, 8 membranes; AP5, 9 membranes; TTX, 5 membranes; α-bungarotoxin, 5 membranes; L685,458, 5 membranes; ANOVA, F = 7.54, P = 0.0004; t test between control and drug-treated groups, * P < 0.01). Error bars represent s.e.m.
We found that action potentials and nAChR were able to regulate Aβ secretion; however, our results do not exclude the possibility that they can also regulate the effect of Aβ on dendritic spines. To address this, we added synthetic Aβ42, prepared in a manner that promotes formation of oligomeric structures (see Online Methods), to the media of EGFP virus–infected slice cultures for 24 h in the presence of TTX, α-bungarotoxin or AP5. Neither TTX nor α-bungarotoxin prevented Aβ-mediated spine loss (Fig. 4), indicating that action potentials and nAChRs regulate Aβ secretion, but not the effect of Aβ on spine loss. Although AP5 had no effect on Aβ secretion (Fig. 3a,b), AP5-treated neurons had normal spine density in the presence of synthetic Aβ (Fig. 4), indicating that NMDA receptor blockade modulated the effect of Aβ on spine density, but not secretion of Aβ. Together, these results indicate that different forms of activity can modulate Aβ-mediated spine loss through the regulation of Aβ secretion and by regulating the effects of Aβ on dendritic spines.
Figure 4.
Synthetic Aβ-induced spine loss can be rescued by blockade of NMDA receptors, but not by blockade of action potentials or nAChRs. The CA1 region of hippocampal slice cultures was infected with EGFP virus and incubated in normal medium or in medium containing Aβ (1–42) and AP5, TTX or α-bungarotoxin as indicated. Spine density was measured after 24 h of infection and drug treatment (no treatment: 17 dendrites, 9 slices; Aβ42: 24 dendrites, 10 slices; Aβ42 + AP5: 15 dendrites, 9 slices; Aβ42 + TTX: 13 dendrites, 7 slices; Aβ42 + α-bungarotoxin: 14 dendrites, 7 slices; ANOVA, F = 7.27, P < 0.0001; t test between no treatment and drug-treated groups, *P < 0.002). Error bars represent s.e.m.
Aβ reduces spine structural plasticity
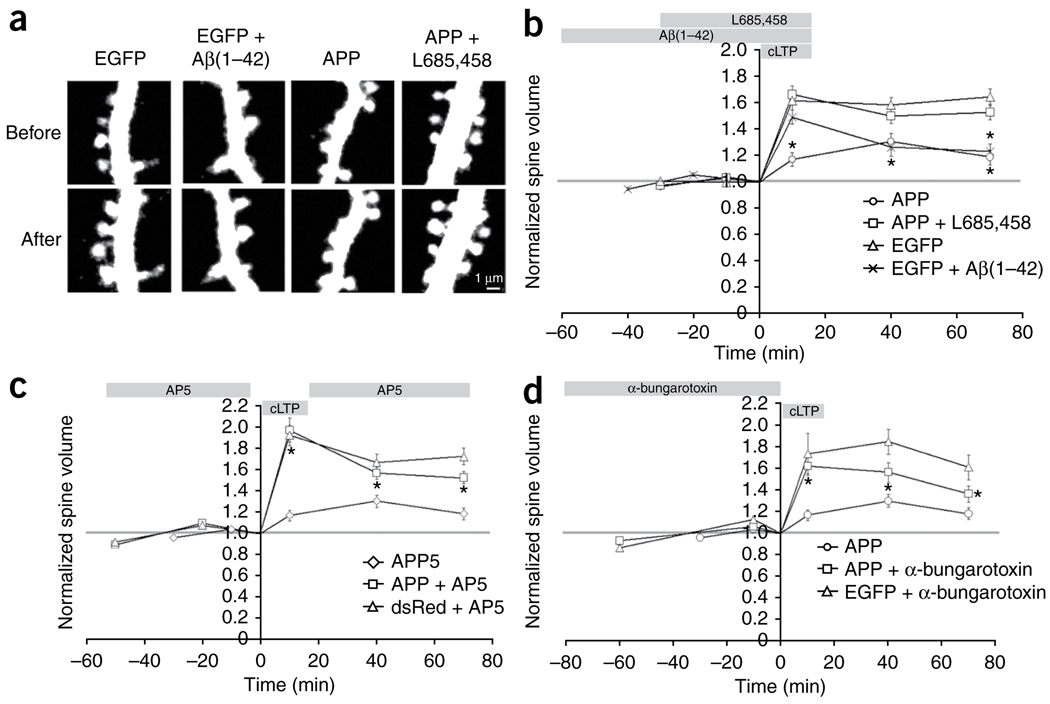
These results indicate that a substantial fraction of dendritic spines are lost over time in the presence of Aβ from dendrites or axons. But what is the effect of Aβ on the plasticity of the remaining spines? We next examined the structural plasticity of spines in the presence of Aβ. LTP and a long-lasting spine enlargement can be reliably induced in slices by a brief bath application of a solution that favors NMDA receptor activation and global neuronal bursting25,39. Using this chemical LTP (cLTP) induction protocol, we examined spine enlargement in neurons that had been transfected with both APP and the