Abstract
Repeated administration of low doses of ethanol gradually increases locomotor responses to ethanol in adult Swiss mice. This phenomenon is known as behavioral sensitization. However, we have shown that adolescent Swiss mice show either behavioral tolerance or no sensitization after repeated ethanol injections. Although the mesolimbic dopamine system has been extensively implicated in behavioral sensitization, several studies have demonstrated an important role of glutamatergic transmission in this phenomenon. In addition, relatively few studies have examined the role of developmental factors in behavioral sensitization to ethanol. To examine the relationship between age differences in behavioral sensitization to ethanol and the neurochemical adaptations related to glutamate within nucleus accumbens (NAc), in vivo microdialysis was conducted in adolescent and adult Swiss mice treated with ethanol (1.8 g/kg) or saline for 15 days, and subsequently challenged with an acute dose (1.8 g/kg) of ethanol six days later. Consistent with previous findings, only adult mice demonstrated evidence of behavioral sensitization. However, ethanol-treated adolescent mice demonstrated a 196.1 ± 40.0% peak increase in extracellular levels of glutamate in the NAc following ethanol challenge in comparison with basal values, whereas ethanol-treated adult mice demonstrated a 52.2 ± 6.2% reduction in extracellular levels of glutamate in the NAc following ethanol challenge. These observations suggest an age-dependent inverse relationship between behavioral and glutamatergic responses to repeated ethanol exposure.
Keywords: adolescent, glutamate, behavioral sensitization, mice, microdialysis, nucleus accumbens
INTRODUCTION
During adolescence, maturational changes in the brain contribute to numerous age-specific behaviors that are unique to this stage of development. Brain reward systems related to drug dependence also undergo significant structural and neurochemical rearrangements during this period. Adolescent individuals are particularly prone to initiate use of alcohol and other drugs of abuse (Spear, 2000). Drug use during adolescence may result in critical neuroadaptive changes that subsequently increase the vulnerability of developing addiction later in life (Crews et al., 2007; Spear, 2000; Grant and Dawson, 1997).
According to the 2007 U.S. National Survey on Drug Use and Health, the rate of current alcohol use among youths aged 12 to 17 was 15.9 percent in 2007 (Substance Abuse and Mental Health Services Administration, 2008). Estimates obtained in a household survey conducted by the “Centro Brasileiro de Informações sobre Drogas Psicotrópicas” in Brazil, showed that 54.4 percent of adolescents age 12 to 17 have used alcoholic drinks at least once in their lifetime (Galduróz and Carlini, 2007). Higher rates of adolescent alcohol consumption by some adolescent populations may increase the frequency of alcohol abuse problems later in adulthood. In animals, adolescent rats consume more ethanol than adults in voluntary drinking paradigms under continuous (Brunell and Spear, 2005; Truxell et al., 2007) and limited access conditions (Maldonado et al., 2008).
Repeated exposure to drugs of abuse in laboratory animals, including ethanol, produce long-lasting neuroadaptations in reward systems critically involved with incentive motivation that persist long after drug exposure has been discontinued (Nestler, 2001). Behavioral adaptations to repeated drug administration include the development of tolerance and sensitization, which are characterized by reduced and increased responsiveness to the drug, respectively. Neurochemical adaptations are related to alterations in reward-related neuronal signaling pathways and are relevant to drug craving and compulsive aspects of addiction (Ron and Jurd, 2005). In animal models, such alterations are manifested behaviorally. For example, repeated administration of the same dose of a drug results in a progressive increase in the locomotor response to drug administration, a phenomenon known as behavioral sensitization. This phenomenon has been associated with sensitization to the incentive motivational effects of drugs (Wise and Bozarth, 1987; Robinson and Berridge, 2001).
Behavioral ontogenic studies have demonstrated that adolescent animals show reduced development or expression of behavioral sensitization to ethanol (Faria et al., 2008; Stevenson et al., 2008), increased sensitivity to the acute locomotor stimulant, anxiolytic-like, and ataxic effects of ethanol (Hefner and Holmes, 2007); increased sensitivity to the appetitive motivation effects of ethanol (Pautassi et al., 2008) and consume more ethanol (Brunell and Spear, 2005; Doremus et al., 2005; Maldonado et al., 2008) when compared to adults. It is known that ethanol is a potent inhibitor of the N-methyl-D-aspartate (NMDA) receptor subtype for glutamate, and prolonged ethanol exposure leads to a compensatory up-regulation of NMDA receptor function (Hoffman et al., 1989; Lovinger et al., 1989; Melendez et al., 2005). Co-administration of the non-competitive NMDA receptor antagonist MK-801 with ethanol blocks the development of ethanol-induced behavioral sensitization (Broadbent and Weitemier, 1999; Camarini et al., 2000). These findings suggest that glutamatergic transmission is involved in the behavioral alterations produced by repeated ethanol exposure. Moreover, studies have shown that acute ethanol exposure increases extracellular levels of glutamate in the nucleus accumbens (NAc) (Szumlinski et al., 2007; Kapasova and Szumlinski, 2008), a forebrain region critically involved in the reinforcing properties of various drugs of abuse. Moreover, blockade of glutamatergic signaling in the NAc attenuates ethanol consumption and reinforcement (Gass and Olive, 2009; Cozzoli et al., 2009)
Taken together, these findings suggest that glutamatergic transmission plays an important role in behavioral responses to repeated ethanol exposure, as well as the motivational effects of ethanol. However, little is known about the effects of repeated ethanol exposure on glutamatergic transmission in the NAc at different stages of development (i.e., adolescence vs. adulthood). Therefore, the present study was designed to examine the relationship between age-related differences in the expression of behavioral sensitization to ethanol and changes in extracellular glutamate levels in the NAc produced by ethanol in adolescent and adult mice.
