Abstract
The relative anticonvulsant potential of the γ-aminobutyric acid (GABA) agonist, muscimol, was compared after microinjection into either the inferior colliculus, substantia nigra or medial septum of ethanol-dependent rats. Bilateral microinjection of muscimol (10–30 ng) into the inferior colliculus 15 to 60 min before testing suppressed all sound-induced seizure components (wild running, clonus and tonus) in rats withdrawn from ethanol for 6.5 to 8.5 hr. However, forelimb tremors were not altered. Audiogenic seizures were suppressed for at least 3 hr after muscimol (30 ng). In the medial septum and substantia nigra, microinjection of muscimol (30–100 ng) only partially reduced the tonic component of audiogenic seizures and exerted no effect on the frequency of wild running or clonus. GABA (10 μg) and two other GABA agonists [4,5,6,7-tetrahydroisoxa-zolo[5,40c]pyridin-3-ol (THIP), 300 ng and chlordiazepoxide, 10–30 μg], microinjected into the inferior colliculus, also reduced audiogenic seizure susceptibility. However, 1,3-butanediol, which suppresses ethanol withdrawal seizures after peripheral administration in rats, was inactive. The relative proconvulsant potential of the GABA antagonist, bicuculline methiodide, also was compared after microinjection into either the inferior colliculus, substantia nigra or medial septum of ethanol naive rats. In each animal, audiogenic seizure-like wild running, clonus and tonus were evoked by microinjecting bicuculline methiodide into the inferior colliculus at the rate of 6.0 ng/6 min. However, these reactions did not occur when bicuculline methiodide was applied at a slower rate (1.8 ng/6 min). Similar injections of bicuculline methiodide (600 ng/6 min) into the substantia nigra caused only clonus and tonus without wild running. A smaller dose (180 ng/6 min) had no effect. In the medial septum, microinjection of this GABA antagonist (1800 ng/6 min) did not exert any obvious seizure-like activity. These results suggest that the inferior colliculus is important in GABAmimetic suppression of audiogenic seizures and that reduced GABAergic activity in this nucleus may be responsible for the increased susceptibility to audiogenic seizures in rats during ethanol withdrawal.
The cellular mechanism by which ethanol modifies the function of the CNS is of great interest in the search for the biological basis of alcoholism (Kalant, 1975; DeLuca, 1981). Recent investigations suggest that an ethanol-evoked adaptive reduction in CNS inhibition mediated by diminished GABA neurotransmission may play an important role in the development of physical dependence and the expression of the ethanol withdrawal syndrome (see review by Hunt, 1983). For example, there are fewer binding sites for GABA in the brains of rodents exposed continuously to ethanol (Ticku, 1980; Ticku and Burch, 1980; Volicer, 1980). This may indicate an adaptive reduction of GABAergic function during ethanol dependence. In addition, drugs which facilitate GABAergic inhibition by activating GABA receptors can suppress ethanol withdrawal-induced audiogenic seizures in laboratory rodents (Cooper et al., 1979; Frye et al., 1979, 1983). Finally, other support for this hypothesis comes from the clinical finding that seizure-susceptible alcoholics have reduced cerebral spinal fluid GABA concentrations during acute ethanol withdrawal (Goldman et al., 1981). Thus, continuous ethanol intoxication leading to the development of physical dependence may involve, in part, a general reduction in normal GABA-mediated CNS inhibition in both man and laboratory animals.
Despite evidence for a link between reduced GABAergic inhibition and the ethanol withdrawal syndrome, studies to date suggest that all abstinence signs cannot be reversed by activation of GABA receptors. “Convulsions on handling” in mice (Goldstein, 1979) and tremors in rats and monkeys (Frye et al., 1983; Tarika and Winger, 1980) are not suppressed by drugs which activate GABA receptors, as are audiogenically induced seizures (Cooper et al., 1979; Frye et al., 1983). This dramatic difference in the sensitivity of two distinct ethanol withdrawal signs to GABAmimetic agents is not due to strain differences or varying experimental conditions (Frye et al., 1983). Differential suppression of ethanol withdrawal signs by GABAmimetics suggests that individual withdrawal reactions are unique and the result of distinct types of CNS adaptation in response to ethanol, possibly within specialized parts of the nervous system.
