Abstract

In an effort to identify a new class of druglike HIV-1 protease inhibitors, four different stereopure β-hydroxy γ-lactam-containing inhibitors have been synthesized, biologically evaluated, and cocrystallized. The impact of the tether length of the central spacer (two or three carbons) was also investigated. A compound with a shorter tether and (3R,4S) absolute configuration exhibited high activity with a Ki of 2.1 nM and an EC50 of 0.64 μM. Further optimization by decoration of the P1′ side chain furnished an even more potent HIV-1 protease inhibitor (Ki = 0.8 nM, EC50 = 0.04 μM). According to X-ray analysis, the new class of inhibitors did not fully succeed in forming two symmetric hydrogen bonds to the catalytic aspartates. The crystal structures of the complexes further explain the difference in potency between the shorter inhibitors (two-carbon spacer) and the longer inhibitors (three-carbon spacer).
Introduction
More than 25 years after the identification of the causative agent of AIDS,1,2 HIV/AIDS is still a major challenge to society. The latest WHO/UNAIDS report (2010) states that the number of people living with HIV has risen to 33.3 million, with more than 2.6 million new cases annually and almost 5000 AIDS-related deaths per day.3
With the introduction of the first HIV-1 protease inhibitor (PI) (saquinavir4) in 1995 and the development of highly active antiviral therapy (HAART)5−7 the clinical outcome of HIV/AIDS changed from a lethal to a manageable, but chronic, disease in the developed world.8−10 The early PIs suffered from poor pharmacokinetic profiles and caused severe side effects such as hepatic toxicity and lipodystrophy.11,12 For these reasons and with a frequent daily dosing regimen, they were not the first-hand choice in HAART. The most common combinations in early HAART were instead two nucleoside reverse transcriptase inhibitors (NRTIs) together with a non-nucleoside reverse transcriptase inhibitor (NNRTI). The development of NNRTI- and/or NRTI-resistant HIV strains and the introduction of new PIs, with a once-daily dose regime and improved effect profiles, have made the combination of a PI together with two NRTIs a more frequent choice for first line treatment in HAART.8,13,14
Although saquinavir has, to date, been followed by eight other PIs (ritonavir, indinavir, fosamprenavir, nelfinavir, lopinavir, atazanavir, tipranavir, and darunavir),15 improving pharmacokinetic properties and reducing adverse effects are still issues that need to be addressed.16−18 Further, the rapid replication and the high mutation rate of the HIV-1 virus,19,20 together with the mutation pressure induced by today’s pharmacotherapies, will lead to an increase in the problems associated with resistant virus strains. Thus, we cannot expect the good results currently seen with HAART to continue if new drugs are not developed and introduced onto the market.17,21
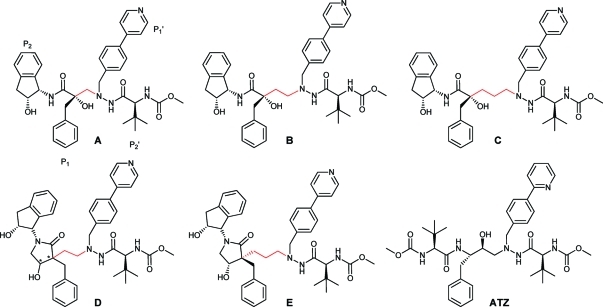
We have been engaged in the development of novel HIV-1 PIs since 1997.22 In our most recent program we developed novel classes of potent HIV-1 PIs incorporating a shielded tertiary alcohol as part of the transition state mimic.23−28 Inspired by the structure of the potent inhibitor Atazanavir (ATZ)29,30 (Figure 1), we used a similar hydrazide moiety in the prime side31 of our new tert-hydroxy-containing PIs. By altering the length of the central backbone, using a one-, two-, or three-carbon spacer (Figure 1, series A,23−25B,26 and C,27,28 respectively), we focused on optimizing the interaction with the catalytically active aspartic acid residues of the enzyme.
Figure 1.
Examples of the previous series of tertiary alcohol-containing HIV-1 PIs. Spacers are indicated in red: A, one-carbon spacer (Ki = 5.5 nM);23−25B, two-carbon spacer (Ki = 2.3 nM);26C, three-carbon spacer (Ki = 2.8 nM);27D, novel lactam-based inhibitors with two-carbon spacer (Ki = 0.8 nM) and altered stereocenters indicated by asterisks; E, three-carbon spacer (Ki = 4.2 nM). ATZ is included for comparison (Ki = 2.7 nM).37
The prepared tert-hydroxy comprising PIs rendered good affinity and potency.23−28 Class B, with the two-carbon spacer, yielded the best results, with values of Ki and EC50 as low as 1 and 3 nM, respectively.26 In all three series (A–C), inhibitors with high membrane permeability were identified, as well as inhibitors with good metabolic stability,23−28 providing pharmacokinetic properties well in the range of HIV PIs already on the market, e.g., ATZ.23
X-ray analyses of inhibitors in series A–C cocrystallized with the enzyme revealed binding modes that were not completely successful in establishing strong symmetric hydrogen bonds (<3.0 Å) with both the catalytic residues Asp25 and or Asp125, originating from each monomer of the HIV-1 protease.32−34 Therefore, we decided to further elaborate the central transition-state mimic by relocating the hydroxyl group one position away from the backbone. This strategy was implemented by making use of a β-hydroxy γ-lactam moiety equipped with a secondary alcohol. It was hypothesized that the β-hydroxy γ-lactam would provide a better hydrogen bond arrangement for the catalytic Asp residues and at the same time reduce the flexibility, providing a more rigid inhibitor.35,36
Modeling studies supported the hypothesis that a hydroxyl group in the 4-position of the γ-lactam might provide a new conformationally constrained transition-state-mimicking scaffold for the development of novel HIV-1 PIs. Since both the (3R,4S) and the (3R,4R) stereoisomers provided good docking poses, we decided to synthesize and evaluate all four stereoisomers of the γ-lactam (Figure 1, D). In addition, two different lengths of the central tether (two or three carbons) were investigated (Figure 1, D and E). The prime-side31 hydrazide moiety, inspired by ATZ, has been successfully used in inhibitors in series A–C and was therefore retained in the new series of lactam-based inhibitors.
Here we present the synthetic protocols and the inhibitory potency on enzyme level, as well as the activity in a cell-based assay, of the new inhibitors (D and E). Also included are stability and permeability studies of selected compounds, together with X-ray analyses of three of the inhibitors cocrystallized with the HIV-1 protease.
Chemistry
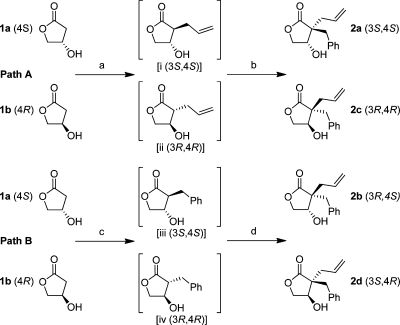
Starting from (S)-4-hydroxydihydrofuran-2(3H)-one (1a) or (R)-4-hydroxydihydrofuran-2(3H)-one (1b), four HIV-1 PR inhibitors with a two-carbon spacer and with varied stereochemistry in the lactam ring were synthesized (Scheme 1). Encouraged by previously reported alkylations,38−401a and 1b were chosen as starting substrates for the two-step alkylation process. Upon treatment with DMPU, LDA, and the first alkylating agent (allyl bromide or benzyl bromide) at −50 °C followed by a second portion of LDA and the addition of the second alkylating agent (benzyl bromide or allyl bromide) at −40 °C, the dialkylated β-hydroxy γ-lactams 2a–d were synthesized in isolated yields of 2–49% (Scheme 1, paths A and B).
Scheme 1. Dialkylation of Lactones 1a and 1b.