MATERIALS AND METHODS
Subjects
Adult male Swiss mice (postnatal day (PND) 57-58 for adults, PND 27-28 for adolescents at the start of the experiments, (CEDEME, São Paulo, SP, Brazil) were used as subjects. Mice were housed in groups of 5 in standard Plexiglas cages in a colony room with controlled lighting (12:12 light-dark cycle, with lights on at 06:30 h) and temperature (22 ± 2 °C) conditions. Food and water were provided ad libitum. Mice were allowed to adapt to the colony room for at least five days prior to experimentation. All experiments were carried out between 8:30 and 11:30 h. All procedures were approved by the Ethical Committee for Animal Research (CEEA) of the Institute of Biomedical Sciences of the University of São Paulo.
Drugs
Ethanol (EtOH) (95% v/v, Merck do Brasil, SA) was dissolved in 0.9% w/v sodium chloride (physiological saline) to produce a 20% v/v ethanol solution and was administered via the intraperitoneal (i.p) route at a dose of 1.8 g/kg. Control animals received equivalent volumes of saline (SAL) by i.p. injection.
Procedures
A summary of the experimental schedule is shown in Figure 1. Adolescent or adult mice (n=12 per group) were treated with either saline or ethanol for 15 consecutive days. On the 2 first days before starting the ethanol treatment, mice received an injection of saline and were placed in the open field apparatus 5 min after the injection for a period of 5 min in the apparatus to habituate the animals to injection procedures and the locomotor activity monitoring environment. Locomotor activity was measured on the first, 7th and 15th day of the experiment. Microdialysis was conducted six days after the last ethanol injection. On day 21 (6 days following the last ethanol injection), all mice received an acute i.p. injection of ethanol (1.8 g/kg). Two additional control groups (adolescent and adult) were administered an equivalent volume of saline on day 21 to control for possible effects of handling and/or injection stress on extracellular glutamate levels.
Figure 1.
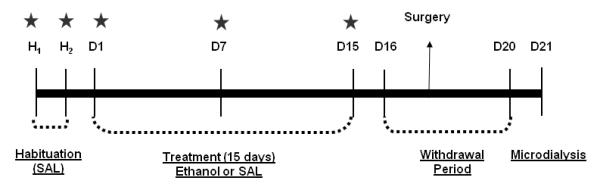
Summary of the experimental paradigm. n=12 mice/group were used in this experiment. On the first 2 habituation days (H1, H2), all mice received SAL. During the treatment phase, mice were treated daily with SAL or 1.8 g/kg i.p EtOH (D1-D15). On the day following the last ethanol injection (i.e., Day 16), mice underwent stereotaxic implantation of microdialysis guide cannula. On Day 20, microdialysis probes were implanted, and, microdialysis sample collection was performed on Day 21. A separate set of animals underwent identical ethanol or saline treatment but in lieu of microdialysis procedures, BECs were measured on Day 21. Star denotes assessment of open field locomotor activity.
For each locomotor activity assessment, animals were placed in the center of a cylindrical open-field arena (40 cm diameter). The open-field employed consists of a circular wooden arena (40 cm in diameter and 35 cm high) painted blue. A video camera installed 230 cm above the apparatus was connected to a computer located outside of the experimental chamber. Horizontal locomotor activity was quantified by Ethovision software (Noldus, The Netherlands) for 5 min after a post-ethanol or saline injection time of 5 min.
Surgery
Following locomotor sensitization procedures, mice were implanted with intracerebral guide cannula for in vivo microdialysis sampling. Anesthesia was induced with 5% isoflurane with oxygen as a carrier gas and maintained at 2-3% during surgery. Mice were placed into a stereotaxic frame and the animal’s head was shaved and scrubbed with a betadine solution, and an incision was made to expose the skull. Two holes were drilled in the skull approximately 3.0 mm lateral and 3.0 mm posterior to bregma where miniature skull screws were inserted. An additional hole was drilled anterior to bregma and stainless steel guide cannulae (15 mm in length) aimed that the NAc were lowered to a depth of 5.0 mm from skull surface using the following coordinates from bregma for the NAc (antero-posterior: +1.7 mm; lateral: + 0.9 mm) (Paxinos and Franklin, 2001). The guide cannulae were fixed to the skull using dental resin and a dummy probe was inserted into the guide cannula to prevent obstruction and contamination from external debris. After surgery, animals were individually housed and allowed to recover for 4 days prior to microdialysis procedures.
In vivo microdialysis procedures
On the afternoon prior to microdialysis procedures, the dummy probe was removed and microdialysis probes (CMA/7, CMA Microdialysis, North Chelmsford, MA) equipped with 1 mm cuprophane membranes were inserted into the guide cannula. Mice anesthetized during probe implantation. Probes were perfused with artificial cerebrospinal fluid (aCSF, Harvard Apparatus, Holliston, MA) via syringes located in a syringe pump (KD Scientific Inc, New Hope, PA). Probes were connected to the syringe pump via fluorinated ethylene-propylene tubing (FEP, 0.005” ID). The composition of the aCSF was (in mM): Na 150; K 3.0; Ca 1.4; Mg 0.8; P 1.0; Cl 155. Microdialysis procedures were conducted six days after the last ethanol injection. Following implantation of the microdialysis probe, microdialysis tubing was connected to a dual channel liquid swivel (Instech Laboratories, Plymouth Meeting, MA) via FEP tubing, and the flow rate was set to 0.5 μl/min. Following overnight equilibration, the flow rate was adjusted to 2.0 μl/min. One hour after this adjustment, dialysis samples were collected at 15-min intervals. After four baseline samples were collected, all mice received an acute i.p. injection of ethanol (1.8 g/kg; 20% w/v), followed by collection of eight additional samples. Saline control groups received an i.p. injection of saline. Samples were stored at −80°C until analysis by HPLC (see below).