Recent reports suggest that susceptibility to some types of seizures may be reduced by drug treatments applied at a small number of brain sites. For example, susceptibility to seizures induced in rodents by electro-shock, chemical excitants or electrical kindling is markedly reduced by discrete microinjection of GABAmimetic drugs into the substantia nigra (Iadarola and Gale, 1981a,b, 1982; McNamara et al., 1982, 1983). Similarly, microinjection of GABAmimetic drugs or lesions of the inferior colliculus, as well as lesions of the medial septal nucleus, reduce susceptibility to audiogenic seizures in rats with a genetic predisposition for epileptic responses to loud noises (Kesner, 1966; Duplisse et al., 1974). The present study was undertaken to determine whether the inferior colliculus, substantia nigra or medial septum might be important in the ability of GABAmimetics to suppress withdrawal-related audiogenic seizures in ethanol-dependent rats.
Materials and Methods
Animals and surgical treatments
Male Sprague-Dawley rats [Crl:CD(SD)BR] weighing 125 to 150 g were purchased from Charles River Laboratories (Somerville, MA) and housed singly under environmentally controlled conditions (7:00 a.m.-7:00 p.m. light-dark cycle; 22–25°C). Wayne Blox rodent laboratory chow and water were freely available unless otherwise stated. One day after arrival, rats were anesthetized with sodium pentobarbital (40 mg/kg i.p.) and mounted in a stereotaxic apparatus fitted with blunt “guinea-pig” ear bars (David Kopf Instruments, Tujunga, CA) to prevent tympanic membrane rupture. After opening the scalp and placing small stainless-steel anchoring screws in the skull, a 26-gauge stainless-steel guide tube was lowered into the brain through a small burr hole to a depth 1 mm dorsal to the intended microinjection site. Methyl methacrylate cement was used to anchor the guide tube to the screws and skull. Stylets consisting of 32-gauge tubing slightly shorter than the guide tube with a small right angle bend at one end were inserted into each guide tube to prevent clogging. Coordinates for microinjection were derived from the atlas of Paxinos and Watson (1982); substantia nigra: AP, 3.5; L, ±2.0; V, −8.0 (below skull surface); inferior colliculus: AP, 0; L, ±1.5; V, −4.0; medial septum: AP, 9.2; L, 0.0; V, −6.0. After surgery, rats were allowed to recover for a period of 2 weeks during which time they were fed a liquid diet containing ethanol (see below).
Chronic ethanol treatment
Immediately after stereotaxic surgery, rats were housed singly. In addition to rat chow and water, each animal was supplied with 35 ml of a nutritionally complete liquid diet (dextrose diet) prepared from purified materials which met or exceeded the nutrient requirements for rats of the National Research Council (1972). The composition of the diet was based on the “low fat” diet described by Thompson and Reitz (1978) with minor modification of the soluble mineral salts (Peng et al., 1982). The 1st day after surgery, Wayne Blox Rodent Lab Chow was removed but an additional 35 ml of dextrose liquid diet and water were again made available. Beginning the next day, animals were offered water, as well as a liquid diet in which ethanol replaced part of the dextrose, isocalorically (1 g of ethanol = 1.75 g of dextrose) as well as water. This treatment was continued for 12 consecutive days. An ethanol concentration of 0.06 g/ml was used for the first 2 days of this period. For the next 5 days and for the last 5 days the ethanol concentrations were increased to 0.07 and 0.08 g/ml, respectively. This compensated for the development of metabolic tolerance to ethanol. Dextrose or ethanol liquid diets were provided fresh daily between 12:00 noon to 1:00 p.m. in clean, graduated cylinders fitted with ball-bearing drinking spouts (Wahmann Manufacturing Co., Timonium, MD). This regimen of ethanol diet feeding was found to induce daily ethanol consumption of 12 to 16 g/kg/24 hr, maintain large amounts of ethanol in the blood stream (up to 2 mg/ml) and allow continued weight gain (1–2 g/day) without first having to fast the experimental animals (Frye et al., 1981).