cytoplasmic marker dsRed by biolistics transfection for 3 d. Images of spines were taken at multiple time points before, during and after cLTP induction. The volume of each spine at each time point was measured and normalized to the baseline value before cLTP induction. EGFP-transfected cells had stable cLTP-induced spine enlargement for at least 70 min after induction. However, APP-transfected neurons showed less cLTP-induced spine enlargement (Fig. 5a,b), indicating that structural plasticity was impaired in the APP-overexpressing cells. This compromised plasticity was reversible, as addition of L685,458 for 30 min before and during cLTP induction rescued the spine enlargement deficit in APP-overexpressing neurons (Fig. 5b). This indicates that continuous Aβ production is necessary to impair structural plasticity.
Figure 5.
Acute overproduction of Aβ reduces spine structural plasticity by NMDAR- and nAChR-dependent mechanisms. (a) Spine volumes from neurons transfected with EGFP or APP/dsRed before and after cLTP induction. Aβ (1–42) or L685,458 were applied as indicated. The signals from the cytoplasmic markers are displayed. Scale bar represents 1 µm. (b) Acute overproduction of Aβ reduced spine structural plasticity. The time of cLTP induction, Aβ (1–42) or L685,458 application is indicated at the top of the graph (APP: n = 166 spines, 5 slices; APP + L685,458: n = 312 spines, 8 slices; EGFP: n = 247 spines, 6 slices; EGFP + Aβ (1–42): n = 275 spines, 6 slices; ANOVA, F = 7.99, P < 0.0001; t test between APP and APP + L685,458 and between EGFP and EGFP + Aβ (1–42), *P < 0.0004). (c) AP5 rescued Aβ-mediated impairment of cLTP spine enlargement. Data from untreated APP-expressing neurons in b is also shown (APP + AP5: n = 254 spines, 8 slices; dsRed + AP5: n = 185 spines, 5 slices; ANOVA, F = 1.34, P < 0.0001; t test between APP and APP + AP5, *P < 0.001). (d) α-bungarotoxin rescued Aβ-mediated impairment of spine enlargement after cLTP induction. Data from untreated APP-expressing neurons in b is also shown (APP + α-bungarotoxin: n = 153 spines, 5 slices; EGFP + α-bungarotoxin: n = 119 spines, 4 slices; ANOVA, F = 7.62, P < 0.0001; t test between APP and APP + α-bungarotoxin, * P < 0.01). Error bars represent s.e.m.
To further test the acute effect of Aβ on structural plasticity, we briefly added synthetic oligomeric Aβ(1–42) to EGFP-transfected neurons 1 h before and during cLTP induction. Compared with the untreated control, oligomeric Aβ(1–42)-treated neurons showed less spine enlargement after induction (Fig. 5a,b). Thus, short exposure of wild-type neurons to synthetic oligomeric Aβ is sufficient to impair cLTP-induced spine enlargement.