Reagents and conditions. Path A: (a) DMPU, LDA, allyl bromide, dry THF, added at −50 °C, stirred at −50 °C for 1 h; (b) LDA, benzyl bromide added at −40 °C, stirred at −30 °C for 1 h, giving 2a and 2c in 49% and 33% isolated yield, respectively. Path B: (c) DMPU, LDA, benzyl bromide, dry THF, added at −50 °C, stirred at −40 °C for 1 h; (d) LDA, allyl bromide, added at −40 °C, stirred at −30 °C for 1 h, giving 2b and 2d in 2% and 5% isolated yield, respectively.
In the first alkylation, the allyl group in 2a and 2c (or the benzyl group in 2b and 2d) was introduced trans to the controlling 4-hydroxyl group as expected, showing facial selectivity, as previously reported by Meyers et al.41 and others.39,40,42 In the second alkylation, the benzyl group (or the allyl group in 2b and 2d) was introduced trans to the 4-hydroxyl functionality. Consequently, the second alkylation changed the stereochemistry of the first inserted group, forcing it to end up cis to the 4-hydroxyl group.40
To be able to collect enough material of 2b and 2d, with their low-yielding synthetic pathway, a method was developed to alter the stereochemistry at the hydroxyl group in 2a and 2c. Oxidation of 2a and 2c with Dess–Martin reagent to the corresponding ketones was followed by reduction using NaBH4, affording 2d and 2b following paths A and B, respectively (Scheme 2), with ratios 2d/2a of 5.7:1 and 2b/2c of 5.9:1. The diastereomers were separated on a silica flash column.
Scheme 2. Diastereomeric Enrichment of 2b and 2d.
Reagents and conditions: (a) 2a or 2c, Dess–Martin, DCM, rt, 1 h; (b) NaBH4, 1% methanol in THF, rt 2 h, 2d + 2a (5.9:1) 92%, 2b + 2c (5.7:1) 85%.
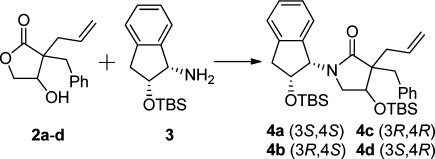
Lactamization of the lactones 2a–d with TBS-protected indanolamine (3) was performed by adopting the methodology developed by Orrling et al.43 (Scheme 3). Lactams 4a–d were isolated in good yields using the ionic liquid 1-butyl-3-methylimidazolium tetrafluoroborate ([bmim]BF4)44 under microwave irradiation at 180 °C for 35 min, followed by protection of the alcohol moiety with TBSOTf under basic conditions.45,46 (Scheme 3). Although the mixture was heated to 180 °C, these lactamization conditions are relatively mild compared to those previously reported.47 The use of highly polar [bmim]BF4 allowed lactamization to proceed smoothly without the need of Brønstedt acid.43
Scheme 3. Lactamization of Lactones 2a–d.
Reagents and conditions: (i) [bmim]BF4, 180 °C, 35 min; (ii) triethylamine, TBSOTf, DCM, 0–25 °C, overnight, giving isolated yields of 4a 64%, 4b 53%, 4c 72%, and 4d 50%.
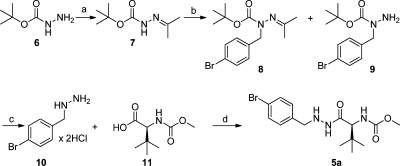
To synthesize the prime-side moiety 5a, hydrazone 7 was prepared in almost quantitative yield starting from the BOC-protected hydrazine 6, as previously reported in the literature48 (Scheme 4). Benzylation of 7 using KOH and 4-bromobenzyl bromide in anhydrous toluene afforded 8 in good yield. Catalytic quantities of the phase-transfer catalyst tetrabutylammonium hydrogen sulfate (TBAHS) were used to improve solubility and increase the rate of the reaction.49,50
Scheme 4. Synthesis of the Prime Side (5a).
Reaction conditions: (a) acetone, MgSO4, AcOH (cat.), reflux, 1 h, 98%; (b) (i) KOH, anhydrous toluene, TBAHS, 50 °C, 20 min; (ii) 3, 100 °C, 2 h, 81%; (c) HCl, THF, reflux, 3 h, quantitative yield; (d) EDCI, HOBt, NMM, DCM, 0–25 °C, 15 h, 77% (61% isolated yield over four steps.). Steps a and c required no purification.
After the initial workup of the alkylation reaction only compound 8 was generated, but after flash chromatography purification, compound 9 was also formed (owing to hydrolysis of the hydrazone). However, purification in this step was necessary to remove excess quantities of 4-bromobenzyl bromide, which was foreseen to cause problems in the later steps. The mixture of 8 and 9 was deprotected with 4 M HCl in THF to yield the pure hydrochloride salt of 10.
Owing to the photosensitivity of the free nitrogen in the p-bromobenzylhydrazine 10, the coupling of 10 with 11, synthesized as previously reported,23 was performed in a reaction vessel wrapped in aluminum foil. Moreover, 10, 11, and HOBt were added under a nitrogen atmosphere at 0 °C, and the mixture was stirred for 30 min. Subsequently, 4-methylmorpholine (NMM) and N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC) were added and the reaction mixture was gradually heated to 25 °C and stirred under a nitrogen atmosphere for 15 h, giving 5a in good isolated yield (77%, 61% overall isolated yield starting from 38 mmol of 6).
Next the allylic double bonds in lactams 4a–d were oxidatively cleaved to give the corresponding aldehydes v–viii using osmium tetraoxide and sodium periodate in THF/water (3:1) at room temperature36,51 (Scheme 5). Note that the nomenclature for the absolute configuration for the lactam carbon in position 3 changes when comparing the lactams 4a–d, the intermediates v–viii, and 12 and 13 because of changes in the assigned priority according to the sequence rule.52,53
Scheme 5. Oxidation and Reductive Amination To Give Two-Carbon-Spacer-Containing HIV-1 PIs 13a–d.
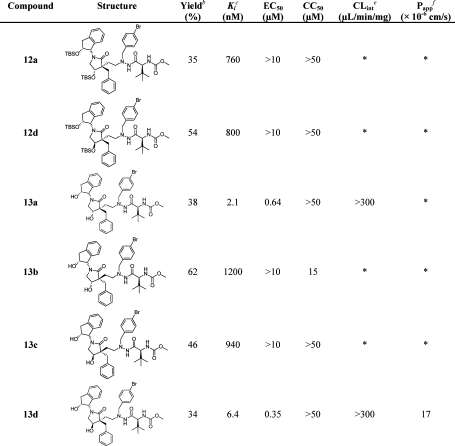
Reagents and conditions: (a) 4a–d, OsO4, NaIO4, THF/H2O, rt, overnight; (b) 5a, acetic acid, Na(OAc)3BH, dry THF, rt, overnight, provided 12a in 35% and 12d in 54% isolated yield from 4a and 4d, respectively; (c) TBAF, THF, rt, overnight, provided 13a in 38%, 13b in 60%, 13c in 46%, 13d in 34% isolated yield from 4a–d, respectively.
The aldehydes were quickly flushed through a short silica column. Reductive amination between the crude aldehydes and the prime side (5a) was performed in dry THF using acetic acid, followed by treatment with Na(OAc)3BH, to afford the crude TBS-protected products. The TBS protecting groups were removed using TBAF, and the inhibitors 13a–d, carrying a two-carbon tether, were isolated in good yields (Scheme 5). The TBS-protected inhibitors 12a and 12d (but not 12b and 12c) were isolated, purified, and fully characterized before the final deprotection.