HPLC Analysis of Glutamate
Dialysate glutamate content was determined by a high performance liquid chromatography system coupled with electrochemical detection (HPLC-ED, ESA Inc., North Chelsmford, MA) as described previously (Olive et al., 2000; Camarini et al., 2008). Microdialysis samples were derivatized with o-pthalaldehyde and beta-mercaptoethanol (Sigma-Aldrich, St. Louis, MO) automatically by a refrigerated autosampler (ESA, Inc.) immediately prior to injection onto a HR-80 column (ESA, Inc.) (Olive et al., 2000; Camarini et al., 2008). The mobile phase consisted of 0.1 M NaH2PO4 and 25% (v/v) methanol (pH=6.75) and was pumped at a flow rate of 0.6 ml/min. Glutamate levels in microdialysis samples were quantified by comparing computer-integrated peak areas of samples with those of L-glutamate standards using a three point calibration curve (0, 0.3 μM and 1.47 μM) run every 20 samples. The detection limit of the HPLC assay for glutamate was approximately 0.03 μM at a signal-to-noise ratio of 3 to 1. Samples from seven mice with undetectable levels of glutamate were excluded from analyses.
Microdialysis data analysis
Absolute levels of glutamate in the microdialysis samples were converted to percent of baseline dialysate glutamate content. Baseline dialysate levels of glutamate were calculated by averaging the absolute concentration of glutamate in each of the four pre-injection dialysate samples of each individual animal, and then all concentrations of dialysate glutamate were converted to percent of this baseline according to the animal’s individual basal dialysate levels of glutamate.
Histological verification of probe placement
Following microdialysis procedures, animals were anesthetized with an overdose of ketamine/xylazine solution. The animal’s heart was then exposed and a heparin-containing solution was injected in the left ventricle. Animals were then perfused transcardially with 4% w/v paraformaldehyde in phosphate buffered saline (pH=7.4). After perfusion, brains were removed and placed in a solution containing 4% paraformaldehyde and 10% (w/v) sucrose overnight. Brains were then cut on a cryostat at 30 μm thickness and the sections were mounted on gelatin-coated slides, then allowed to air-dry overnight. The slides were rehydrated in double-distilled water and stained with thionin (Nissl stain) for 15-45 s until the desired staining intensity was reached, then gradually dehydrated in 5-min immersions in ethanol at increasing concentrations (50, 75, 90, 95 and 100%, v/v). Probe placement in the NAc was verified according to the atlas of Paxinos and Franklin (2001). Only animals with probe placements restricted to the NAc were included in the data analysis. Eight animals were excluded from the study due to improper probe placement.
Blood ethanol concentration (BEC)
To address the possibility that age could influence blood-ethanol concentrations (BECs) by altered ethanol metabolism, separate groups of mice underwent identical behavioral sensitization procedures as outlined in Figure 1. Ethanol (1.8 g/kg) was injected via the i.p. route. At 15 and 60 minutes after ethanol injection, blood samples were obtained from the retro-orbital sinus. At 180 minutes, animals were sacrificed and trunk blood was collected for BEC determination. Immediately following collection, blood samples were centrifuged for 10 minutes at 10,000 rpm, and 5 μl of the plasma supernatant from each sample was analyzed for ethanol concentration using an Analox GL6 Multiassay Analyser (Analox Instruments, MA, USA). BECs were expressed in mg/dl.
Statistical Analyses
All data were analyzed by a mixed three-way analysis of variance (ANOVA), with time as the repeated measures factor (i.e., day of locomotor activity assessment for behavioral sensitization studies or minutes following ethanol injection for the BEC determination or time following ethanol injection for the glutamate quantification) and treatment (i.e., ethanol or saline administration on days 1-15) and age group (i.e., adolescent or adult) as the between subjects factor. Significant interactions were deconstructed for main effects. Levene’s test was used to assess the homogeneity of variances and if the data passed this test, Newman-Keuls comparison to a mean was used for planned comparisons, where appropriate. The significance level was α ≤ 0.05.
RESULTS
Age Differences in Behavioral Responses to Ethanol
A diagram of the timeline of the experimental procedures is shown in Figure 1. Figure 2 shows the locomotor activity of adolescent and adult animals treated with ethanol (1.8 g/kg i.p.) or saline for 15 consecutive days. Locomotor activity was quantified on the two habituation days that preceded treatment, on the first treatment day (acute), in the middle of the treatment (7th day) and the last day (15th day). During the two habituation days, decline in activity was observed consistent with habituation to the testing apparatus. This observation was supported by a significant difference between treatment days [Fdays(1,44)=26.03; p<0.01].
Figure 2.
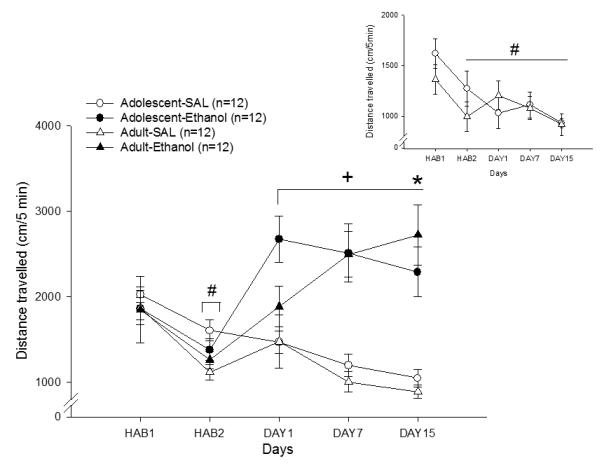
Effects of repeated treatment with ethanol (1.8 g/kg) or saline on locomotor activity in adolescent and adult Swiss mice. #HAB2 < HAB1, indicating habituation to the apparatus. +Ethanol-treated mice presented increased locomotor activity as compared with saline-treated mice. *Ethanol-treated adult mice displayed increased locomotor activity on Day 15 as compared with the first ethanol injection (Day 1). Inset, control adolescent and adult mice that received saline on Day 21 showed a similar habituation response (#HAB2 and afterwards < HAB1).