Given the modulatory roles of NMDAR and nAChR in Aβ-mediated spine loss, we examined their involvement in cLTP-induced spine enlargement in neurons overexpressing APP. To block NMDAR activation, we added AP5 1 h before cLTP induction, briefly removing it during cLTP induction (as NMDAR activity is required for cLTP induction) and replacing it after cLTP induction. This treatment led to normal spine enlargement in APP-overexpressing neurons (Fig. 5c), indicating that an AP5-sensitive process is continuously activated by APP overexpression to prevent cLTP-induced spine plasticity.
Blockade of nAChR was achieved by the addition of α-bungarotoxin 1 h before cLTP induction. Although the subsequent cLTP induction and expression was performed in solutions free of α-bungarotoxin, nAChRs were likely blocked as a result of the very slow off-rate of α-bungarotoxin40. Following treatment with α-bungarotoxin, spines from APP-overexpressing neurons showed more spine enlargement after cLTP induction (Fig. 5d). These results indicate that blockade of NMDAR and nAChR can substantially reduce the Aβ-mediated deficit in cLTP-induced spine enlargement.
Axonal and dendritic Aβ reduces plasticity at nearby dendrites
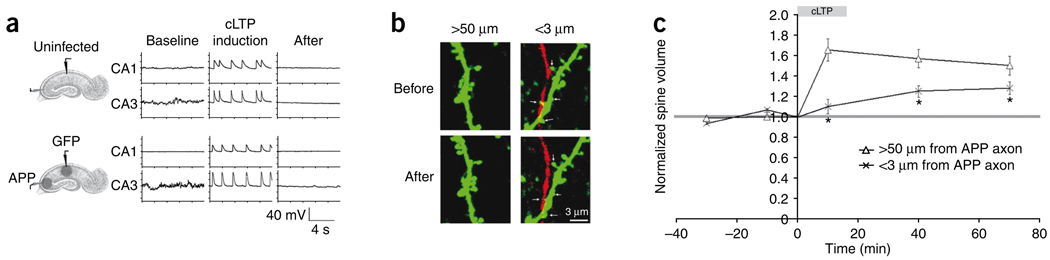
The effects of axonal Aβ on spine plasticity were examined in a population of spines close to APP-overexpressing axons. EGFP and APP/dsRed were expressed in the CA1 and CA3 regions, respectively. We first conducted a control experiment to determine whether slices with APP-overexpressing CA3 neurons would exhibit similar synchronous bursting during cLTP induction as slices without APP-overexpressing CA3 neurons. This control experiment was necessary, as APP-overexpression can produce synaptic depression13 and synaptic activity is important for synchronizing CA3 bursting. Cell-attached recordings were performed simultaneously in pairs of CA1 and CA3 neurons before and during cLTP induction. Compared with uninfected controls, slices infected with EGFP and APP viruses showed similar synchronous bursting in both CA1 and CA3 neurons (Fig. 6a and Supplementary Fig. 2). This indicates that the APP overexpression in the CA3 region did not inhibit the neuronal bursting induced by the cLTP protocol. Indeed, normal spine enlargement was observed in EGFP-labeled CA1 cells far from APP-overexpressing axons (>50 µm) in the infected slices (Fig. 6b,c). However, EGFP-labeled spines within 3 µm of APP-overexpressing axons showed a reduction in spine enlargement (Fig. 6b,c). This indicates that axonal Aβ impairs nearby spine plasticity.
Figure 6.
Axonal secretion of Aβ reduces local spine structural plasticity. (a) Example traces of cell-attached recordings from a pair of uninfected CA1 and CA3 pyramidal neurons (top) or a pair of EGFP-infected CA1 cells and APP-infected CA3 cells (bottom) before, during and after cLTP induction. (b) Left, examples of EGFP-labeled spines more than 50 µm away from APP-labeled axons before and after cLTP. Right, EGFP-labeled spines within 3 µm of an APP-labeled axon before and after cLTP (arrows). Scale bar represents 3 µm. (c) The volume change of spines >50 µm from APP axons or <3 µm from APP axons before, during and after cLTP induction (>50 µm from APP axon: n = 139 spines, 25 slices; <3 µm from APP axon: n = 143 spines, 25 slices; ANOVA, F = 6.13, P = 0.0001; t test between >50 um from APP axon and <3 um from APP axon, *P < 0.0005). Error bars represent s.e.m.