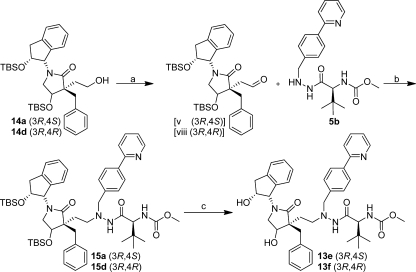
To evaluate the effect of different P1′ side chains, a small series of P1′ p-phenyl- and p-pyridyl-substituted inhibitors was produced. The known problem of rapid protodeboronation of 2-pyridylboronic acid54 prevented us from conducting functionalization of 12a and 12d directly via Suzuki–Miyaura cross-coupling. Thus, to introduce the 2-pyridyl as a para-substituent in P1′, the 2-pyridine-substituted hydrazide 5b (Scheme 6) was synthesized starting from the 4-(2-pyridinyl)benzaldehyde, as previously described.27
Scheme 6. Synthesis of the 2-Pyridyl-Substituted Two-Carbon-Spacer-Containing HIV-1 PIs 13e–f.
Reagents and conditions: (a) 14a or 14d, Dess–Martin reagent, dry DCM, rt, 1 h; (b) acetic acid, Na(OAc)3BH, dry THF, rt, overnight; (c) TBAF, THF, rt, overnight, provided isolated yields of 63% 13e and 38% 13h from 14a and 14d, respectively.
The alcohols 14a and 14d were isolated as side products in reductive amination reactions to produce 12a and 12d, respectively. Dess–Martin reagent was used to oxidize 14a and 14d to the corresponding aldehyde intermediates (Scheme 5, v and viii, respectively), followed by reductive amination with 5b using acetic acid and Na(OAc)3BH in dry THF and subsequent TBAF-mediated deprotection to give useful yields of the inhibitors 13e and 13f (Scheme 6).
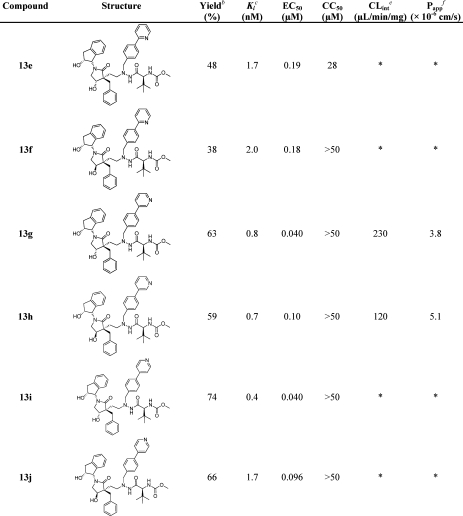
The TBS-protected inhibitors 12a and 12d were decorated using the corresponding phenyl- or pyridylboronic acids in Suzuki–Miyaura cross-coupling in which Herrmann’s palladacycle (0.1 equiv) was used as a palladium precatalyst together with K2CO3 (3.3 equiv) and [HP(t-Bu)3]BF4 (0.2 equiv) in DME/water. The reaction mixtures were heated to 140 °C for 20 min under focused microwave irradiation in sealed reaction vessels.55−57 Cross-coupling was followed by deprotection of the hydroxyl groups using TBAF in THF at room temperature, giving inhibitors 13g–j in good isolated yields (Scheme 7 and Table 2).
Scheme 7. Suzuki–Miyaura Decoration of 12a and 12d To Provide the Two-Carbon-Spacer-Containing HIV-1 PIs 13g–j.
Reagents and conditions: (a) (i) 12a or 12d, Herrmann’s palladacycle, K2CO3, 3- or 4-pyridylboronic acid, [HP(t-Bu)3]BF4, DME, water, microwave 140 °C, 20 min; (ii) TBAF, THF, rt, overnight, providing isolated yields of 63% 13g, 59% 13h, 74% 13i, and 66% 13j.
Table 2. Isolated Yields, Enzyme Inhibition Data, and Antiviral Activity of Compounds 13g–ja.
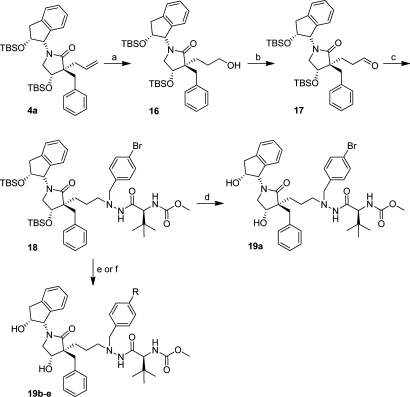
To be able to incorporate the new lactam scaffold into inhibitors with the three-carbon spacer, corresponding to the previously published C series (Figure 1), the allylic compound 4a was refluxed in THF at 80 °C with 9-BBN for 6 h. After addition of NaOH, H2O2, and ethanol at room temperature and another 2 h stirring, the primary alcohol 16 was isolated in good yield (Scheme 8).58 The alcohol 16 was oxidized to the corresponding aldehyde (17) using 50% SO3Py in DMSO together with triethylamine in DCM at 0–25 °C.59,60
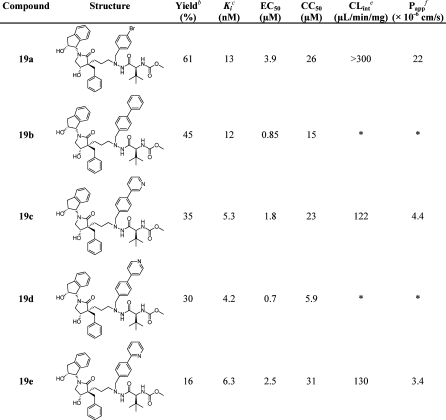
Scheme 8. Synthesis of Three-Carbon-Spacer-Containing HIV-1 PIs 19a–e.
Reagents and conditions: (a) (i) 9-BBN, dry THF, 80 °C, 6 h; (ii) 2 M NaOH, 30% H2O2 in H2O, ethanol, rt, 2 h, 78%; (b) Et3N, 50% SO3Py in DMSO, dry DCM, 0–20 °C, 3 h; (c) 5a, acetic acid, Na(OAc)3BH, dry THF, 35 °C, 3 h, 35%; (d) TBAF, THF, rt, overnight, 19a 61%; (e) (i) Herrmann’s palladacycle, K2CO3, arylboronic acid, [HP(t-Bu)3]BF4, 105 °C, 1.5 h; (ii) TBAF, THF, rt, overnight, 19b 45%, 19c 35%, and 19d 30%; (f) (i) 2-(tributylstannyl)pyridine, Pd(PPh3)2Cl2, CuO, DMF, 105 °C, 2 h; (ii) TBAF, THF, rt, overnight, 19e 16%.
The aldehyde was thereafter used in a reductive amination reaction with 5a using Na(OAc)3BH as reducing agent at 35 °C to give 18 in moderate isolated yield (Scheme 8). The nomenclature for the absolute configuration of the lactam carbon in position 4 changes when comparing the 13a–j and 19a–e series because of changes in the assigned priority according to the sequence rule in IUPAC’s guidelines.52,53
Deprotection of 18 using TBAF in THF gave inhibitor 19a in a good yield. Inhibitor 18 was also used as starting material in Suzuki–Miyaura cross-coupling with phenyl- and pyridylboronic acids together with Herrmann’s palladacycle, K2CO3, and [HP(tBu3)]BF4, heated by microwave irradiation to 105 °C for 1.5 h. Deprotection of the TBS groups using TBAF in THF gave 19b–d in 35–40% isolated yields. By use of 2-(tributylstannyl)pyridine, compound 18 was subjected to Stille type coupling in DMF under microwave irradiation (105 °C, 2 h) using CuO and with Pd(PPh3)2Cl2 as precatalyst.61−63 The Stille coupling was followed by TBAF-mediated deprotection giving inhibitor 19e in moderate isolated yield.
Results
Since the preliminary docking studies suggested that two of the stereoisomers in the lactam moiety ((3R,4S) and (3R,4R)), in the two-carbon-tethered inhibitors would fit well in the enzyme, all four stereoisomers were synthesized and evaluated regarding binding and in a cell-based assay, giving the results summarized in Table 1. Comparisons with previous series of tertiary-alcohol-based HIV-1 PIs (A–C) could easily be conducted by using the indanolamide in the P2 position and the p-bromophenyl as the P1′ side chain.