Significant increases in locomotor activity were observed in mice treated with ethanol as compared to those treated with saline [Ftreatment(1,44) = 51.54; p < 0.01], providing evidence of a stimulant effect of ethanol at this dose. This effect was confirmed by the interaction between treatment and days [F(2,88) = 3.77; p < 0.05]. An interaction between age, treatment, and treatment day [F(2,88) = 3.14; p < 0.05] revealed a significant increase in the locomotor activity of ethanol-treated adult animals on the fifteenth treatment day in comparison to the first treatment day. However, adolescent mice showed no differences in locomotor activity across the 15 days of treatment, suggesting that adult but not adolescent mice developed behavioral sensitization to ethanol.
Analysis of the locomotor data from the saline control groups in a two-way ANOVA revealed a main effect of days [F(4,48) = 6.39; p < 0.01], indicating habituation to the testing apparatus, but no age effect [F(1,12) = 0.0005; p > 0.05] nor an interaction between age and days[F(4,48) = 1.28; p > 0.05].
Basal Extracellular Levels of Glutamate in the NAc
A three-way ANOVA (age X treatment X time), with time as a repeated measure, was performed for the 4 basal levels of glutamate and indicated no differences among groups (all p-values > 0.05), and no significant interactions were observed. The average ± S.E.M. of the dialysate glutamate levels in the first four samples (baseline) for adolescent (saline and ethanol) and adult (saline and ethanol) groups were, respectively, 0.153 ± 0.08, 0.185 ± 0.06; 0.192 ± 0.05 and 0.13 ± 0.12 μM. The average ± S.E.M. of the glutamate levels in the first four samples (baseline) for the adolescent and adult saline control groups were 0.192 ± 0.04 and 0.196 ± 0.02 μM, respectively, and no intergroup differences were observed (p > 0.05).
Age Differences in Glutamate Responses to Ethanol
A three-way ANOVA (age X treatment X time), with time as a repeated measure, performed for the 12 microdialysis samples collected, indicated a significant effect of age [Fage(1,36) = 7.93; p < 0.01], time [Ftime(11,396) = 6.1; p < 0.01], an age X treatment interaction [F(1,36) = 28.04; p < 0.051], an age X time interaction [F(11,396) = 2.18; p < 0.05]; a treatment X time interaction [F(11,396) = 2.19; p < 0.05], and age X treatment X time interaction [F(11,396) = 3.92; p < 0.01]. Analysis of the age X treatment interaction revealed that ethanol-treated adolescent mice demonstrated higher dialysate levels of glutamate than saline-treated adolescent mice, while adult mice presented an inverse relationship.
As shown in Fig. 3, marked age differences were observed regarding the effect of repeated ethanol administration during adolescence and adulthood in extracellular levels of glutamate in the NAc following a subsequent ethanol challenge. Post-hoc analysis of the age X treatment X time interaction revealed that in ethanol-pretreated adolescent mice, a subsequent ethanol challenge produced increases in extracellular levels of glutamate from baseline at 15 min post ethanol challenge. In contrast, ethanol-pretreated adult mice showed a reduction in extracellular glutamate levels from baseline at 45 min following ethanol challenge. Ethanol-treated adolescent mice demonstrated a 196.1 ± 40.0% peak increase in extracellular levels of glutamate in the NAc following ethanol challenge in comparison with basal values, whereas ethanol-treated adult mice demonstrated a 52.2 ± 6.2% peak reduction in extracellular levels of glutamate in the NAc following ethanol challenge.
Figure 3.
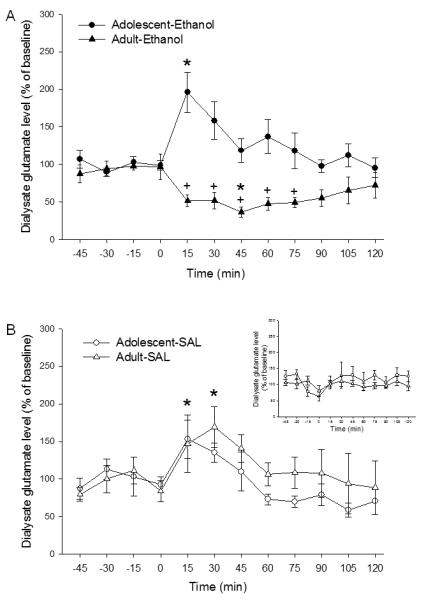
Age-related differences in dialysate levels of glutamate in the NAc after an ethanol challenge (1.8 g/kg) following the behavioral sensitization procedures. (A). Adolescent mice treated repeatedly with ethanol followed by an ethanol challenge (Adolescent-EtOH) demonstrated an increase in dialysate levels of glutamate at 15 min post-injection (*p<0.05 vs. last baseline sample), whereas adult mice reated repeatedly with ethanol followed by an ethanol challenge (Adult-EtOH) showed a reduction in dialysate levels of glutamate at 45 min post-injection (*p<0.05 vs. last baseline sample),. Intergroup differences in dialysate glutamate levels were found at 15-75 min post-injection (+p<0.05 vs. Adolescent-EtOH mice at the same timepoint. (B). Adolescent and adult mice treated repeatedly with saline (Adolescent-sal and adult-sal, respectively) showed similar increases in dialysate glutamate levels at 15 and 30 min post ethanol challenge (*p<0.05 vs. last baseline sample). However, no intergroup differences were observed.. (Inset) Administration of saline had no effect on dialysate levels of glutamate in the adolescent and adult saline control groups..