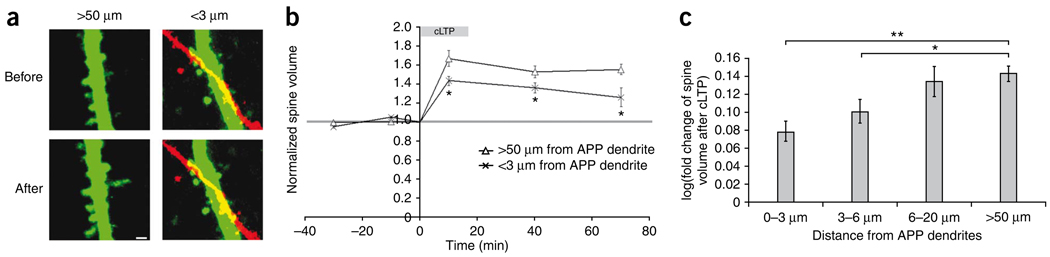
To test whether dendritic Aβ affects plasticity in neighboring spines, we examined cLTP-induced spine enlargement in spines close to APP-overexpressing dendrites. The CA1 region of a hippocampal slice culture was infected with the EGFP and APP viruses. EFGP-labeled spines within 3 µm of an APP-overexpressing dendrite were grouped as spines close to APP dendrites (Fig. 7a). Spines more than 50 µm away from any APP-overexpressing structure were used as a control group. Following cLTP induction, spines near APP-overexpressing dendrites showed reduced enlargement compared with the control group (Fig. 7b), indicating that Aβ from APP-overexpressing dendrites reduces spine plasticity at nearby dendrites.
Figure 7.
Dendritic secretion of Aβ reduces local spine structural plasticity. (a) EGFP-labeled spines before and after cLTP induction. Left, spines more than 50 µm away from APP-labeled structures. Right, EGFP-labeled spines within 3 µm of an APP-labeled dendrite. Scale bar represents 1 µm. (b) The volume change of spines >50 µm from APP dendrites and of those <3 µm from APP dendrites before, during and after cLTP induction (>50 µm from APP dendrite: n = 194 spines, 42 slices; <3 µm from APP dendrite: n = 243 spines, 42 slices; ANOVA, F = 33.1, P < 0.0001; t test between >50 um from APP dendrite and <3 um from APP dendrite, *P < 0.01). (c) Bar graph of spine volume change after cLTP (log transformed) in groups of spines 0–3 µm, 3–6 µm, 6–20 µm or >50 µm from APP dendrites (0–3 µm: 0.079 ± 0.011, 358 spines; 3–6 µm: 0.101 ± 0.013, 235 spines; 6–20 µm: 0.134 ± 0.016, 180 spines; >50 µm: 0.143 ± 0.009, 580 spines, from 68 slices; ANOVA, F = 7.77, P < 0.0001; t test, *P = 0.008, **P < 0.001). Error bars represent s.e.m.
These results predict that the further away the spines are from the Aβ production site, the less impaired their structural plasticity will be. To address this, we grouped EGFP-labeled spines on the basis of their distance from APP-overexpressing dendrites and compared them for volume change after cLTP (Fig. 7c). For spines within 3 µm of APP dendrites or those 3–6 µm from APP dendrites, spine enlargement after cLTP was reduced compared with EGFP spines far away from APP dendrites (>50 µm) (Fig. 7c). However, normal spine plasticity was observed in the group of spines 6–20 µm from APP dendrites (Fig. 7c), which suggests that the effective distance of dendritic Aβ on spine plasticity lies somewhere around 5–10 µm from the APP-expressing dendrites in our experimental system. A similar distance-dependent effect was not attempted for experiments in which presynaptic CA3 axons were driven to overexpress APP, as large groups of axons were generally infected in any region, making it impossible to determine the distance from source to spine. The observed effect on spine plasticity (Figs. 4–7) was not a result of a difference in initial spine size among groups, as similar spine size distributions were observed among them (Supplementary Fig. 3).
