Abstract
Ventricular myocytes are continuously exposed to fluid shear in vivo by relative movement of laminar sheets and adjacent cells. Preliminary observations have shown that neonatal myocytes respond to fluid shear by increasing their beating rate, which could have an arrhythmogenic effect under elevated shear conditions. The objective of this study is to investigate the characteristics of the fluid shear response in cultured myocytes and to study selected potential mechanisms. Cultured neonatal rat ventricular myocytes that were spontaneously beating were subjected to low shear rates (5–50/s) in a fluid flow chamber using standard culture medium. The beating rate was measured from digital microscopic recordings. The myocytes reacted to low shear rates by a graded and reversible increase in their spontaneous beating rate of up to 500%. The response to shear was substantially attenuated in the presence of the β-adrenergic agonist isoproterenol (by 86 ± 8%), as well as after incubation with integrin-blocking RGD peptides (by 92 ± 8%). The results suggest that the β-adrenergic signaling pathway and integrin activation, which are known to interact, may play an important role in the response mechanism.
Keywords: Cardiomyocytes, cell culture, flow chamber, shear rate, mechanotransduction
INTRODUCTION
The present study is designed to explore the response to short-term fluid shear in a well-established model of spontaneously contracting isolated neonatal rat ventricular myocytes (NRVM). We provide evidence that the myocytes respond to low shear rates with a reversible and graded increase in their spontaneous beating frequency, and that the underlying mechanism may be mediated by both an integrin-dependent and a β-adrenergic signaling pathway.
As with most other cell types, cardiac myocytes are exposed to fluid shear in vivo. Myofiber shortening during contraction causes ventricular wall thickening and relative movement of laminar sheets of myocytes, which gives rise to fluid shear between the fiber sheets (1). Fluid shear in the myocardium probably also occurs in the interstitium between individual myocytes within fiber sheets because of relative motion. This mechanical stimulus on the cell membranes may play an important role in the physiological or pathological function in cardiac myocytes, similar to other cell types. In various other cell types, exposure and adaptation to fluid shear has been extensively studied. Examples of these include adjustments in shape and orientation in vascular endothelial cells (2), alignment in smooth muscle cells (3), pseudopod retraction or projection in leukocytes (4,5), upregulation of prostaglandin production in osteocytes (6), induction of platelet aggregation (7), and increased calcium influx in kidney epithelial cells (8). Various cellular components have been proposed to play a role in mechanotransduction of fluid shear in these cell types. In endothelial cells alone, they include potassium ion channels and stretch-activated channels, integrins and the cytoskeleton, G protein activation, and indirect signaling molecules such as nitric oxide (reviewed in Davies, 1995 [9]). In contrast, a response to fluid shear in cardiac myocytes has only recently been suggested (10), whereas most studies of mechanotransduction in ventricular myocytes have focused on stretch as the mechanical stimulus (11–13).
Our preliminary observations have shown that isolated NRVM increase their spontaneous beating rate when exposed to low shear (5–50/s or 50–500 mdyn/cm2). We estimated the approximate amount of shear in vivo to be on the same order of magnitude, based on a model of parallel plates which represent the fiber sheets. The phenomenon was first observed in isolated embryonic mouse hearts, in which heart rate could be regulated by the amount of physiological solution flowing over its epicardium (14). A recent study has also shown that propagating action potentials can be induced by fluid jet pulses in isolated cardiac myocytes (10). Although ventricular myocytes in vivo do not contract spontaneously, and although the response to fluid shear in ventricular myocytes has thus only been directly observed in culture, the fundamental mechanism leading to this response is likely also present in vivo. It is therefore plausible that pathologically elevated interstitial fluid shear in the myocardium, which may occur in hypertrophy and heart failure because of detachment of myocytes from the extracellular matrix and ensuing myocyte slippage (for review, see Paul, 2003 [15]), could cause various abnormalities in myocyte mechanotransduction and mechanoelectric feedback. Particularly interesting is the idea that the mechanism underlying the response to fluid shear, under elevated shear conditions, could spark ectopic action potentials and lead to arrhythmias.
MATERIALS AND METHODS
Cell Culture
Primary cultured myocytes were prepared from ventricles of neonatal (1–2 d old) Sprague-Dawley rats using a standard isolation kit (Cellutron Life Technology, Highland Park, NJ). Briefly, 30 neonatal rats were decapitated, their hearts quickly excised and trimmed to remove atrial tissue. The ventricles were gently stirred for 20-min periods in digestion buffer at 37°C. The cell suspension was centrifuged at 250g for 1 min, the super-natant discarded and the pellet cells resuspended in buffer solution. The cycle was repeated a total of five times or until all of the tissue was digested. The cells were preplated in uncoated plates for 1–2 h at 37°C to reduce the contamination of cardiac fibroblasts. The cardiac myocytes were collected and plated on microscope slides coated with SureCoat (a combination of collagen and laminin, Cellutron Life Technology, Highland Park, NJ) at a plating density of 8–9 × 104 cells/cm2, and cultured in plating medium containing 15% serum (horse and fetal bovine). The plating medium was exchanged after 24 h with standard culture (control) medium containing 6% serum. The culture was allowed to incubate for 7 d on average, after which the cells had spread to a monolayer and were beating spontaneously and synchronously at a stable rate.