In addition, ethanol-pretreated adult mice displayed lower levels of extracellular glutamate than ethanol-pretreated adolescent mice from 15 to 75 min post-ethanol challenge. No statistical differences were found between saline pre-treated adult and adolescent mice. Post-hoc analysis of the age X treatment X time interaction showed that dialysate glutamate levels peak at 15 min over basal levels for saline-treated adolescent mice (153 ± 13.5%) and at 30 min for saline-treated adult mice (169.3. ± 18.7%) after ethanol challenge.
Two-way ANOVA performed on the microdialysis data from the saline control groups revealed no age effect [F(1,12) = 1.34; p > 0.05], no time effect [F(11,132) = 1.36; p > 0.05] nor an interaction of age and time [F(11,132) = 0.75; p > 0.05], demonstrating a lack of effect of handling and/or injection stress on extracellular glutamate levels in the NAc.
Blood ethanol concentration (BEC)
Table 1 summarizes the BECs measured in saline or ethanol-treated adolescent and adult mice on ethanol test day at 15, 60 and 180 minutes following the administration of 1.8 g/kg ethanol.
Table 1.
BEC in saline- or ethanol-treated adolescent and adult mice after an acute ethanol challenge injection (1.8 g/kg) on day 21.
| Groups | 15 min | 60 min | 180 min |
|---|---|---|---|
| Adolescent-SAL | 206.68 ± 14.89 | 130.21 ± 19.63 | 7.69 ± 2.19 |
| Adolescent-Ethanol | 226.29 ± 7.48 | 140.49 ± 14.69 | 7.34 ± 0.69 |
| Adult-SAL | 208.08 ± 22.13 | 163.00 ± 19.05 | 20.44 ± 6.17 |
| Adult-Ethanol | 241.70 ± 5.66 | 149.95 ± 14.32 | 10.12 ± 0.37 |
SAL = saline; BEC = blood ethanol concentration. BECs were measured at 15, 60 and 180 min postinjection. BEC data (expressed in mg/dL) are shown as mean ± standard error of the mean of each treatment group (n=8 per group). No differences were found between adolescent and adult mice (saline or ethanol treated).
Statistical analysis of BECs did not reveal significant effects of age or treatment when comparing the adolescent and adult saline or ethanol-treated mice [Fage(1,28) = 4.06; p > 0.05]; [Ftreatment(1,28) = 1.43; p > 0.05]. The only significant effect observed was an effect of time [Ftime(2,56) = 260.2; p < 0.01], consistent with ethanol clearance from the blood. There were no significant interactions observed.
Histological results
Diagrams of coronal mouse brain sections showing approximate locations of microdialysis probe membranes located in the NAc are shown in Figure 4. Animals with incorrect probe placements were excluded from analyses.
Figure 4.
Diagrams of mouse brain coronal sections from the atlas of Franklin and Paxinos (2001) showing approximate locations of microdialysis probes in the NAc. Numbers alongside each section represents the distance (in mm) of the section from bregma (B).
DISCUSSION
Our results show that adult, but not adolescent, Swiss mice developed behavioral sensitization to repeated administration of 1.8 g/kg of ethanol. In the current study, adolescent demonstrated an trend towards an elevated locomotor response to acute ethanol on day 1 of treatment compared to the adults. However, this difference did not reach statistical significance, which could be interpreted as a ceiling effect which masked the sensitized response to the repeated treatment with ethanol. According to the literature, contradictory behavioral responses to psychostimulant drugs have been demonstrated in adolescent rodents when compared to adults, showing increased or decreased locomotor activity in response to cocaine or amphetamine (Lanier and Isaacson, 1997; Spear and Brick, 1979; Spear and Brake, 1983; Laviola et al., 1995; Witt, 1994; Catlow and Kirstein, 2005; Camarini et al., 2008). The intense neural development period of adolescence may account, in part, to the variability in behavioral responses to drugs of abuse. Regardless of the mechanism, the present locomotor results are in accordance with previous findings from our laboratory (Faria et al., 2008) and that of others using DBA/2 mice (Stevenson et al., 2008). We also found that the ability of repeated ethanol to induce behavioral sensitization was inversely related to the effects of a subsequent ethanol challenge on extracellular levels of glutamate in the NAc. That is, adolescent mice that did not show evidence of locomotor sensitization to ethanol, a subsequent ethanol challenge increased extracellular glutamate levels in the NAc of these mice. In contrast, adult mice, which did show evidence of behavioral sensitization, exhibited a decrease in extracellular levels of glutamate in the NAc following a subsequent ethanol challenge.
Although it could be speculated that the neurochemical measurements were not conducted in close temporal proximity to the final locomotor assessment, the correlation between these two factors should be strongly considered. We demonstrated that a withdrawal period did not change the pattern of behavioral responses to a challenge dose of ethanol in adolescent and adult mice pretreated with ethanol (i.e, adolescent mice did not demonstrate locomotor sensitization in contrast to adults (Faria et al., 2008), which usually express a long-lasting sensitized response to ethanol (Lessov and Phillips, 1998).