DISCUSSION
Numerous studies indicate that Aβ peptides are important for initiating the pathogenesis of Alzheimer’s disease2. The mechanisms by which this occurs are not known, although recent studies have indicated that Aβ can reduce synaptic transmission and lead to the loss of synapses4,5,16. Although Aβ is produced in vesicles from the secretory and endosomal system (reviewed in ref. 41), it has not been clear whether the Aβ that produces synaptic deficits originates from pre- or postsynaptic compartments. Previous studies found that APP can be transported anterogradely in axons32,33,35 and that Aβ can be made in axonal terminal fields29; however, these studies did not demonstrate synaptic defects from axonally released Aβ. Other studies have shown that surgical lesions of axons decreased Aβ-rich plaques in axonal terminal regions of APP transgenic animals31,42. However, these ablations should also reduce postsynaptic depolarization, which could reduce dendritic Aβ. Indeed, some studies have shown that overexpression of APP in dendritic compartments can reduce synaptic transmission in nearby neurons that do not overexpress APP, suggesting that dendritic Aβ can affect nearby synapses13.
We found that overexpression of APP in either dendritic or axonal compartments led to a reduction in spine density and plasticity in nearby neurons. This effect is relevant to Alzheimer’s disease, as Aβ application at levels reached in the brains of individuals with Alzheimer’s disease36 produced a similar reduction in spine density and plasticity5 that could similarly be prevented by AP5. This effect was likely a result of secreted Aβ, as blockade of Aβ secretion by a γ-secretase inhibitor blocked spine reduction and impaired plasticity by APP overexpression. Furthermore, expression of APP(MV), a mutant form of APP that does not produce Aβ, but makes other cleavage products of APP, failed to produce a reduction in spine density4. It is possible that production of Aβ in axons or dendrites leads to secretion of additional toxic substances or prevents normal synaptic function and thereby leads to the local effects that we observed. Identifying the local target of Aβ that leads to reduction of spines and their plasticity would help to elucidate the mediators of these synaptic effects. Our findings indicate that Aβ from axonal or dendritic compartments can lead to a loss of synaptic structure and function. However, we employed acute production or delivery of Aβ. Thus, the effects that we observed may not be entirely representative of events that occur in a chronic condition such as Alzheimer’s disease. Nevertheless, the effects that we observed in spine reduction were similar in magnitude and in pharmacological sensitivity to effects produced by concentrations of Aβ that are estimated to be present in the brains of individuals with Alzheimer’s disease36,43. Our results do not provide information regarding the source of Aβ in nonpathological conditions, where lower levels are likely produced. It is noteworthy that in Alzheimer’s disease regions in the brain that are synaptically connected (for example, entorhinal cortex and hippocampus) can be affected in temporally linked manner. Our findings suggest that neurons in entorhinal cortex with increased Aβ production would affect the entorhinal cortex through its Aβ-rich dendrites and the dentate gyrus through its Aβ-rich axons.
Blockade of nAChRs with α-bungarotoxin led to a reduction of Aβ secretion from APP-overexpressing neurons. α7-containing nAChRs are present at synaptic and extrasynaptic sites on pre- and postsynaptic compartments, as well as in the cell bodies of hippocampal pyramidal neurons44,45. However, organotypic hippocampal slices have few or no cholinergic neurons. Therefore, there is probably a ligand other than acetylcholine acting on nAChRs that is blocked by α-bungarotoxin. One possibility is that Aβ acts on nAChRs46 that are close to the site of Aβ release, leading to increased intracellular calcium47, which leads to an increase in Aβ production and/or secretion. That is, Aβ could be part of a positive feedback loop that is mediated by nAChR activation and leads to increased Aβ secretion. Blockade of nAChRs would decrease Aβ secretion and reduce the effects of APP overexpression on spine density and plasticity. Additional protective mechanisms by which α-bungarotoxin acts cannot be ruled out. It is notable that a widely used treatment strategy for Alzheimer’s disease is to increase brain acetylcholine levels (reviewed in ref. 48). Our results suggest that this may lead to increase Aβ secretion and would therefore be detrimental. Indeed the long-term effect of enhancing acetylcholine levels on Alzheimer’s disease progress is still in question48.