Laminar Flow Assay
The glass slides with attached myocytes were assembled into a parallel plate flow chamber apparatus with dimensions of 1.38 cm × 7.62 cm × 127 μm. The height of the flow chamber was set by placing a silastic gasket between the slide and the upper lid of the chamber. The narrow slots at input and output ports allowed medium flow to be introduced as a uniform laminar flow field over the entire width of the chamber. Myocytes were sheared for a duration of 3–5 min by introducing medium into the flow chamber apparatus using a mechanical syringe infusion pump (Harvard Apparatus, Holliston, MA), which allowed step changes in flow rate. The time to reach steady-state flow was estimated to take approx 5 s. The chamber as well as bath and flow medium were equilibrated to a temperature of 36 ± 1°C before beginning the flow protocol.
The response to different amounts of shear was tested in control medium and in medium containing (in separate experiments) Ficoll type 400, streptomycin sul-fate, isoproterenol, propranolol (all from Sigma, St. Louis MO), RGD-containing peptide (GRGDSP) and PKI peptide (Calbiochem, San Diego, CA), RGE-containing peptide (GRGESP, AnaSpec, San Jose, CA), and in serum-free medium. Before each intervention, the shear response in control medium was measured in the same group of myocytes because of a high variability in the magnitude of the response between individual myocytes cultures. (Some PKI, propranolol, and Ficoll experiments were performed in reverse order.) For the same reason of variability, the shear rate was adjusted to the response of the particular culture in some cases, thus leading to different magnitudes of shear for different series of experiments. The necessary medium changes during the experiments were introduced by infusing at least twice the chamber volume of new medium, thereby shearing the cells. After each medium change, the beating rate was allowed to stabilize for 10–15 min before repeating the shear protocol. Although both the baseline beating rate and the magnitude of response were highly variable between cultures, they were verified in some cases to be consistent across the slide.
Beating Rate Measurements
Myocyte beating rates were determined by using a pixel value thresholding algorithm. First, a rectangular region of interest of the microscopic image was selected in the frame grabber software. A movie of 10-s or 1-min length was recorded at 10 frames/s and compiled into a stack of digital images (TIF format). The algorithm was used to construct an M-mode image of the stack by mapping selected pixels from each image over time. From this M-mode map, the location with the greatest contrast in pixel value was determined and used to calculate the beating rate. The halfway point between the maximum and minimum pixel value at that location was determined and used as a threshold to establish whether the cells had contracted. The number of contractions in the M-mode image was thus counted and the average beating rate calculated.
Statistical Analysis
All data are expressed as means ± SEM. Repeated measures analysis of variance (2-by-2 or 2-by-3: two media by two or three shear rates) was performed to compare the response with shear before and after medium change. Differences at p < 0.05 were considered statistically significant. Statistical power for nonsignifi-cant differences was calculated for a 50% difference except where stated otherwise.
RESULTS
Characteristics of the Fluid Shear Response
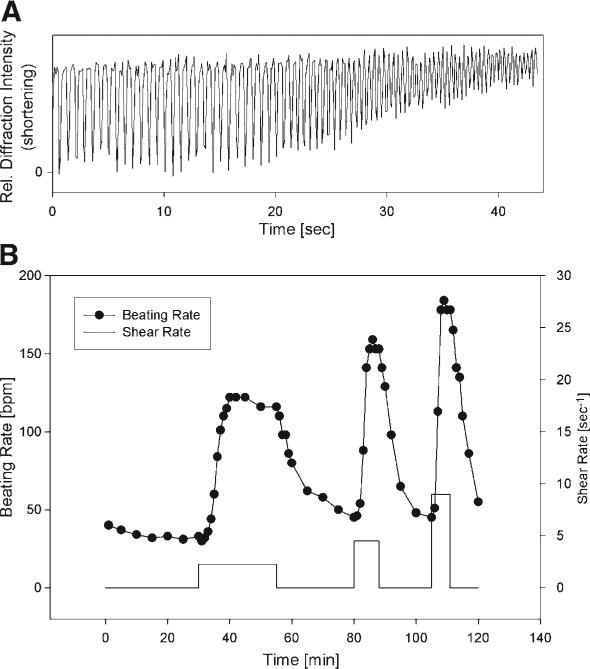
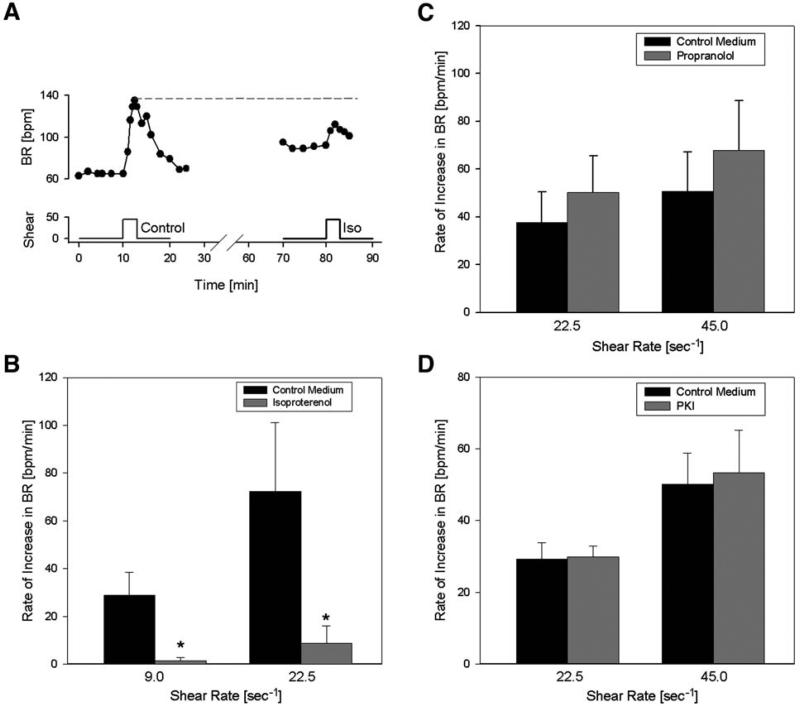
NRVM respond to small magnitudes of fluid shear stress by increasing their spontaneous beating rate (Fig. 1A). The increase of contraction frequency with shear is clearly evident. As seen in Fig. 1A, myocytes typically did not reach complete relaxation as the contractions accelerated, leading to a decrease in shortening amplitude. A representative time course of beating rate with subsequent exposure to shear rates of 2.3–9/s is depicted in Fig. 1B, showing the typical reversible and graded response to fluid shear.