A major neural system essential for drug reward and reinforcement involves dopaminergic projections from the ventral tegmental area (VTA) to the NAc. However, increasing evidence suggests a role for glutamatergic transmission in ethanol reward and reinforcement (Bäckström and Hyytiä, 2004; Biala and Kotlinska, 1999; Camarini et al., 2000; Stephens and Brown, 1999), particularly glutamatergic input from the prefrontal cortex (PFC) to the NAc (Kalivas and Volkow, 2005). The NAc plays a role in the pathophysiology of drug addiction and receives projections from different areas in the PFC. During adolescence, the PFC is relatively underdeveloped which likely results in the impaired impulse control that is characteristic of this age population. The ability of ethanol pretreated animals to show greater increases in extracellular levels of glutamate in response to an ethanol challenge as compared to similarly treated adult animals may be a result of enhanced excitability of glutamatergic afferents from the PFC to the NAc in response to ethanol exposure. This may be related to developmental changes in the PFC. For example, in rats, levels of NMDA glutamate receptors in the PFC peak at infancy and subsequently decline with age (Insel et al., 1990). Recently, Henson et al (2008) demonstrated that NR3A levels change over development in human PFC, with peak levels of expression during early childhood followed by a gradual decrease into adulthood.
Using in vivo microdialysis to measure extracelullar glutamate levels following ethanol treatment, Yan et al. (1998) observed that rats receiving i.p. injection of ethanol at a dose of 2.0 g/kg demonstrated significantly decreased extracellular glutamate levels in the NAc. However, recent studies have demonstrated that this effect is dependent on ethanol dose and genetic background of the animal (Kapasova and Szumlinski, 2008; Szumlinski et al., 2005, 2008; Dahchour et al., 2000; Selim and Bradberry, 1996). Low doses of ethanol generally elevate extracellular glutamate in the NAc (Lominac et al., 2006; Moghaddam and Bolinao, 1994) while higher doses of ethanol produce reductions in extracellular glutamate levels in this region (Moghaddam and Bolinao, 1994).
While less well-studied than the mesolimbic dopamine system, evidence supports a potential role for glutamatergic transmission in the NAc in mediating behavioral sensitization to ethanol. Differences in the NAc glutamate response to ethanol are observed between rats and mice that exhibit different behavioral responses to ethanol (Selim and Bradberrry, 1996; Dahchour et al, 2000; Kapasova and Szumlinski, 2008). For example, acute ethanol (1.0 g/kg) enhanced extracellular levels of glutamate in the NAc in Lewis rats (Selim and Bradberry, 1996). Interestingly, animals selected for differences in sensitivity to the hypnotic effects of ethanol (low-alcohol sensitivity rats (LAS) and high-alcohol sensitivity rats (HAS)) also differed in their responses to ethanol with regard to extracellular glutamate levels in the NAc, as ethanol administration produced an increase in extracellular glutamate levels in the NAc of LAS rats and a decrease in HAS rats (Dahchour et al., 2000).
Recently, Kapasova and Szumlinski (2008) demonstrated strain differences in changes in extracellular levels of glutamate in the NAc after acute and repeated ethanol administration. In this study, microdialysis was conducted in two mice strains: the alcohol-preferring C57BL/6J mice and alcohol-avoiding DBA2/J mice. Besides differences in ethanol preference, these strains also differ in behavioral responses to i.p. injections of ethanol, with DBA2/J mice showing sensitization to the locomotor stimulant effects of ethanol, while in C57BL/6J mice this phenomenon is not observed. The authors demonstrated that C57BL/6J exhibited a sensitized glutamate response to ethanol following repeated treatment, whereas DBA2/J presented tolerance to this effect. Interestingly, our data showed that adult mice, which presented the typical behavioral sensitization, failed to demonstrate an ability of ethanol to enhance extracellular levels of glutamate in the NAc. On the other hand, adolescent mice, which did not exhibit sensitization to the locomotor effects of ethanol, displayed an increased glutamate response to repeated ethanol treatment. The age-dependent differences in glutamate response to ethanol could not be attributed to alterations induced by handling or injection stress, since we found no changes in dialysate glutamate levels following a saline challenge on day 21 in adolescent or adult mice. Taken together, the present data are in agreement with Kapasova and Szumlinski (2008) and reinforce the hypothesis that glutamatergic transmission plays an important role in behavioral sensitization to ethanol.
In conclusion, we show that there is an inverse relationship between the development of ethanol-induced behavioral sensitization and the ability of a subsequent ethanol challenge to increase extracellular levels of glutamate in the NAc in adolescent versus adult mice. Considering that it has been demonstrated that pre-exposure to ethanol during adolescence can promote alcohol intake in adult rodents (Pascual et al., 2009), these findings may be of relevance to the enhanced propensity to develop substance abuse problems in adults who are repeatedly exposed to ethanol during adolescence.
Acknowledgments
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and grant AA013852 from the National Institute on Alcohol Abuse and Alcoholism (USA). The authors would like to thank André Veloso Lima Rueda for expert technical assistance.