Table 1. Isolated Yields, Enzyme Inhibition Data, and Antiviral Activity of 12a, 12d, and 13a–da.
In accordance with the initial docking studies, inhibitors 13a (3R,4S) and 13d (3R,4R) exhibited good activity in the enzyme assay (Ki of 2.1 and 6.4 nM, respectively) as well as in the cell-based evaluation (EC50 of 0.64 and 0.35 μM, respectively). The stereoisomers 13b (3S,4S) and 13c (3S,4R) did not show any activity and, as expected, neither did the TBS-protected inhibitors 12a and 12d. The metabolic stability and permeability of the two active inhibitors were investigated. High metabolic clearance was observed for both 13a and 13d. However, 13d also showed good results in the Caco-2 permeability study. Because of its low solubility, inhibitor 13a was not tested in the permeability assay. Compound 13b exhibited slight cell toxic properties, with a CC50 of 15 μM.
The lactam scaffold inhibitors 13a and 13d ((3R,4S) and (3R,4R), respectively) yielded the most potent inhibitors and were therefore selected for further optimization.
When the P1′ position is optimized by replacing the p-bromo substituent of the P1′ phenyl group in 13a and 13d with heteroaromatic moieties, the inhibitors showed improved protease inhibitor potency and, most importantly, increased antiviral activity (Table 2, 13e–j).
The best inhibitors, having (3R,4S) configuration and 3- or 4-pyridylbenzyl as the P1′ moiety (13g and 13i), exhibited 10 times higher potency than 13a in the cell-based antiviral activity assay, the best EC50 values being 40 nM (Table 2). The 2-pyridyl-substituted inhibitor (13e) showed lower activity than the 3- and 4-pyridyl-substituted analogues (13g and 13j, respectively). The improved EC50 upon decorations with 2-, 3-, and 4-pyridyls has previously been demonstrated showing the same trend.24
The (3R,4R) compounds showed less improvements, but all inhibitors decorated with pyridine functionalized in P1′ were observed to have higher potency than the precursor bromo compound 13d. The position of the nitrogen in the heteroaromatic P1′ group showed the same general trend as in the (3R,4S) inhibitors, with the meta- and para-positions providing the best potency (Table 2).
Heteroaromatic functionalization of P1′ provided inhibitors with increased stability compared to 13a and 13d. Compound 13h gave the best result (Clint of 120 μL min–1 mg–1). Both 13g and 13h were observed to possess moderate permeability in the Caco-2 studies, with Papp of 3.8 × 10–6 and 5.1 × 10–6 cm/s, respectively.
When the backbone spacer was elongated from two to three carbons, as in 19a–e, inhibitors with lower potency than the 13 series were obtained (Table 3). This is in accordance with results previously reported for the linear series of tertiary alcohol inhibitors, e.g., comparing the B(26) and C(27) series (Figure 1). However, with the p-phenyl or p-4-pyridyl groups in the P1′ position, submicromolar values of EC50 were observed in the antiviral cell based assay (19b and 19d).
Table 3. Isolated Yields, Enzyme Inhibition Data, and Antiviral Activity for Compounds 19a–ea.
As mentioned above, permeability (Caco-2) and stability (Clint) studies were performed on some of the inhibitors prepared (13a, 13d, 13g, 13h, 19a, 19c, and 19e). Compound 19a showed high permeability (>20 × 10–6 cm/s), while all other inhibitors investigated showed moderate permeability ((3–20) × 10–6 cm/s). The value of Clint varied from 120 to >300 μL min–1 mg–1 (Tables 1–3). These results are in the same range as those previously reported for ATZ (Papp = 5.3 × 10–6 cm/s, Clint = 90 μL min–1 mg–1 23 [140 μL min–1 mg–1 64]). There was no major difference between the 13 and the 19 series with respect to Clint and Papp, and the rigidification of the backbone seemed to be well tolerated compared to the linear inhibitors.23,27,28 The metabolic stability was improved when the bromo group in 13a and 19a was substituted by the heteroaromatic pyridyls, although the permeability was unfortunately reduced at the same time.
X-ray Structure Analysis
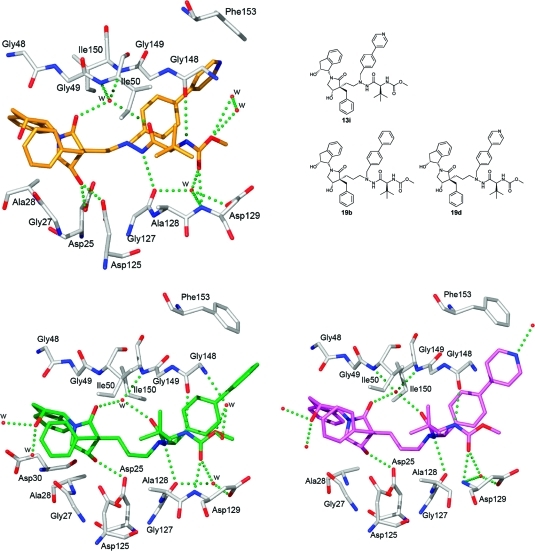
A drug-resistant strain of the HIV-1 protease (Leu63Pro, Val82Thr, Ile84Val)65 was cocrystallized with the active PIs 13i, 19b, and 19d for X-ray crystallographic studies of the complexes. Data were obtained for all complexes, and the structures were refined to high resolution (for refinement statistics, see Supporting Information). The resulting electron density maps allowed unambiguous modeling of the inhibitors within the binding site. Overviews of the binding patterns are presented in Figure 2. Previously published structures of HIV-1 PIs 20,2721,28 and ATZ29 are included for comparison (Figure 3).
Figure 2.
Comparison of the overall X-ray conformations and binding patterns of compounds 13i (top left, PDB code 2uxz), 19b (bottom left, PDB code 4a6c), and 19d (bottom right, PDB code 4a6b) in the active site of HIV-1 protease. Compound 13i forms five direct hydrogen bonds to the protease and five more via water molecules. The corresponding binding interactions for 19b and 19d are four direct bonds and six more through water bridges. In all three complexes, two of the interactions via water are due to the structural water coordinating Ile50 and Ile150 in the protein flaps.
Figure 3.
Previously published PIs for comparison: ATZ (PDB code 3EL9), 20 (PDB code 2uxz),27 and 21 (PDB code 2xye).28
A complicating factor for the comparisons of the inhibitor complexes was the fact that compounds 20 and ATZ were rotated 180° compared to compounds 13i, 19b, 19d, and 21.66−68
The amino acids are labeled according to the novel inhibitor–enzyme complexes presented herein (13i, 19b, and 19d). The overall binding configurations for 13i, 19b, and 19d to the protease are, as expected, in good accordance with those of previously published linear inhibitors 20,2721,28 as well as with ATZ, despite the novel β-hydroxy γ-lactam moiety.
Lactam Moiety
On the basis of the modeling studies, it was postulated that the β-hydroxyl group of the lactam moieties forms hydrogen bonds with the catalytic aspartic acids (Asp25 and Asp125).