Fig. 1.
(A) Shortening of a representative group of cultured neonatal rat cardiomyocytes, showing the increase in contraction frequency during shear exposure (shear rate: 45/s, shear stress: 450 mdyn/cm2). Because shortening was measured as relative light diffraction intensity from randomly oriented myocytes, the unit is arbitrary. As the beating rate increased, the myocytes became unable to relax completely, leading to a diminished amplitude of shortening. (B) Representative changes in spontaneous beating rate from different shear rates (2.25, 4.5, and 9/s) in cultured ventricular myocytes. The response is consistent, reversible, and graded by the magnitude of shear.
In general, the magnitude of the response was consistent in the same culture of myocytes but could differ between cultures. On average, in 10 experiments, the relative increase in beating rate amounted to 80 ± 14% at a shear rate of 45/s and could reach up to 500%. The initial beating rate in unsheared myocytes was also variable between cultures (25–140 bpm, on average 67 ± 30 bpm). Because of the variability in the data, we chose to perform a control test (shear in control medium) for each experiment, before or after the intervention. In many myocyte cultures, the beating rate increased to such high frequencies with shear (>220 bpm), that shortening ceased below resolution of measurement and the contractions became undetectable.
As Fig. 1B shows, the absolute increase in beating rate was relatively insensitive to the amount of shear, whereas the rate of increase in beating rate changed several-fold, which was also most consistent between cultures. We thus represent the response as the rate of increase in beating rate, measured as the slope of the beating rate between the onset of shear and the maximum beating rate during shear (usually after 3–5 min).
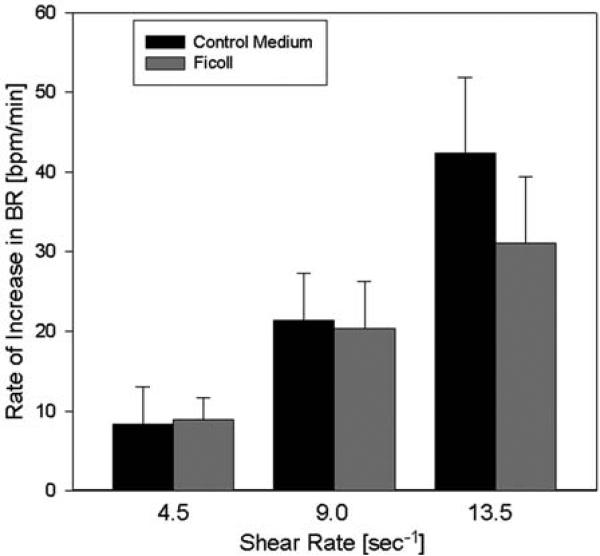
The culture media we used are Newtonian fluids with constant viscosity. Therefore, shear stress equals shear rate multiplied by viscosity. To test whether the myocytes responded to shear stress or to shear rate, we compared their response to shear with media of different viscosity. Medium with a 2.5-fold increased viscosity compared to control medium was produced by adding the nonreactive macromolecule Ficoll to the medium (0.1 nM). Figure 2 shows that addition of Ficoll did not produce an increase in the response (p = 0.50, n = 6), although shear stress was elevated 2.5-fold at the same shear rate. The power for these experiments was sufficient to detect a twofold increase in the response. This evidence suggests that NRVM are sensitive to changes in shear rate rather than shear stress.
Fig. 2.
Effect of viscosity changes on the shear response. The viscosity was increased 2.5-fold by addition of the macromolecule ficoll (0.1 nM), resulting in higher shear stress at the same shear rate. For instance, at a shear rate of 9.0/s, shear stress amounted to 90 mdyn/cm2 in control medium and to about 225 mdyn/cm2 with added Ficoll. The rate of shear response was not significantly affected by the increase in viscosity (n = 6).
It is conceivable that the myocytes would raise their beating frequency simply because of an increased oxygen supply to the myocytes during shear in the enclosed chamber. To test this idea, we measured the oxygen concentration in the chamber using a microdissolved oxygen probe (Lazar Research Laboratories, Los Angeles, CA) during the shear protocol. There was a minimal continuous loss of oxygen (2.5%/h, n = 4) that did not correlate with the shear rate, suggesting that the stimulus of the shear response is not dependent on oxygen concentration in the myocytes’ immediate environment.
We also performed tests on cultured myocytes to examine the fluid shear response, in this case with fluid flow through a micropipet directed over the top of the cells as described by Moazzam et al. (4). Thus the fluid flow distribution was radial, exposing the center myocytes to the maximum amount of shear, which was on the same order of magnitude as the maximum laminar shear in the flow chamber. Spontaneously contracting cells were prepared as described for the flow chamber experiments and imaged for contractile rate changes from nonhomogeneous fluid flow on the top surface of the cells. Changes in rate were similar to those reported here with the shear chamber. Thus a paracrine phenomenon is unlikely to be the cause of the increase in contractile rate.