REFERENCES
- Bäckström P, Hyytiä P. Ionotropic glutamate receptor antagonists modulate cue-induced reinstatement of ethanol-seeking behavior. Alcohol. Clin. Exp. Res. 2004;28:558–565. doi: 10.1097/01.alc.0000122101.13164.21. [DOI] [PubMed] [Google Scholar]
- Biala G, Kotlinska J. Blockade of the acquisition ethanol-induced conditioned place preference by N-methyl-D-aspartate receptor antagonists. Alcohol Alcohol. 1999;34:175–182. doi: 10.1093/alcalc/34.2.175. [DOI] [PubMed] [Google Scholar]
- Broadbent J, Weitemier AZ. Dizolcipine (MK-801) prevents the development of sensitization to ethanol in DBA/2J mice. Alcohol Alcohol. 1999;34:283–288. doi: 10.1093/alcalc/34.3.283. [DOI] [PubMed] [Google Scholar]
- Brunell SC, Spear LP. Effect of stress on voluntary intake of a sweetened ethanol solution in pair-housed adolescent and adult rats. Alcohol. Clin. Exp. Res. 2005;29:1641–1653. doi: 10.1097/01.alc.0000179382.64752.13. [DOI] [PubMed] [Google Scholar]
- Camarini R, Griffin WC, Yanke AB, Santos BR, Olive MF. Effects of adolescent exposure to cocaine on locomotor activity and extracellular dopamine and glutamate levels in nucleus accumbens of DBA/2J mice. Brain Res. 2008;1193:34–42. doi: 10.1016/j.brainres.2007.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camarini R, Frussa-Filho R, Monteiro MG, Calil HM. MK-801 blocks the development of behavioral sensitization to the ethanol. Alcohol. Clin. Exp. Res. 2000;24:285–290. [PubMed] [Google Scholar]
- Cozzoli DK, Goulding SP, Zhang PW, Xiao B, Hu JH, Ary AW, Obara I, Rahn A, Abou-Ziab H, Tyrrel B, Marini C, Yoneyama N, Metten P, Snelling C, Dehoff MH, Crabbe JC, Finn DA, Klugmann M, Worley PF, Szumlinski KKJ. Binge drinking upregulates accumbens mGluR5-Homer2-PI3K signaling: functional implications for alcoholism. J. Neurosci. 2009;29:8655–8668. doi: 10.1523/JNEUROSCI.5900-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews F, He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacol. Biochem. Behav. 2007;86:189–199. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahchour A, Hoffman A, Deitrich R, De Witte P. Effects of ethanol on extracellular amino acid levels in high- and low-alcohol sensitive rats: a microdialysis study. Alcohol Alcohol. 2000;35:548–553. doi: 10.1093/alcalc/35.6.548. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol. Clin. Exp. Res. 2005;29:1796–1808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- Faria RR, Rueda A.V. Lima, Sayuri C, Soares SL, Malta MB, Carrara-Nascimento PF, da Silva Alves A, Marcourakis T, Yonamine M, Scavone C, et al. Environmental modulation of ethanol-induced locomotor activity: correlation with neuronal activity in distinct brain regions of adolescent and adult Swiss mice. Brain Res. 2008;1239:127–140. doi: 10.1016/j.brainres.2008.08.056. [DOI] [PubMed] [Google Scholar]
- Galduróz JC, Carlini EA. Use of alcohol among the inhabitants of the 107 largest cities in Brazil – 2001. Braz. J. Med. Biol. Res. 2007;40:367–375. doi: 10.1590/s0100-879x2007000300012. [DOI] [PubMed] [Google Scholar]
- Gass JT, Olive MF. Role of protein kinase C epsilon (PKCε) in the reduction of ethanol reinforcement due to mGluR5 antagonis in the nucleus accumbens shell. Psychopharmacology. 2009;204:587–597. doi: 10.1007/s00213-009-1490-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J. Subst. Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Hefner K, Holmes A. An investigation of the behavioral actions of ethanol across adolescence in mice. Psychopharmacology. 2007;191:311–322. doi: 10.1007/s00213-006-0646-2. [DOI] [PubMed] [Google Scholar]
- Henson MA, Roberts AC, Salimi K, Vadlamudi S, Hamer RM, Gilmore JH, Jarskog LF, Philpot BD. Developmental regulation of the NMDA receptor subunits, NR3A and NR1, in human prefrontal cortex. Cereb. Cortex. 2008;18:2560–2573. doi: 10.1093/cercor/bhn017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman PL, Rabe C, Moses F, Tabakoff B. N-methyl-D-aspartate receptors and ethanol: inhibition of calcium flux and cyclic GMP production. J. Neurochem. 1989;52:1937–1940. doi: 10.1111/j.1471-4159.1989.tb07280.x. [DOI] [PubMed] [Google Scholar]
- Insel TR, Miller LP, Gelhard RE. The ontogeny of excitatory amino acid receptors in rat forebrain—I. N-methyl-D-aspartate and quisqualate receptors. Neuroscience. 1990;35:31–43. doi: 10.1016/0306-4522(90)90117-m. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am. J. Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kapasova Z, Szumlinski K. Strain differences in alcohol-induced neurochemical plasticity: a role for accumbens glutamate in alcohol intake. Alcohol. Clin. Exp. Res. 2008;32:617–631. doi: 10.1111/j.1530-0277.2008.00620.x. [DOI] [PubMed] [Google Scholar]
- Lominac KD, Kapasova Z, Hannun RA, Patterson C, Middaugh LD, Szumlinski KK. Behavioral and neurochemical interactions between Group 1 mGluR antagonists and ethanol: potential insight into their anti-addictive properties. Drug Alcohol. Depend. 2006;85:142–156. doi: 10.1016/j.drugalcdep.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA-activated ion currents in hippocampal neurons. Science. 1989;243:1721–1724. doi: 10.1126/science.2467382. [DOI] [PubMed] [Google Scholar]