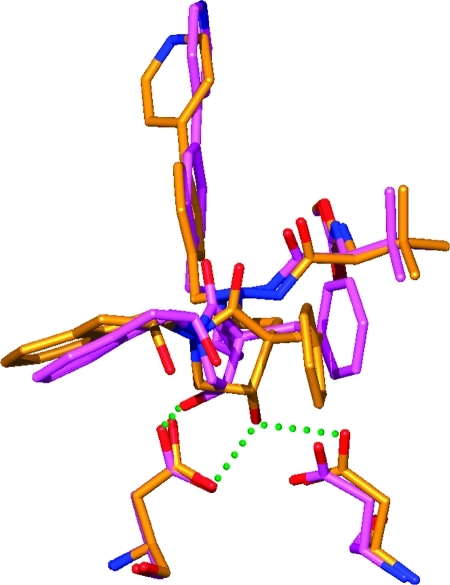
In the two-carbon linker compound 13i this β-hydroxy group forms hydrogen bond interactions to the two catalytic aspartic acids with 2.7 and 3.0 Å. The β-hydroxy group in the three-carbon inhibitors 19b and 19d only form hydrogen bonds to Asp25, with 2.7 and 2.6 Å, respectively (Figure 2). This loss of a hydrogen bond for the 19 series compounds is due to the different spatial conformation of the lactam ring, apparently as a result of the longer central backbone (Figure 4). As the only difference between the structures of 13i and 19d is the length of the backbone tether, this is a likely explanation of the lower antiviral potency of compound 19d compared with 13i.
Figure 4.
The position of the β-hydroxy group of the lactame ring, involved in hydrogen binding to both Asp25 and Asp125 in 13i (gold), is different in 19d (purple) and 19b (not shown) exhibiting the three-carbon linker. This leads to a loss of a hydrogen bond to one of the catalytic aspartates. The position of the β-hydroxy group involved in hydrogen binding in 19d is 2.1 Å from the position observed in 13i.
None of the cocrystallized PIs in these novel series formed a symmetrical binding pattern with the catalytic aspartic acids (Asp25 and Asp125) such as that seen in ATZ. Together with the hydrazide carbonyl oxygen, the carbonyl oxygen in the lactam ring in both 13i and 19d creates hydrogen bonds to the structural water bridging the inhibitors and the Ile50 and Ile150 in the flap region with hydrogen bond lengths of 2.7–3.3 Å (Figure 2).
The position of the P2–P3 indanolamide in 13i, 19b, and 19d is not markedly affected by the introduction of the β-hydroxy γ-lactam, absent in 20. While in 20 the indanolhydroxyl group was close enough to form a hydrogen bond to Arg108 and for the Arg108 to make an edge-on cation−π interaction with the P1 phenyl group, the distance to the indanol group in 13i seems to prevent this bond from forming (Figure 5).
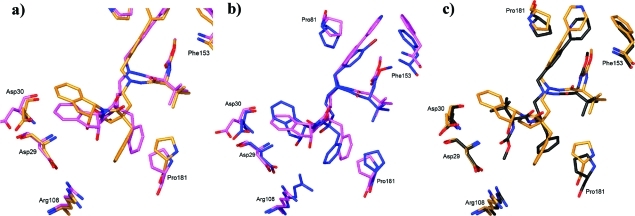
Figure 5.
Comparison of the positioning of the cocrystallized inhibitors in the S2–S3 pocket and interaction with Pro81 and Phe153 in the S1′ pocket. The effect on the S2–S3 site is visualized at residues Asp29, Asp30, Arg108, and Pro181. (a) Superimposition of 13i (gold) and 19d (purple). As a result of an additional CH2 group in 19d, the indanol group of 19d dislocates Asp30 compared to 13i. (b) Superimposition of 19d and 20 (blue). The lactam group present in the new series of compounds as in 19d mimics the conformation of 20, also exhibiting the three-carbon linker, very well. With the lactam ring present, the position of the indanol ring, and therefore also Asp30/130, is more similar to the situation in 13i comprising the two-carbon linker. (c) Superimposition of 13i and ATZ (black). Despite the differences of functional elements between 13i and ATZ in the S2–S3 site, the common ribbon of the compounds overlap well. In the P1′ site 13i and 19d extend further than ATZ, inducing spatial rearrangement of Phe153 and Pro81. Compound 19d induced side chain and main chain atom displacements in Phe153 and Pro81 up to 2.5 and 1.7 Å, respectively, compared to ATZ complex positions. Corresponding displacements with 13i as substrate are 0.9 and 0.5 Å. None of the new compounds induced a shift in the position of Arg108, as was seen in compound 20.27
P1′ Site
In accordance with the previously observed results for 21,28 the P1′ outer phenyl group in both 13i and 19d interacts through a hydrophobic interaction with Pro81 (3.3–3.8 Å) and an edge–face π–π interaction to Phe153 (3.7–3.8 Å). The differences in length of the central motif as well as in the length of the extension of 13i, 19d, 20, 21, and ATZ in the P1′ site are nicely accommodated through corresponding shifts in the positions of Phe153 and Pro81 (Figure 5).28
These interactions are likely to improve the binding constant and is the most likely explanation of the better binding of compound 13i than 13d, differing only in the length of the extension in the P1′ site. In a previously examined complex with compound 20, the interaction with Phe153 was not possible, as the corresponding moiety only reached far enough for a van der Waal interaction with Pro81. Neither is the interaction with Phe153 observed in the complex with ATZ. Since the binding modes of 19b and 19d are very similar (Figure 2), only 19d was included in the analysis, as the structure of the complex could be interpreted at higher resolution.
Discussion
Chemistry
The introduction of the β-hydroxy γ-lactams as new scaffolds was intended to provide more rigid PIs and to relocate the hydroxyl group from the backbone to enable more symmetric binding to the catalytically active Asp25 and Asp125 of the HIV-1 protease. The outcome of the dialkylation reactions performed to obtain 2a–d was in accordance with the results described by Amat et al. in 2007, although they observed a larger substrate-dependent variability.69 When introducing the benzyl moiety in the first alkylation, as in the cases of 2b and 2d, the yields were lower (2% and 5%, respectively) than when the allyl group was introduced before the benzyl moiety (as in 2a and 2c, with yields of 49% and 33%, respectively). The same trend has been reported by Johnson et al. with 4-substitued lactams40 but was not observed in the 5-substitued examples presented by Meyers et al., in which the order of addition did not affect the yields.41 Probable reasons for the lower yields observed by Johnson et al. were steric and/or electrostatic interactions between the 4-hydroxy group and the bulkier 3-benzyl moiety present after the first alkylation, compared to the smaller allyl group. These findings followed the reasoning presented by Huang et al.,70 who proposed stereoelectronic factors to be the major explanation in this class of stereoselective two-step alkylation reactions.
In the present work, the diastereoselectivity controlled by the stereochemistry of the 4-hydroxy group was strong enough to allow highly enantiomerically enriched isomers to be obtained in all cases.
There was an urgent need for a robust method for the synthesis of the prime side hydrazide moiety (5a). The procedures used previously were cumbersome and low yielding because of the use of toxic and environmentally hazardous hydrazine hydrate and/or tedious purification protocols. Previously used synthetic procedures were not satisfactory, since the quantities of prime side were not sufficient to support our lead optimization program throughout. The synthetic route to the prime side hydrazide moiety 5a presented here provided an efficient way of producing sufficient amounts and constitutes an improvement in yield as well as a reduction in work compared to previous methods.27,71 With this convenient method, there was no need to use hazardous hydrazine hydrate, and the purification protocol resulted in a good yield.
Biological Evaluation and X-ray Structure Analysis
The biological results obtained from the novel lactam-containing inhibitors are summarized in Tables 1–3. Evaluation of the four stereoisomers (13a–d) gave two active and two nonactive PIs (Table 1). The (3R,4R) and (3R,4S) stereoisomers in the lactam ring showed the best results, with 13a and 13d being the most potent compounds (Ki < 10 nM and EC50 < 1 μM).
The most important structure–activity feature appears to be the direction of the benzyl in the P1 position. With R-stereochemistry at the α-carbon (13a and 13d), the direction of the β-hydroxy substituent (position 4) appears to be of less importance for inhibition with 13a and 13d being almost equipotent. With S-stereochemistry at the α-carbon, 13b and 13c showed almost no inhibiting effect on the enzyme or in the cell-based antiviral activity assay (Table 1) and, as expected, the TBS-protected inhibitors 12a and 12d did not show any inhibitory potency.