Possible Mechanism
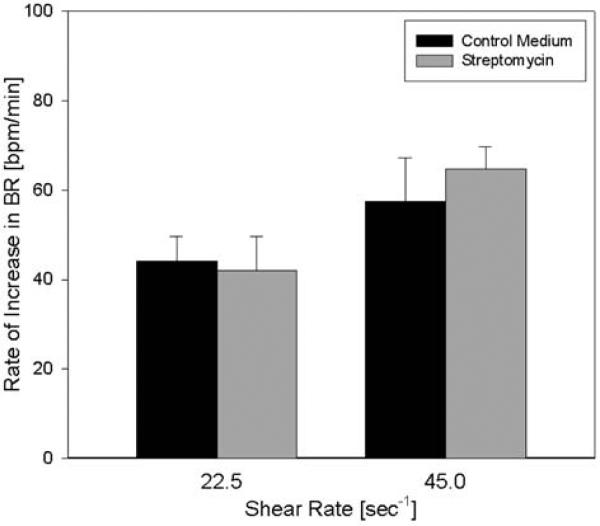
To explore possible mechanisms for the fluid shear response, we first hypothesized that stretch-activated channels may mediate this process. We tested this hypothesis by exposing the cultured myocytes to fluid shear in the presence of streptomycin (Fig. 3). Streptomycin was added to the medium as an antibiotic but is also commonly used to block mechanosensitive channel currents at concentrations higher than 40 μM. After incubation with medium containing 70 μM streptomycin, the response to shear was comparable to the response in medium not containing the antibiotic (Fig. 3, n = 3, p = 0.40, power >0.95), suggesting that the shear-induced effect is not dependent on stretch-activated channels.
Fig. 3.
Effect of streptomycin on the shear response. Myocytes were incubated with the stretch-activated channel blocker streptomycin (70 μM) and exposed to shear. Streptomycin did not significantly affect the rate of response to shear at either shear rate compared with control medium (n = 3).
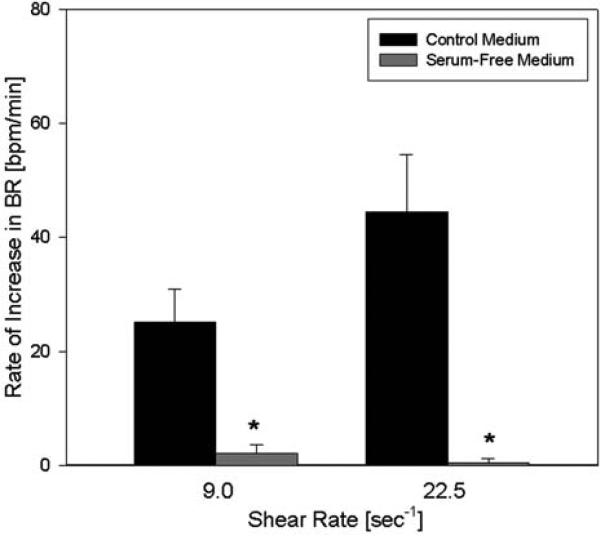
We further examined the fluid shear response in the absence of serum in the medium. Several different serum-free media, containing NaHCO3 or HEPES as a buffer and containing different amounts of amino acids were compared with control medium (Fig. 4). In all serum-free media, the response to shear was almost completely abolished and indistinguishable from noise (p = 0.004, n = 4). This indicates that a component in the serum is required for the increase in beating rate with shear or may induce the response itself.
Fig. 4.
Effect of serum-free medium on the response to shear. Myocytes were exposed to shear with control medium and serum-free medium. The increase in beating rate with shear was nearly abolished at both shear rates in the absence of serum compared with the control medium (n = 4). Asterisks denote statistical significance from control (p < 0.05).
Figure 5A shows a representative response of the beating rate to shear in NRVM in the absence and presence of the nonspecific β-adrenergic agonist isoproterenol. After incubation with isoproterenol, when the contraction rate had risen to a higher level, the response was diminished. On average, the myocytes showed a significantly blunted response at two different shear rates in the presence of isoproterenol (1 μM) compared with shear by control medium (p < 0.01, n = 6, Fig. 5B). This seems to suggest that the shear pathway and β-adrenergic pathway merge, but this does not necessarily have to be the case. Contraction rate could have been maximally increased by isoproterenol such that any further significant increase was not possible by any mechanism. However, this possibility is ruled out by the observation that in all individual experiments (e.g., Fig. 5A), the maximum beating rate during shear in control medium (or during wash-in of isoproterenol, not shown here) exceeded the highest beating rate during shear in isoproterenol-containing medium. Therefore, the maximum beating rate had not been reached with isoproterenol, confirming that the shear pathway does not function separately from the β-adrenergic pathway.
Fig. 5.
Impact of isoproterenol, propranolol and PKI on the response to shear. (A) Example of the contraction rate response to shear in the absence and presence of isoproterenol (iso). Note that the increase with isoproterenol does not reach the maximum beating rate. (B) Summarized response to isoproterenol: after incubation with isoproterenol (1 μM), the response to shear was significantly blunted compared with control medium at both shear rates (n = 6). Asterisks denote statistical significance from control medium (p < 0.05). (C) Shear response in control medium compared with medium containing the nonspecific β-blocker propranolol (1 μM). Propranolol (n = 5) did not affect the rate of response to shear significantly. (D) Effect of PKA-inhibiting peptide PKI (5 μM) on the response. No statistical difference was detected (n = 4).