Blockade of NMDA receptors produced effects different from blockade of nAChRs. AP5 did not reduce the secretion of Aβ. However, AP5 did prevent spine loss in cells overexpressing APP, on nearby cells and in slices exposed to exogenous Aβ. Thus, NMDA receptor activation is required for Aβ to exert its effects on spines, consistent with recent results5. It is noteworthy that the protective effects of AP5 or a γ-secretase inhibitor on spine plasticity in cells overexpressing APP could be seen with only 1 h pretreatment of slices with the drug. Apparently, the effects of Aβ during this period are important for its effects on plasticity and they require NMDA receptor activity. This effect is reminiscent of findings that mild activation of NMDA receptors before induction can block LTP49. It is not clear whether Aβ produces NMDA receptor potentiation50 or weakening5,14, either of which could potentially mediate this effect.
In conclusion, we found that overproduction of axonal or dendritic Aβ can lead to a decrease in spine number and plasticity. The effects of Aβ were local and could be modulated by action potentials, nAChR and NMDA receptors. Thus, neurons that overproduce Aβ will control the gain of inputs and outputs to nearby neurons that do not overproduce Aβ. Although this could be a protective physiological mechanism, in excess, it could substantially reduce the function of a circuit.
METHODS
Methods and any associated references are available in the online version of the paper at http://www.nature.com/natureneuroscience/.
Supplementary Material
ACKNOWLEDGMENTS
We thank members of the Malinow laboratory for helpful discussions, J. Huang for careful reading of the manuscript and N. Dawkins and I. Hunton for expert technical assistance. This work was supported by grants from the US National Institutes of Health (R.M.), the Cure Alzheimer’s Fund (R.M.), Eisai (H.H.) and the Leslie C. Quick Fellowship (W.W.).
Footnotes
Note: Supplementary information is available on the Nature Neuroscience website.
AUTHOR CONTRIBUTIONS
W.W., L.N.N., H.W.K. and H.H. designed and conducted the experiments and analyzed data. S.S. and R.M. designed experiments and supervised the project.
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions/.
References
- 1.Terry RD, et al. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 2.Walsh DM, Selkoe DJ. Deciphering the molecular basis of memory failure in Alzheimer’s disease. Neuron. 2004;44:181–193. doi: 10.1016/j.neuron.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 3.Lacor PN, et al. Synaptic targeting by Alzheimer’s-related amyloid beta oligomers. J. Neurosci. 2004;24:10191–10200. doi: 10.1523/JNEUROSCI.3432-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsieh H, et al. AMPAR removal underlies Abeta-induced synaptic depression and dendritic spine loss. Neuron. 2006;52:831–843. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shankar GM, et al. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J. Neurosci. 2007;27:2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shrestha BR, et al. Amyloid beta peptide adversely affects spine number and motility in hippocampal neurons. Mol. Cell. Neurosci. 2006;33:274–282. doi: 10.1016/j.mcn.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 7.Calabrese B, et al. Rapid, concurrent alterations in pre- and postsynaptic structure induced by naturally-secreted amyloid-beta protein. Mol. Cell. Neurosci. 2007;35:183–193. doi: 10.1016/j.mcn.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evans NA, et al. Abeta(1–42) reduces synapse number and inhibits neurite outgrowth in primary cortical and hippocampal neurons: a quantitative analysis. J. Neurosci. Methods. 2008;175:96–103. doi: 10.1016/j.jneumeth.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Lacor PN, et al. Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer’s disease. J. Neurosci. 2007;27:796–807. doi: 10.1523/JNEUROSCI.3501-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lanz TA, Carter DB, Merchant KM. Dendritic spine loss in the hippocampus of young PDAPP and Tg2576 mice and its prevention by the ApoE2 genotype. Neurobiol. Dis. 2003;13:246–253. doi: 10.1016/s0969-9961(03)00079-2. [DOI] [PubMed] [Google Scholar]