When the P1′ side chain was decorated with heteroaromatic moieties (Table 2), at best a 10-fold improvement in inhibitory potency was observed (13g and 13i, EC50 = 0.04 μM). Compared to the 2-pyridyl inhibitor 13e (EC50 = 0.190 μM), the 3- and 4-pyridyl-substituted inhibitors (13g and 13i, respectively) with (3R,4S) stereochemistry afforded 5 times higher potency, with EC50 of 40 nM. Despite the fact that ATZ contains a 2-pyridinyl in position P1′,37 our previous series with one- or three-carbon spacers showed better potency for the 3- and 4-pyridinyl-substituted inhibitors.24,27,28 With the linear two-carbon spacer the 2-, 3-, and 4-pyridinyls gave equipotent inhibitors.26 This result was also obtained with the lactam-containing inhibitors with the three-carbon extended PIs in the 19 series.
Comparing p-bromide functionalized inhibitors 13a and 19a, a 5-fold loss of potency within measured Ki and EC50 values were observed. However, the same trend is present in both series (13 and 19, Table 3) as seen with the shorter inhibitors. The pyridyls (19c–e) showed slightly better inhibition compared to the p-bromo compound 19a. The p-phenyl substituted 19b was among the most potent inhibitors, concurring with recent reports.26,28
Conclusions
We have successfully introduced β-hydroxy γ-lactams providing a rigid backbone moiety and replaced the previously used tert-hydroxy group with a sec-hydroxy group. All four stereoisomers were synthesized, incorporated into the full inhibitor and evaluated. In addition, the length of the central spacer was varied (two or three carbons). Functionalization of the two most potent stereoisomers (3R,4S) (13a) and (3R,4R) (13d) with heteroaromatic moieties in the p-benzyl P1′ position improved the potency, rendering Ki values down to 0.7 nM and EC50 values down to 0.04 μM. Three inhibitors were cocrystallized with the HIV-1 protease enzyme providing information about the binding of the hydroxy lactams to the enzyme. The change in binding pattern between the inhibitors with two- and three-carbon spacers was in good agreement with the observed variation in enzyme binding activity.
Acknowledgments
We thank the Swedish Research Council (VR) and the Swedish Foundation for Strategic Research (SSF) for financial support, and we thank Prof. Torsten Unge for help with preparation of the crystals.
Supporting Information Available
Details of syntheses and reaction conditions, X-ray statistics, and description of biological assays. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Supplementary Material
References
- Barre-Sinoussi F.; Chermann J. C.; Rey F.; Nugeyre M. T.; Chamaret S.; Gruest J.; Dauguet C.; Axler-Blin C.; Vezinet-Brun F.; Rouzioux C.; Rozenbaum W.; Montagnier L. Isolation of a T-Lymphotropic Retrovirus from a Patient at Risk for Acquired Immune Deficiency Syndrome (AIDS). Science 1983, 220, 868–871. [DOI] [PubMed] [Google Scholar]
- Gallo R. C.; Sarin P. S.; Gelmann E. P.; Robert-Guroff M.; Richardson E.; Kalyanaraman V. S.; Mann D.; Sidhu G. D.; Stahl R. E.; Zolla-Pazner S.; Leibowitch J.; Popovic M. Isolation of Human T-Cell Leukemia Virus in Acquired Immune Deficiency Syndrome (AIDS). Science 1983, 220, 865–867. [DOI] [PubMed] [Google Scholar]
- Global Report: UNAIDS Reoport on the Global AIDS Epidemic 2010; UNAIDS: Geneva, 2010; 364 pp. [Google Scholar]
- Roberts N. A.; Martin J. A.; Kinchington D.; Broadhurst A. V.; Craig J. C.; Duncan I. B.; Galpin S. A.; Handa B. K.; Kay J.; Krohn A.; Lambert R. W.; Merrett J. H.; Mills J. S.; Parkes K. E. B.; Redshaw S.; Ritchie A. J.; Taylor D. L.; Thomas G. J.; Machin P. J. Rational Design of Peptide-Based HIV Proteinase-Inhibitors. Science 1990, 248, 358–361. [DOI] [PubMed] [Google Scholar]
- Hammer S. M.; Squires K. E.; Hughes M. D.; Grimes J. M.; Demeter L. M.; Currier J. S.; Eron J. J. Jr.; Feinberg J. E.; Balfour H. H. Jr.; Deyton L. R.; Chodakewitz J. A.; Fischl M. A. A Controlled Trial of Two Nucleoside Analogues plus Indinavir in Persons with Human Immunodeficiency Virus Infection and CD4 Cell Counts of 200 per Cubic Millimeter or Less. AIDS Clinical Trials Group 320 Study Team. N. Engl. J. Med. 1997, 337, 725–733. [DOI] [PubMed] [Google Scholar]
- Palella F. J. Jr.; Delaney K. M.; Moorman A. C.; Loveless M. O.; Fuhrer J.; Satten G. A.; Aschman D. J.; Holmberg S. D. Declining Morbidity and Mortality among Patients with Advanced Human Immunodeficiency Virus Infection. HIV Outpatient Study Investigators. N. Engl. J. Med. 1998, 338, 853–860. [DOI] [PubMed] [Google Scholar]
- Mocroft A.; Vella S.; Benfield T. L.; Chiesi A.; Miller V.; Gargalianos P.; d’Arminio Monforte A.; Yust I.; Bruun J. N.; Phillips A. N.; Lundgren J. D. Changing Patterns of Mortality across Europe in Patients Infected with HIV-1. Lancet 1998, 352, 1725–1730. [DOI] [PubMed] [Google Scholar]
- Yeni P. Update on HAART in HIV. J. Hepatol. 2006, 44, S100–S103. [DOI] [PubMed] [Google Scholar]
- Pokorna J.; Machala L.; Rezacova P.; Konvalinka J. Current and Novel Inhibitors of HIV Protease. Viruses 2009, 1, 1209–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh R. K.; Ghosh S. M.; Chawla S. Recent Advances in Antiretroviral Drugs. Expert Opin. Pharmacol. 2011, 12, 31–46. [DOI] [PubMed] [Google Scholar]
- Carr A. Toxicity of Antiretroviral Therapy and Implications for Drug Development. Nat. Rev. Drug Discovery 2003, 2, 624–634. [DOI] [PubMed] [Google Scholar]
- Hawkins T. Understanding and Managing the Adverse Effects of Antiretroviral Therapy. Antiviral Res. 2010, 85, 201–209. [DOI] [PubMed] [Google Scholar]
- Walmsley S. Protease Inhibitor-Based Regimens for HIV Therapy: Safety and Efficacy. JAIDS, J. Acquired Immune Defic. Syndr. 2007, 45, S5–S13. [DOI] [PubMed] [Google Scholar]
- Mehellou Y.; De Clercq E. Twenty-Six Years of Anti-HIV Drug Discovery: Where Do We Stand and Where Do We Go?. J. Med. Chem. 2010, 53, 521–538. [DOI] [PubMed] [Google Scholar]
- Wensing A. M. J.; van Maarseveen N. M.; Nijhuis M. Fifteen Years of HIV Protease Inhibitors: Raising the Barrier to Resistance. Antiviral Res. 2010, 85, 59–74. [DOI] [PubMed] [Google Scholar]