Next we tested whether β-adrenergic receptors themselves may be involved by exposing NRVM to shear after incubation with the nonspecific β-blocker propranolol (Fig. 5C). Propranolol (1 μM) did not significantly affect the response to shear (p = 0.1, n = 5, power = 0.8 for 60% decrease), suggesting that β-adrenergic receptors are not required to elicit the shear response and that a possible overlap of the pathways must occur further downstream. PKA activation does not appear to be required in the response, because application of the PKA-inhibiting peptide PKA (5 μM), the response to shear was not significantly affected (n = 4, p = 0.6, power = 0.95, Fig. 5D).
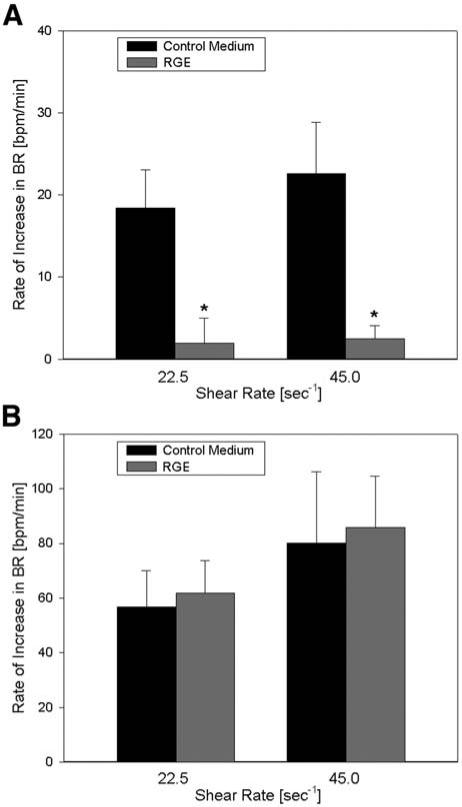
Finally, we tested the hypothesis that the response to shear may be integrin-mediated by blocking β-inte-grins using RGD peptides. After incubation with 100 μM RGD peptide, the response to shear was nearly abolished (p < 0.001, n = 5), indicating that integrins may play a central role in the signaling process (Fig. 6A). We observed that the myocytes tended to detach from the slide after long incubation with RGD peptide (>1 h), suggesting that the peptide was beginning to replace adhesion sites on the slide coating. The nonintegrin-binding control peptide RGE (100 μM) did not block the response to shear (Fig. 6B) and even led to a slight increase in the response (p = 0.6, n = 5, power = 0.8), further supporting the view that inte-grins may mediate the response to shear in NRVM.
Fig. 6.
(A) Effect of RGD peptide on the rate of response to shear. β1-integrin-blocking RGD peptide (100 μM) nearly abolished the shear response (n = 5) compared with control medium at both shear rates. The asterisk denotes a statistical difference (p < 0.05) from control. (B) Effect of RGE peptide on the response to shear. The non-integrin-binding RGE peptide (100 μM) was used as an additional control (n = 5) and did not have a significant effect on the beating rate increase at either shear rate.
DISCUSSION
We demonstrated in this study that spontaneously beating NRVM consistently respond to short-term fluid shear stress by raising their beating rate. This response was reversible and graded by the amount of shear and probably regulated by shear rate rather than shear stress. We showed that stretch-activated channels are probably not involved in the process, whereas the β-adrenergic signaling pathway and particularly β1 inte-grins may play an important role.
Spontaneous Beating in NRVM
The spontaneous beating of NRVM is a well-known phenomenon, with synchronous contractions beginning at about day 3 in culture, although the exact basis remains unclear. Loss of the inwardly rectifying potassium current IK1 appears to be a requirement for spontaneous beating (16). Calcium transients in NRVM have been shown to be due mainly to extracellular Ca2+ influx but not to Ca2+ release from the sarcoplasmic reticulum in NRVM (17). Additionally, the cardiac sarcolemmal L-type Ca2+ channel, the Na+/Ca2+ exchanger and the pacemaker (or funny) current If, which is still present in NRVM after several days of culture (18), have been implicated in providing the inward current to spark an action potential (19–22). Factors that can affect the spontaneous beating rate of cultured myocytes include the β-adrenergic pathway and the muscarinic cholinergic pathway, which change beating rate by reg ulating Ca2+ influx through the L-type Ca2+ channel (22–25). Our finding that the shear-induced increase in beating rate may be overlapping with the β-adrenergic pathway supports the view that an increase in the L-type channel Ca2+ current may also be responsible for the shear-induced response. However, our observation that the rate of change in beating rate is more consistent than the absolute increase seems to point toward a possible accumulative Ca2+ current during shear (e.g., through the Na+/Ca2+ exchanger operating in reverse mode, which is also affected by β-adrenergic stimulation). These possibilities will be tested in the future.
It is not clear why both the baseline beating rate and the shear response varied to a high degree. We speculate that fibroblast contamination may be responsible, because the number of fibroblasts in a myocyte culture has been shown to affect beating rate (26). Although our current flow chamber system does not allow for simultaneous action potential measurements, this variation would have been of interest and could be performed in the future.
As a limitation of this study, it is possible that few pacemaker cells remained in the culture, which may be responding to the fluid shear instead of nonpacemaker ventricular myocytes. However, this is unlikely, because the effect can also be observed when shear is applied locally using a micropipet.