- Maldonado AM, Finkbeiner LM, Alipour KK, Kirstein CL. Voluntary ethanol consumption differs in adolescent and adult male rats using a modified sucrose-fading paradigm. Alcohol. Clin. Exp. Res. 2008;32:1574–1582. doi: 10.1111/j.1530-0277.2008.00733.x. [DOI] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Glutamate, not dopamine, in the accumbens core mediates cocaine-induced reinstatement of drug-seeking behavior. J. Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez RI, Hicks MP, Cagle SS, Kalivas WP. Ethanol Exposure Decreases Glutamate Uptake in the Nucleus Accumbens. Alcohol. Clin. Exp. Res. 2005;29:326–333. doi: 10.1097/01.alc.0000156086.65665.4d. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Bolinao ML. Biphasic effect of ethanol on extracellular accumulation of glutamate in the hippocampus and the nucleus accumbens. Neurosci. Lett. 1994;178:99–102. doi: 10.1016/0304-3940(94)90299-2. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular bases of long-term plasticity underlying addiction. Nat. Rev. Neurosci. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- O’Donnell P, Grace AA. Tonic D2-mediated attenuation of cortical excitation in nucleus accumbens neurons recorded in vitro. Brain Res. 1994;634:105–112. doi: 10.1016/0006-8993(94)90263-1. [DOI] [PubMed] [Google Scholar]
- Olive MF, Mehmert KK, Hodge CW. Microdialysis in the mouse nucleus accumbens: a method for detection of monoamine and amino acid neurotransmitters with simultaneous assessment of locomotor activity. Brain Res. 2000;5:16–24. doi: 10.1016/s1385-299x(99)00054-9. [DOI] [PubMed] [Google Scholar]
- Pautassi RM, Myers M, Spear LP, Molina JC, Spear NE. Adolescent but not adult rats exhibit ethanol-mediated appetitive second-order conditioning. Alcohol. Clin. Exp. Res. 2008;32:2016–2027. doi: 10.1111/j.1530-0277.2008.00789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. 2nd Academic Press; San Diego, CA: 2001. [Google Scholar]
- Rao PA, Molinoff PB, Joyce JN. Ontogeny of dopamine D1 and D2 receptor subtypes in rat basal ganglia: a quantitative autoradiographic study. Brain Res. Dev. Brain Res. 1991;60:161–177. doi: 10.1016/0165-3806(91)90045-k. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Ron D, Jurd R. The “ups and downs” of signaling cascades in addiction. Sci. STKE re14. Review. 2005 doi: 10.1126/stke.3092005re14. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Durstewitz D, Christie BR, Stevens CF, Sejnowski TJ. Dopamine D1/D5 receptor modulation of excitatory synaptic inputs to layer V prefrontal cortex neurons. Proc. Natl. Acad. Sci. USA. 2001a;98:301–306. doi: 10.1073/pnas.011518798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Gorelova N, Durstewitz D, Yang CR. Bidirectional dopamine modulation of GABAergic inhibition in prefrontal cortical pyramidal neurons. J. Neurosci. 2001b;21:3628–3638. doi: 10.1523/JNEUROSCI.21-10-03628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selim M, Bradberry CW. Effect of ethanol on extracellular 5-HT and glutamate in the nucleus accumbens and prefrontal cortex: comparison between Lewis and Fischer 344 rat strains. Brain Res. 1996;716:157–164. doi: 10.1016/0006-8993(95)01385-7. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Stephens DN, Brown G. Disruption of operant oral self-administration of ethanol, sucrose, and saccharin by the AMPA/kainite antagonist NBQX, but not the AMPA antagonist, GYKI 52466. Alcohol. Clin. Exp. Res. 1999;23:1914–1920. [PubMed] [Google Scholar]
- Stevenson RA, Besheer J, Hodge CW. Comparison of ethanol locomotor sensitization in adolescent and adult DBA/2J mice. Psychopharmacology. 2008;197:361–370. doi: 10.1007/s00213-007-1038-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Office of Applied Studies . Results from the 2007 National Survey on Drug Use and Health: National Findings. Rockville, MD: 2008. (NSDUH Series H-34). DHHS Publication No. SMA 08-4343. [Google Scholar]
- Szumlinski KK, Diab ME, Friedman R, Henze LM, Lominac KD, Bowers MS. Accumbens neurochemical adaptations produced by binge-like alcohol consumption. Psychopharmacology. 2007;190:415–431. doi: 10.1007/s00213-006-0641-7. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Lominac KD, Oleson EB, Walker JK, Mason A, Dehoff MH, Klugmann M, Cagle S, Welt K, During M, et al. Homer2 is necessary for EtOH-induced neuroplasticity. J. Neurosci. 2005;25:7054–7061. doi: 10.1523/JNEUROSCI.1529-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski KK, Ary AW, Lominac KD, Klugmann M, Kippin TE. Accumbens Homer2 overexpression facilitates alcohol-induced neuroplasticity in C57BL/6 mice. Neuropsychopharmacology. 2008;33:1365–1378. doi: 10.1038/sj.npp.1301473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truxell EM, Molina JC, Spear NE. Ethanol intake in the juvenile, adolescent, and adult rat: effects of age and prior exposure to ethanol. Alcohol. Clin. Exp. Res. 2007;31:755–765. doi: 10.1111/j.1530-0277.2007.00358.x. [DOI] [PubMed] [Google Scholar]
- Tseng KY, O’Donnell P. D2 dopamine receptors recruit a GABA component for their attenuation of excitatory synaptic transmission in the adult rat prefrontal cortex. Synapse. 2007a;61:843–850. doi: 10.1002/syn.20432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, O’Donnell P. Dopamine modulation of prefrontal cortical interneurons changes during adolescence. Cereb. Cortex. 2007b;17:1235–1240. doi: 10.1093/cercor/bhl034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol. Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- Yan QS, Reith MEA, Yan SG, Jobe PC. Effect of systemic ethanol on basal and stimulated glutamate releases in the nucleus accumbens of freely moving Sprague–Dawley rats: a microdialysis study. Neurosci. Lett. 1998;258:29–32. doi: 10.1016/s0304-3940(98)00840-4. [DOI] [PubMed] [Google Scholar]