- Randolph J. T.; DeGoey D. A. Peptidomimetic Inhibitors of HIV Protease. Curr. Top. Med. Chem. 2004, 4, 1079–1095. [DOI] [PubMed] [Google Scholar]
- Anderson J.; Schiffer C.; Lee S.-K.; Swanstrom R. Viral Protease Inhibitors. Handb. Exp. Pharmacol. 2009, 189, 85–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Este J. A.; Cihlar T. Current Status and Challenges of Antiretroviral Research and Therapy. Antiviral Res. 2010, 85, 25–33. [DOI] [PubMed] [Google Scholar]
- Roberts J. D.; Bebenek K.; Kunkel T. A. The Accuracy of Reverse-Transcriptase from HIV-1. Science 1988, 242, 1171–1173. [DOI] [PubMed] [Google Scholar]
- Mastrolorenzo A.; Rusconi S.; Scozzafava A.; Barbaro G.; Supuran C. T. Inhibitors of HIV-1 Protease: Current State of the Art 10 Years after Their Introduction. From Antiretroviral Drugs to Antifungal, Antibacterial and Antitumor Agents Based on Aspartic Protease Inhibitors. Curr. Med. Chem. 2007, 14, 2734–2748. [DOI] [PubMed] [Google Scholar]
- Clavel F.; Hance A. J. HIV drug resistance. N. Engl. J. Med. 2004, 350, 1023–1035. [DOI] [PubMed] [Google Scholar]
- Hulten J.; Bonham N. M.; Nillroth U.; Hansson T.; Zuccarello G.; Bouzide A.; Aqvist J.; Classon B.; Danielson U. H.; Karlen A.; Kvarnstrom I.; Samuelsson B.; Hallberg A. Cyclic HIV-1 Protease Inhibitors Derived from Mannitol: Synthesis, Inhibitory Potencies, and Computational Predictions of Binding Affinities. J. Med. Chem. 1997, 40, 885–897. [DOI] [PubMed] [Google Scholar]
- Ekegren J. K.; Unge T.; Safa M. Z.; Wallberg H.; Samuelsson B.; Hallberg A. A New Class of HIV-1 Protease Inhibitors Containing a Tertiary Alcohol in the Transition-State Mimicking Scaffold. J. Med. Chem. 2005, 48, 8098–8102. [DOI] [PubMed] [Google Scholar]
- Ekegren J. K.; Ginman N.; Johansson A.; Wallberg H.; Larhed M.; Samuelsson B.; Unge T.; Hallberg A. Microwave-Accelerated Synthesis of P1′-Extended HIV-1 Protease Inhibitors Encompassing a Tertiary Alcohol in the Transition-State Mimicking Scaffold. J. Med. Chem. 2006, 49, 1828–1832. [DOI] [PubMed] [Google Scholar]
- Ekegren J. K.; Gising J.; Wallberg H.; Larhed M.; Samuelsson B.; Hallberg A. Variations of the P2 Group in HIV-1 Protease Inhibitors Containing a Tertiary Alcohol in the Transition-State Mimicking Scaffold. Org. Biomol. Chem. 2006, 4, 3040–3043. [DOI] [PubMed] [Google Scholar]
- Mahalingam A. K.; Axelsson L.; Ekegren J. K.; Wannberg J.; Kihlstrom J.; Unge T.; Wallberg H.; Samuelsson B.; Larhed M.; Hallberg A. HIV-1 Protease Inhibitors with a Transition-State Mimic Comprising a Tertiary Alcohol: Improved Antiviral Activity in Cells. J. Med. Chem. 2010, 53, 607–615. [DOI] [PubMed] [Google Scholar]
- Wu X.; Öhrngren P.; Ekegren J. K.; Unge J.; Unge T.; Wallberg H.; Samuelsson B.; Hallberg A.; Larhed M. Two-Carbon-Elongated HIV-1 Protease Inhibitors with a Tertiary-Alcohol-Containing Transition-State Mimic. J. Med. Chem. 2008, 51, 1053–1057. [DOI] [PubMed] [Google Scholar]
- Öhrngren P.; Wu X.; Persson M.; Ekegren J. K.; Wallberg H.; Vrang L.; Rosenquist A.; Samuelsson B.; Unge T.; Larhed M. HIV-1 Protease Inhibitors with a Tertiary Alcohol Containing Transition-State Mimic and Various P2 and P1′ Substituents. MedChemComm 2011, 2, 701–709. [Google Scholar]
- Bold G.; Fässler A.; Capraro H.-G.; Cozens R.; Klimkait T.; Lazdins J.; Mestan J.; Poncioni B.; Rösel J.; Stover D.; Tintelnot-Blomley M.; Acemoglu F.; Beck W.; Boss E.; Eschbach M.; Hurlimann T.; Masso E.; Roussel S.; Ucci-Stoll K.; Wyss D.; Lang M. New Aza-Dipeptide Analogues as Potent and Orally Absorbed HIV-1 Protease Inhibitors: Candidates for Clinical Development. J. Med. Chem. 1998, 41, 3387–3401. [DOI] [PubMed] [Google Scholar]
- Piliero P. J. Atazanavir: A Novel HIV-1 Protease Inhibitor. Expert Opin. Invest. Drugs 2002, 11, 1295–1301. [DOI] [PubMed] [Google Scholar]
- Schechter I.; Berger A. On the Size of the Active Site in Proteases. I. Papain. Biochem. Biophys. Res. Commun. 1967, 27, 157–162. [DOI] [PubMed] [Google Scholar]
- Miller M.; Schneider J.; Sathyanarayana B. K.; Toth M. V.; Marshall G. R.; Clawson L.; Selk L.; Kent S. B. H.; Wlodawer A. Structure of Complex of Synthetic HIV-1 Protease with a Substrate-Based Inhibitor at 2.3 Å Resolution. Science 1989, 246, 1149–1152. [DOI] [PubMed] [Google Scholar]
- Wlodawer A.; Miller M.; Jaskolski M.; Sathyanarayana B. K.; Baldwin E.; Weber I. T.; Selk L. M.; Clawson L.; Schneider J.; Kent S. B. H. Conserved Folding in Retroviral Proteases: Crystal Structure of a Synthetic HIV-1 Protease. Science 1989, 245, 616–621. [DOI] [PubMed] [Google Scholar]
- Brik A.; Wong C.-H. HIV-1 Protease: Mechanism and Drug Discovery. Org. Biomol. Chem. 2003, 1, 5–14. [DOI] [PubMed] [Google Scholar]
- Freidinger R. M.; Veber D. F.; Perlow D. S.; Brooks J. R.; Saperstein R. Bioactive Conformation of Luteinizing-Hormone-Releasing Hormone: Evidence from a Conformationally Constrained Analog. Science 1980, 210, 656–658. [DOI] [PubMed] [Google Scholar]
- Thaisrivongs S.; Pals D. T.; Turner S. R.; Kroll L. T. Conformationally Constrained Renin Inhibitory Peptides: γ-Lactam-Bridged Dipeptide Isostere as Conformational Restriction. J. Med. Chem. 1988, 31, 1369–1376. [DOI] [PubMed] [Google Scholar]
- Robinson B. S.; Riccardi K. A.; Gong Y.-F.; Guo Q.; Stock D. A.; Blair W. S.; Terry B. J.; Deminie C. A.; Djang F.; Colonno R. J.; Lin P.-F. BMS-232632, a Highly Potent Human Immunodeficiency Virus Protease Inhibitor That Can Be Used in Combination with Other Available Antiretroviral Agents. Antimicrob. Agents Chemother. 2000, 44, 2093–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann J. L.; Schlessinger R. H. Method for Alkylating Lactones. J. Chem. Soc., Chem. Commun. 1973, 711–712. [Google Scholar]
- Maldaner A. O.; Pilli R. A. Stereoselective Alkylation of N-Boc-2-pyrrolidinones and N-Boc-2-piperidinones. Synthesis and Characterization of Disubstituted Lactams. Tetrahedron 1999, 55, 13321–13332. [Google Scholar]