Fluid Shear In Vivo
The amount of fluid shear to elicit a response in myocytes is very low compared with other cell types. Typical shear stresses in other tissues are on the order of 10 to 30 dyn/cm2 in bone and in large arteries (27,28), and up to 50 dyn/cm2 in the microcirculation (29). In the myocardium, we estimated the magnitude of shear stress in vivo to be almost three orders of magnitude lower, using a model of parallel plates that represent the fiber sheets. A distance between the fiber sheets of 10 μm and relative velocity of 50 μm/s during systole would yield a fluid shear stress of 50 mdyn/cm2. Therefore, the amount of shear stress applied in the present experiments is comparable to the estimated level in vivo and could lead to the demonstrated effects under elevated shear, such as is expected to occur in cardiomyopathy and heart failure due to detachment from the extracellular matrix and myocyte slippage (15,30). Interestingly, failing myocytes also show electrophysio-logic characteristics that are similar to those in isolated neonatal myocytes and may thus harbor the mechanism of shear-induced depolarization. Namely, failing myocytes show a decrease in repolarization reserve from a reduction in several outward potassium currents including IK1 (31–33), rendering them more susceptible to depolarization by leak inward currents. Additionally, the pacemaker current If reoccurs in hypertrophied rat ventricular cells, its density increasing with the severity of myocardial hypertrophy (34). Taken together, these findings support the view that the shear-induced increase in beating rate may constitute a possible arrhythmogenic mechanism in heart failure.
Aspects of the Underlying Mechanism
The myocytes responded in the same way to laminar flow in the chamber and local radial flow introduced by a micropipette. In the flow chamber, downstream cells could be affected by second messengers, such as nitric oxide, produced by cells further upstream, whereas in a radial flow field, the center cells responded to flow of fresh culture medium over a part of their surface. Therefore, a paracrine effect is unlikely to be the cause of the increase in contractile rate; it appears that the mechanical fluid shear is responsible for the phenomenon.
NRVM appear to respond to shear rate rather than shear stress, because we did not find an increase in the rate of response with increased viscosity at the same shear rate. Most other studies of various cell types and their functions have reported shear stress sensitivity (35,36), although shear rate dependence was found in melanoma cell migration, which is also integrin related (37). Compared with these studies, we used very low fluid shear rates, probably causing little or no shear strain in the myocytes. Therefore, the fluid shear response may not be mediated by shear deformation of the myocytes but may be induced by membrane receptors. This would be consistent with our finding that the response is not directly related to shear stress but shear rate. Furthermore, this supports the view that the chronotropic effect reported here in response to fluid shear may be based on a different mechanism than the mechanotransduction of large shear stresses, which are thought to be transmitted via collagen struts and the cytoskeleton.
Preincubation of the NRVM with isoproterenol, which increased the beating rate significantly but not maximally, considerably blunted the shear-induced response in beating rate. This suggests that the shear-induced increase in beating rate may be based on a increase in Ca2+ current (perhaps mediated by the L-type channel), similarly to the β-adrenergic pathway (25,38), and that both signaling pathways may overlap further upstream. The overlap appears to occur downstream of PKA because PKI did not block the shear response.
Our results also indicate that the shear response may be mediated by integrins, because the integrin-binding RGD peptides nearly abolished the effect, whereas nonintegrin-binding RGE peptides did not alter the response. Myocardium primarily expresses β1 integrin subunits that form heterodimers with various α-chains (for review, see ref. 39). Most of these receptors can bind fibronectin at its RGD sequence. Integrins are known to play an important role in mechanotransduction through processes that are similar to cell adhesion, including tyrosine phosphorylation of proteins at focal adhesion sites. Because β1 integrins in particular have themselves been linked to the L-type Ca2+ channel (40), it is reasonable to hypothesize that the shear-induced increase in beating frequency is directly related to increased L-type Ca2+ current. This would also explain the overlap between the shear response and the β-adrenergic pathway. In fact, several studies found evidence for cross-talk between the cellular processes controlling cell adhesion via integrins and β-adrenergic stimulation (41–43), although it is not clear how or at what stage these signaling networks interact. These conclusions also agree with our finding that a component in serum is required for the shear response. We propose that fragments of the extracellular matrix present in the serum may bind to the unattached top surface of the NRVM and activate β1 integrins after shear. Other components of the serum that may have to be present to activate integrins are growth factors and cytokines. Further experiments are needed to identify the required components.
We attempted to block L-type channels using nifedi-pine to further test whether they may play a role in the shear effect. However, this caused the vast majority of cells to stop beating, which had also been observed in other studies. Different agents to block L-type Ca2+ current and other Ca2+ handling currents (such as via the Na+/Ca2+ exchanger) will be used in future studies.
A recent study by Kong et al. (10) reported that action potentials in cultured NRVM can be triggered by fluid jet pulses. This phenomenon of mechanoelectric feedback may be related to the shear response described here in that both are electrophysiologic responses triggered by fluid shear stress. However, there are several major differences between our studies, suggesting that the types of fluid shear response we observed may not be related: propagation of action potentials in Kong's study is dependent largely on Na+ current and, therefore, might not reflect the same sensitivity to shear stress. Furthermore, Kong's study was performed in serum-free medium that, as we have shown, greatly attenuates the shear response. This might also explain the much larger shear stresses needed by Kong. Finally in Kong's study, induction of action potentials was linked with stretch-activated channels and thus stands in contrast to our supposition.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grant HL43026. The authors would like to thank Jeffrey Tsai for developing the beating rate analysis program and collecting preliminary data, and Michelle Pike for the measurements of oxygen concentration.