- 11.Jacobsen JS, et al. Early-onset behavioral and synaptic deficits in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 2006;103:5161–5166. doi: 10.1073/pnas.0600948103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spires TL, et al. Dendritic spine abnormalities in amyloid precursor protein transgenic mice demonstrated by gene transfer and intravital multiphoton microscopy. J. Neurosci. 2005;25:7278–7287. doi: 10.1523/JNEUROSCI.1879-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamenetz F, et al. APP processing and synaptic function. Neuron. 2003;37:925–937. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- 14.Snyder EM, et al. Regulation of NMDA receptor trafficking by amyloid-beta. Nat. Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- 15.Almeida CG, et al. Beta-amyloid accumulation in APP mutant neurons reduces PSD-95 and GluR1 in synapses. Neurobiol. Dis. 2005;20:187–198. doi: 10.1016/j.nbd.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Ting JT, Kelley BG, Lambert TJ, Cook DG, Sullivan JM. Amyloid precursor protein overexpression depresses excitatory transmission through both presynaptic and postsynaptic mechanisms. Proc. Natl. Acad. Sci. USA. 2007;104:353–358. doi: 10.1073/pnas.0608807104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walsh DM, et al. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 18.Chapman PF, et al. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat. Neurosci. 1999;2:271–276. doi: 10.1038/6374. [DOI] [PubMed] [Google Scholar]
- 19.Stéphan A, Laroche S, Davis S. Generation of aggregated beta-amyloid in the rat hippocampus impairs synaptic transmission and plasticity and causes memory deficits. J. Neurosci. 2001;21:5703–5714. doi: 10.1523/JNEUROSCI.21-15-05703.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cleary JP, et al. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat. Neurosci. 2005;8:79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- 21.Klyubin I, et al. Amyloid beta protein immunotherapy neutralizes Abeta oligomers that disrupt synaptic plasticity in vivo. Nat. Med. 2005;11:556–561. doi: 10.1038/nm1234. [DOI] [PubMed] [Google Scholar]
- 22.Hartman RE, et al. Treatment with an amyloid-beta antibody ameliorates plaque load, learning deficits and hippocampal long-term potentiation in a mouse model of Alzheimer’s disease. J. Neurosci. 2005;25:6213–6220. doi: 10.1523/JNEUROSCI.0664-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walsh DM, et al. Certain inhibitors of synthetic amyloid beta-peptide (Abeta) fibrillogenesis block oligomerization of natural Abeta and thereby rescue long-term potentiation. J. Neurosci. 2005;25:2455–2462. doi: 10.1523/JNEUROSCI.4391-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morgan D, et al. A beta peptide vaccination prevents memory loss in an animal model of Alzheimer’s disease. Nature. 2000;408:982–985. doi: 10.1038/35050116. [DOI] [PubMed] [Google Scholar]
- 25.Kopec CD, Li B, Wei W, Boehm J, Malinow R. Glutamate receptor exocytosis and spine enlargement during chemically induced long-term potentiation. J. Neurosci. 2006;26:2000–2009. doi: 10.1523/JNEUROSCI.3918-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Engert F, Bonhoeffer T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature. 1999;399:66–70. doi: 10.1038/19978. [DOI] [PubMed] [Google Scholar]
- 27.Maletic-Savatic M, Malinow R, Svoboda K. Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science. 1999;283:1923–1927. doi: 10.1126/science.283.5409.1923. [DOI] [PubMed] [Google Scholar]
- 28.Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429:761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cirrito JR, et al. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron. 2005;48:913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 30.Buckner RL, et al. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J. Neurosci. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lazarov O, Lee M, Peterson DA, Sisodia SS. Evidence that synaptically released beta-amyloid accumulates as extracellular deposits in the hippocampus of transgenic mice. J. Neurosci. 2002;22:9785–9793. doi: 10.1523/JNEUROSCI.22-22-09785.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buxbaum JD, et al. Alzheimer amyloid protein precursor in the rat hippocampus: transport and processing through the perforant path. J. Neurosci. 