- Johnson T. A.; Jang D. O.; Slafer B. W.; Curtis M. D.; Beak P. Asymmetric Carbon–Carbon Bond Formations in Conjugate Additions of Lithiated N-Boc Allylic and Benzylic Amines to Nitroalkenes: Enantioselective Synthesis of Substituted Piperidines, Pyrrolidines, and Pyrimidinones. J. Am. Chem. Soc. 2002, 124, 11689–11698. [DOI] [PubMed] [Google Scholar]
- Meyers A. I.; Seefeld M. A.; Lefker B. A.; Blake J. F. Origin of Stereochemistry in Simple Pyrrolidinone Enolate Alkylations. J. Am. Chem. Soc. 1997, 119, 4565–4566. [Google Scholar]
- Sprules T. J.; Lavallee J. F. Unexpected Contrasteric Alkylation Leading to a Model for 5-Membered Ring Enolate Alkylation: Short Stereoselective Synthesis of (±)-Acetomycin. J. Org. Chem. 1995, 60, 5041–5047. [Google Scholar]
- Orrling K. M.; Wu X. Y.; Russo F.; Larhed M. Fast, Acid-Free, and Selective Lactamization of Lactones in Ionic Liquids. J. Org. Chem. 2008, 73, 8627–8630. [DOI] [PubMed] [Google Scholar]
- Welton T.; Hallett J. P. Room-Temperature Ionic Liquids: Solvents for Synthesis and Catalysis. 2. Chem. Rev. 2011, 111, 3508–3576. [DOI] [PubMed] [Google Scholar]
- Rassu G.; Zanardi F.; Battistini L.; Gaetani E.; Casiraghi G. Expeditious Syntheses of Sugar-Modified Nucleosides and Collections Thereof Exploiting Furan-, Pyrrole-, and Thiophene-Based Siloxy Dienes. J. Med. Chem. 1997, 40, 168–180. [DOI] [PubMed] [Google Scholar]
- Boger D. L.; Schule G. Synthesis of Acyclic Precursors to (3S,4S)-4-Hydroxy-2,3,4,5-tetrahydro-pyridazine-3-carboxylic acid and Incorporation into a Luzopeptin/Quinoxapeptin Dipeptide. J. Org. Chem. 1998, 63, 6421–6424. [DOI] [PubMed] [Google Scholar]
- Zienty F. B.; Steahly G. W. N-Substituted 2-Pyrrolidones. J. Am. Chem. Soc. 1947, 69, 715–716. [DOI] [PubMed] [Google Scholar]
- Zawadzki S.; Zwierzak A. Simple Approach to 1-Alkyl-2-isopropylhydrazines. Pol. J. Chem. 2003, 77, 315–319. [Google Scholar]
- Freedman H. H. Industrial Applications of Phase-Transfer Catalysis (Ptc): Past, Present and Future. Pure Appl. Chem. 1986, 58, 857–868. [Google Scholar]
- Wang M. L.; Tseng Y. H. Analysis of Phase-Transfer-Catalyzed O-Alkylation Reaction Using Fractional Factorial Design Method. Chem. Eng. Commun. 2005, 192, 557–574. [Google Scholar]
- Pappo R.; Allen D. S.; Lemieux R. U.; Johnson W. S. Osmium Tetroxide-Catalyzed Periodate Oxidation of Olefinic Bonds. J. Org. Chem. 1956, 21, 478–479. [Google Scholar]
- Panico R.; Powell W. H.; Richer J. C.. A Guide to IUPAC Nomenclature of Organic Compounds: Recommendations 1993, 1st ed.; Blackwell Scientific Publications: Oxford, U.K., 1993; 208 pp. [Google Scholar]
- See Supporting Information for illustrations where the stereochemistry is indicated for each molecule.
- Miyaura N.; Suzuki A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar]
- Larhed M.; Hallberg A. Microwave-Promoted Palladium-Catalyzed Coupling Reactions. J. Org. Chem. 1996, 61, 9582–9584. [Google Scholar]
- Larhed M.; Moberg C.; Hallberg A. Microwave-Accelerated Homogeneous Catalysis in Organic Chemistry. Acc. Chem. Res. 2002, 35, 717–727. [DOI] [PubMed] [Google Scholar]
- Kappe C. O.; Dallinger D. The Impact of Microwave Synthesis on Drug Discovery. Nat. Rev. Drug Discovery 2006, 5, 51–63. [DOI] [PubMed] [Google Scholar]
- Brown H. C.; Knights E. F.; Scouten C. G. Hydroboration XXXVI. Direct Route to 9-Borabicyclo[3.3.1]nonane via Cyclic Hydroboration of 1,5-Cyclooctadiene. 9-Borabicyclo[3.3.1]nonane as a Uniquely Selective Reagent for Hydroboration of Olefins. J. Am. Chem. Soc. 1974, 96, 7765–7770. [Google Scholar]
- Parikh J. R.; Doering W. V. E. Sulfur Trioxide in Oxidation of Alcohols by Dimethyl Sulfoxide. J. Am. Chem. Soc. 1967, 89, 5505–5507. [Google Scholar]
- Zhang Q.; Jin H.-X.; Wu Y. A Facile Access to Bridged 1,2,4-Trioxanes. Tetrahedron 2006, 62, 11627–11634. [Google Scholar]
- Gronowitz S.; Bjork P.; Malm J.; Hornfeldt A. B. The Effect of Some Additives on the Stille Pd0-Catalyzed Cross-Coupling Reaction. J. Organomet. Chem. 1993, 460, 127–129. [Google Scholar]
- Flöistrup E.; Goede P.; Strömberg R.; Malm J. Synthesis of estradiol backbone mimics via the Stille reaction using copper(II) oxide as co-reagent. Tetrahedron Lett. 2011, 52, 209–211. [Google Scholar]
- Farina V.; Kapadia S.; Krishnan B.; Wang C. J.; Liebeskind L. S. On the Nature of the Copper Effect in the Stille Cross-Coupling. J. Org. Chem. 1994, 59, 5905–5911. [Google Scholar]
- Wempe M. F.; Anderson P. L. Atazanavir Metabolism According to CYP3A5 Status: An in Vitro–in Vivo Assessment. Drug Metab. Dispos. 2011, 39, 522–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber I. T.; Agniswamy J. HIV-1 Protease: Structural Perspectives on Drug Resistance. Viruses 2009, 1, 1110–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navia M. A.; Fitzgerald P. M. D.; Mckeever B. M.; Leu C. T.; Heimbach J. C.; Herber W. K.; Sigal I. S.; Darke P. L.; Springer J. P. Three-Dimensional Structure of Aspartyl Protease from Human Immunodeficiency Virus HIV-1. Nature 1989, 337, 615–620. [DOI] [PubMed] [Google Scholar]
- Wlodawer A.; Erickson J. W. Structure-Based Inhibitors of HIV-1 Protease. Annu. Rev. Biochem. 1993, 62, 543–585. [DOI] [PubMed] [Google Scholar]
- Bagossi P.; Cheng Y. S. E.; Oroszlan S.; Tozser J. Comparison of the Specificity of Homo- and Heterodimeric Linked HIV-1 and HIV-2 Proteinase Dimers. Protein Eng. 1998, 11, 439–445. [DOI] [PubMed] [Google Scholar]
- Amat M.; Lozano O.; Escolano C.; Molins E.; Bosch J. Enantioselective Synthesis of 3,3-Disubstituted Piperidine Derivatives by Enolate Dialkylation of Phenylglycinol-Derived Oxazolopiperidone Lactams. J. Org. Chem. 2007, 72, 4431–4439. [DOI] [PubMed] [Google Scholar]
- Huang A.; Kodanko J. J.; Overman L. E. Asymmetric Synthesis of Pyrrolidinoindolines. Application for the Practical Total Synthesis of (−)-Phenserine. J. Am. Chem. Soc. 2004, 126, 14043–14053. [DOI] [PubMed] [Google Scholar]
- Otteneder M.; Plastaras J. P.; Marnett L. J. Reaction of Malondialdehyde–DNA Adducts with Hydrazines: Development of a Facile Assay for Quantification of Malondialdehyde Equivalents in DNA. Chem. Res. Toxicol. 2002, 15, 312–318. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.