REFERENCES
- 1.Dou J, Tseng WY, Reese TG, Wedeen VJ. Combined diffusion and strain MRI reveals structure and function of human myocardial laminar sheets in vivo. Magn. Reson. Med. 2003;50:107–113. doi: 10.1002/mrm.10482. [DOI] [PubMed] [Google Scholar]
- 2.Dewey CF, Jr., Bussolari SR, Gimbrone MA, Jr., Davies PF. The dynamic response of vascular endothelial cells to fluid shear stress. J. Biomech. Eng. 1981;103:177–185. doi: 10.1115/1.3138276. [DOI] [PubMed] [Google Scholar]
- 3.Sterpetti AV, Cucina A, D'Angelo LS, Cardillo B, Cavallaro A. Response of arterial smooth muscle cells to laminar flow. J. Cardiovasc. Surg. (Torino) 1992;33:619–624. [PubMed] [Google Scholar]
- 4.Moazzam F, DeLano FA, Zweifach BW, Schmid-Schonbein GW. The leukocyte response to fluid stress. Proc. Natl. Acad. Sci. USA. 1997;94:5338–5343. doi: 10.1073/pnas.94.10.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coughlin MF, Schmid-Schonbein GW. Pseudopod projection and cell spreading of passive leukocytes in response to fluid shear stress. Biophys. J. 2004;87:2035–2042. doi: 10.1529/biophysj.104.042192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein-Nulend J, van der Plas A, Semeins CM, et al. Sensitivity of osteocytes to biomechanical stress in vitro. FASEB J. 1995;9:441–445. doi: 10.1096/fasebj.9.5.7896017. [DOI] [PubMed] [Google Scholar]
- 7.Belval T, Hellums JD, Solis RT. The kinetics of platelet aggregation induced by fluid-shearing stress. Microvasc. Res. 1984;28:279–288. doi: 10.1016/0026-2862(84)90001-3. [DOI] [PubMed] [Google Scholar]
- 8.Nauli SM, Alenghat FJ, Luo Y, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat. Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 9.Davies PF. Flow-mediated endothelial mechanotransduction. Physiol. Rev. 1995;75:519–560. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kong CR, Bursac N, Tung L. Mechano-electrical excitation by fluid jets in monolayers of cultured cardiac myocytes. J. Appl. Physiol. 2005;98:2328–2336. doi: 10.1152/japplphysiol.01084.2004. [DOI] [PubMed] [Google Scholar]
- 11.Gopalan SM, Flaim C, Bhatia SN, et al. Anisotropic stretch-induced hypertrophy in neonatal ventricular myocytes micropatterned on deformable elastomers. Biotechnol. Bioeng. 2003;81:578–587. doi: 10.1002/bit.10506. [DOI] [PubMed] [Google Scholar]
- 12.Torsoni AS, Constancio SS, Nadruz W, Jr., Hanks SK, Franchini KG. Focal adhesion kinase is activated and mediates the early hypertrophic response to stretch in cardiac myocytes. Circ. Res. 2003;93:140–147. doi: 10.1161/01.RES.0000081595.25297.1B. [DOI] [PubMed] [Google Scholar]
- 13.Shyu KG, Chen CC, Wang BW, Kuan P. Angiotensin II receptor antagonist blocks the expression of connexin43 induced by cyclical mechanical stretch in cultured neonatal rat cardiac myocytes. J. Mol. Cell. Cardiol. 2001;33:691–698. doi: 10.1006/jmcc.2000.1333. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka N, Mao L, DeLano FA, et al. Left ventricular volumes and function in the embryonic mouse heart. Am. J. Physiol. 1997;273:H1368–H1376. doi: 10.1152/ajpheart.1997.273.3.H1368. [DOI] [PubMed] [Google Scholar]
- 15.Paul S. Ventricular remodeling. Crit. Care Nurs. Clin. N. Am. 2003;15:407–411. doi: 10.1016/s0899-5885(02)00089-8. [DOI] [PubMed] [Google Scholar]
- 16.Masuda H, Sperelakis N. Inwardly rectifying potassium current in rat fetal and neonatal ventricular cardiomyocytes. Am. J. Physiol. 1993;265:H1107–H1111. doi: 10.1152/ajpheart.1993.265.4.H1107. [DOI] [PubMed] [Google Scholar]
- 17.Gomez JP, Potreau D, Raymond G. Intracellular calcium transients from newborn rat cardiomyocytes in primary culture. Cell Calcium. 1994;15:265–275. doi: 10.1016/0143-4160(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 18.Cerbai E, Pino R, Sartiani L, Mugelli A. Influence of postnatal-development on I(f) occurrence and properties in neonatal rat ventricular myocytes. Cardiovasc. Res. 1999;42:416–423. doi: 10.1016/s0008-6363(99)00037-1. [DOI] [PubMed] [Google Scholar]
- 19.Kimura H, Takemura H, Imoto K, Furukawa K, Ohshika H, Mochizuki Y. Relation between spontaneous contraction and sarcoplasmic reticulum function in cultured neonatal rat cardiac myocytes. Cell Signal. 1998;10:349–354. doi: 10.1016/s0898-6568(97)00134-4. [DOI] [PubMed] [Google Scholar]
- 20.Lakatta EG. Beyond Bowditch: the convergence of cardiac chronotropy and inotropy. Cell Calcium. 2004;35:629–642. doi: 10.1016/j.ceca.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 21.Silva J, Rudy Y. Mechanism of pacemaking in I(K1)-downregulated myocytes. Circ. Res. 2003;92:261–263. doi: 10.1161/01.RES.0000057996.20414.C6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiang Y, Rybin VO, Steinberg SF, Kobilka B. Caveolar localization dictates physiologic signaling of beta 2-adrenoceptors in neonatal cardiac myocytes. J. Biol. Chem. 2002;277:34,280–34,286. doi: 10.1074/jbc.M201644200. [DOI] [PubMed] [Google Scholar]