1998;18:9629–9637. doi: 10.1523/JNEUROSCI.18-23-09629.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koo EH, et al. Precursor of amyloid protein in Alzheimer disease undergoes fast anterograde axonal transport. Proc. Natl. Acad. Sci. USA. 1990;87:1561–1565. doi: 10.1073/pnas.87.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferreira A, Caceres A, Kosik KS. Intraneuronal compartments of the amyloid precursor protein. J. Neurosci. 1993;13:3112–3123. doi: 10.1523/JNEUROSCI.13-07-03112.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sisodia SS, Koo EH, Hoffman PN, Perry G, Price DL. Identification and transport of full-length amyloid precursor proteins in rat peripheral nervous system. J. Neurosci. 1993;13:3136–3142. doi: 10.1523/JNEUROSCI.13-07-03136.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xia W, et al. A specific enzyme-linked immunosorbent assay for measuring beta-amyloid protein oligomers in human plasma and brain tissue of patients with Alzheimer disease. Arch. Neurol. 2009;66:190–199. doi: 10.1001/archneurol.2008.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaether C, Skehel P, Dotti CG. Axonal membrane proteins are transported in distinct carriers: a two-color video microscopy study in cultured hippocampal neurons. Mol. Biol. Cell. 2000;11:1213–1224. doi: 10.1091/mbc.11.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shankar GM, et al. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat. Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Otmakhov N, et al. Forskolin-induced LTP in the CA1 hippocampal region is NMDA receptor dependent. J. Neurophysiol. 2004;91:1955–1962. doi: 10.1152/jn.00941.2003. [DOI] [PubMed] [Google Scholar]
- 40.Changeux JP, Kasai M, Lee CY. Use of a snake venom toxin to characterize the cholinergic receptor protein. Proc. Natl. Acad. Sci. USA. 1970;67:1241–1247. doi: 10.1073/pnas.67.3.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Small SA, Gandy S. Sorting through the cell biology of Alzheimer’s disease: intracellular pathways to pathogenesis. Neuron. 2006;52:15–31. doi: 10.1016/j.neuron.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sheng JG, Price DL, Koliatsos VE. Disruption of corticocortical connections ameliorates amyloid burden in terminal fields in a transgenic model of Abeta amyloidosis. J. Neurosci. 2002;22:9794–9799. doi: 10.1523/JNEUROSCI.22-22-09794.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klyubin I, et al. Amyloid beta protein dimer-containing human CSF disrupts synaptic plasticity: prevention by systemic passive immunization. J. Neurosci. 2008;28:4231–4237. doi: 10.1523/JNEUROSCI.5161-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones IW, Barik J, O’Neill MJ, Wonnacott S. Alpha bungarotoxin-1.4 nm gold: a novel conjugate for visualising the precise subcellular distribution of alpha 7* nicotinic acetylcholine receptors. J. Neurosci. Methods. 2004;134:65–74. doi: 10.1016/j.jneumeth.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 45.Fabian-Fine R, et al. Ultrastructural distribution of the alpha7 nicotinic acetylcholine receptor subunit in rat hippocampus. J. Neurosci. 2001;21:7993–8003. doi: 10.1523/JNEUROSCI.21-20-07993.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dineley KT, Bell KA, Bui D, Sweatt JD. Beta-amyloid peptide activates alpha 7 nicotinic acetylcholine receptors expressed in Xenopus oocytes. J. Biol. Chem. 2002;277:25056–25061. doi: 10.1074/jbc.M200066200. [DOI] [PubMed] [Google Scholar]
- 47.Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu. Rev. Pharmacol. Toxicol. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- 48.Raschetti R, Albanese E, Vanacore N, Maggini M. Cholinesterase inhibitors in mild cognitive impairment: a systematic review of randomized trials. PLoS Med. 2007;4:e338. doi: 10.1371/journal.pmed.0040338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coan EJ, Irving AJ, Collingridge GL. Low-frequency activation of the NMDA receptor system can prevent the induction of LTP. Neurosci. Lett. 1989;105:205–210. doi: 10.1016/0304-3940(89)90038-4. [DOI] [PubMed] [Google Scholar]
- 50.Molnár Z, et al. Enhancement of NMDA responses by beta-amyloid peptides in the hippocampus in vivo. Neuroreport. 2004;15:1649–1652. doi: 10.1097/01.wnr.0000134471.06244.d2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.