- 23.Abi-Gerges N, Fischmeister R, Mery PF. G protein-mediated inhibitory effect of a nitric oxide donor on the L-type Ca2+ current in rat ventricular myocytes. J. Physiol. 2001;531:117–130. doi: 10.1111/j.1469-7793.2001.0117j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balligand JL, Kelly RA, Marsden PA, Smith TW, Michel T. Control of cardiac muscle cell function by an endogenous nitric oxide signaling system. Proc. Natl. Acad. Sci. USA. 1993;90:347–351. doi: 10.1073/pnas.90.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Devic E, Xiang Y, Gould D, Kobilka B. Beta-adrenergic receptor subtype-specific signaling in cardiac myocytes from beta(1) and beta(2) adrenoceptor knockout mice. Mol. Pharmacol. 2001;60:577–583. [PubMed] [Google Scholar]
- 26.Orita H, Fukasawa M, Hirooka S, Uchino H, Fukui K, Washio M. Modulation of cardiac myocyte beating rate and hypertrophy by cardiac fibroblasts isolated from neonatal rat ventricle. Jpn Circ. J. 1993;57:912–920. doi: 10.1253/jcj.57.912. [DOI] [PubMed] [Google Scholar]
- 27.Kroll MH, Hellums JD, McIntire LV, Schafer AI, Moake JL. Platelets and shear stress. Blood. 1996;88:1525–1541. [PubMed] [Google Scholar]
- 28.Weinbaum S, Cowin SC, Zeng Y. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J. Biomech. 1994;27:339–360. doi: 10.1016/0021-9290(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 29.Schmid-Schonbein GW. Biomechanics of microcirculatory blood perfusion. Annu. Rev. Biomed. Eng. 1999;1:73–102. doi: 10.1146/annurev.bioeng.1.1.73. [DOI] [PubMed] [Google Scholar]
- 30.Cohn JN. Critical review of heart failure: the role of left ventricular remodeling in the therapeutic response. Clin. Cardiol. 1995;18:IV4–IV12. doi: 10.1002/clc.4960181603. [DOI] [PubMed] [Google Scholar]
- 31.Lodge NJ, Normandin DE. Alterations in Ito1, IKr and Ik1 density in the BIO TO-2 strain of syrian myopathic hamsters. J. Mol. Cell. Cardiol. 1997;29:3211–3221. doi: 10.1006/jmcc.1997.0548. [DOI] [PubMed] [Google Scholar]
- 32.Knollmann BC, Knollmann-Ritschel BE, Weissman NJ, Jones LR, Morad M. Remodelling of ionic currents in hypertrophied and failing hearts of transgenic mice overexpressing calsequestrin. J. Physiol. 2000;525:483–498. doi: 10.1111/j.1469-7793.2000.t01-1-00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Janse MJ. Electrophysiological changes in heart failure and their relationship to arrhythmogenesis. Cardiovasc. Res. 2004;61:208–217. doi: 10.1016/j.cardiores.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 34.Cerbai E, Barbieri M, Mugelli A. Characterization of the hyperpolarization-activated current, I(f), in ventricular myocytes isolated from hypertensive rats. J. Physiol. 1994;481:585–591. doi: 10.1113/jphysiol.1994.sp020465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reich KM, Gay CV, Frangos JA. Fluid shear stress as a mediator of osteoblast cyclic adenosine monophosphate production. J. Cell. Physiol. 1990;143:100–104. doi: 10.1002/jcp.1041430113. [DOI] [PubMed] [Google Scholar]
- 36.Bakker AD, Soejima K, Klein-Nulend J, Burger EH. The production of nitric oxide and prostaglandin E(2) by primary bone cells is shear stress dependent. J. Biomech. 2001;34:671–677. doi: 10.1016/s0021-9290(00)00231-1. [DOI] [PubMed] [Google Scholar]
- 37.Slattery MJ, Liang S, Dong C. Distinct role of hydrodynamic shear in leukocyte-facilitated tumor cell extravasation. Am. J. Physiol. Cell. Physiol. 2005;288:C831–C839. doi: 10.1152/ajpcell.00439.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reuter H, Cachelin AB, De Peyer JE, Kokubun S. Modulation of calcium channels in cultured cardiac cells by isoproterenol and 8-bromo-cAMP. Cold Spring Harb. Symp. Quant. Biol. 1983;48:193–200. doi: 10.1101/sqb.1983.048.01.022. [DOI] [PubMed] [Google Scholar]
- 39.Ross RS, Borg TK. Integrins and the myocardium. Circ. Res. 2001;88:1112–1119. doi: 10.1161/hh1101.091862. [DOI] [PubMed] [Google Scholar]
- 40.Wang YG, Samarel AM, Lipsius SL. Laminin acts via beta 1 integrin signalling to alter cholinergic regulation of L-type Ca(2+) current in cat atrial myocytes. J. Physiol. 2000;526:57–68. doi: 10.1111/j.1469-7793.2000.t01-1-00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng Q, Ross RS, Walsh KB. Overexpression of the integrin beta(1A) subunit and the beta(1A) cytoplasmic domain modifies the beta-adrenergic regulation of the cardiac L-type Ca(2+)current. J. Mol. Cell. Cardiol. 2004;36:809–819. doi: 10.1016/j.yjmcc.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 42.Communal C, Singh M, Menon B, Xie Z, Colucci WS, Singh K. beta1 integrins expression in adult rat ventricular myocytes and its role in the regulation of beta-adrenergic receptor-stimulated apoptosis. J. Cell. Biochem. 2003;89:381–8. doi: 10.1002/jcb.10520. [DOI] [PubMed] [Google Scholar]
- 43.Wang YG, Samarel AM, Lipsius SL. Laminin binding to beta1-integrins selectively alters beta1-and beta2-adrenoceptor signalling in cat atrial myocytes. J. Physiol. 2000;527:3–9. doi: 10.1111/j.1469-7793.2000.t01-2-00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]