Assessment of audiogenic seizure susceptibility and forelimb tremor
Withdrawal hyperexcitability, in the form of susceptibility to audiogenic seizures, has been considered an index of the presence of physical dependence on ethanol (Majchrowicz, 1975; Freund, 1975). In the present study, susceptibility to audiogenically induced seizures was evaluated between 6.5 to 8.5 hr after ethanol diet was removed (8:00 a.m.) on the final day of liquid diet treatment. Rats made physically dependent on ethanol, as described above, exhibited the greatest audiogenic seizure susceptibility during this time (Frye and Ellis, 1975).
Susceptibility to audiogenic seizures was determined by exposing individual rats to the sound generated by a 98 db electric bell for 1 min (Frye and Ellis, 1975, 1977). The test animal was confined to a cylindrical wire cage (21 cm × 21 cm) within a dimly illuminated, sound-attenuating chamber (44 cm on each side) fitted with a viewing window. An electric bell attached to the chamber ceiling directly above the rat was rung beginning 30 sec after placing the test animal in the wire cage. Sound-susceptible rats exhibited one or more wild running episodes. These consisted of at least three rapid circles of the wire cage within a 3-sec interval and were followed by loss of upright posture and clonic-tonic convulsions.
For purposes of quantifying audiogenic seizures, clonus was considered to be present if, after a wild running episode, the rat lost its upright posture and exhibited continuous hindlimb kicking (rapid cycles of hindlimb extension and retraction for at least 3 sec). Tonus was considered to be present when hindlimb kicking during clonus was arrested (i.e., either continuous extension or retraction of both hind-limbs) for a period of not less than 3 sec. Wild running always preceded clonus, which always preceded tonus. A single trained observer, without knowledge of the specific treatment of the individual animals, evaluated the seizure tests. A test of inter-rater reliability indicated that agreement occurred between two trained observers on the presence of wild running and clonus 100% and on tonus 93.8% of the time during a series of 34 evaluations. The frequency of “wild running,” “clonus” and “tonus” was recorded for analysis by the χ2 test (Winer, 1971); an alpha level of .05 or less was used to determine significance. Ethanol withdrawal-induced tremor in rats was evaluated immediately before all audiogenic seizure tests using a modification of the convulsions on handling method of Goldstein (1972) for mice, as previously described (Frye et al., 1981). “Tremor scores” were determined by lifting rats vertically by the tail and scoring the following reactions: a score of three was assigned to rats showing an immediate tonic extension of the forelimbs characterized by a violent generalized forelimb-whole body tremor; a score of two was assigned to rats that showed this reaction when they were rotated 180° around the axis of the tail; and a score of one was assigned to rats that failed to display forelimb extension but which showed clearly visible forelimb tremors when lifted by the tail and rotated. Animals showing no forelimb extension or tremor were assigned a score of zero. A test of inter-rater reliability showed that two trained observers assigned the same tremor score 85.3% of the time during a series of 34 simultaneous evaluations. Tremor data were expressed as the mean and S.E.M. for each treatment. A randomized group one way analysis of variance was used to determine the presence of significant differences between treatment group means (Winer, 1971). The Newman-Keuls test was used for all post-hoc pair-wise comparisons (Winer, 1971). An alpha level of .05 was used for all statistical tests.
On any one experimental day, no more than 15 rats were tested to assure that all data were collected during the period of peak audiogenic seizure susceptibility 6.5 to 8.5 hr after ethanol withdrawal. Each animal was randomly assigned to either a saline control treatment or to one of several different drug treatments. Each treatment was evaluated on two or more separate test days, along with appropriate saline control treatments.
Drug microinjections
All drugs were dissolved in sterile 0.9% sodium chloride solution and the pH adjusted to 6.5 to 7.5. Bicuculline methiodide was purchased from Pierce Chemical Co. (Rockford, IL), muscimol and GABA from Sigma Chemical Co. (St. Louis, MO) and 1,3-butanediol from Aldrich Chemical Co., Inc. (Milwaukee, WI). THIP (4,5,6,7-tetrahydroisoxazole[5,40c]pyridin-3-ol) was a gift from Lund-beck and Co. A/S (Copenhagen, Denmark) and chlordiazepoxide was a gift from Hoffmann La Roche Inc. (Nutley, NJ).
Microinjections into the inferior colliculus, substantia nigra or medial septum were administered with 33-gauge stainless-steel cannulas, which extended into the target tissue 1 mm beyond the end of the permanent guide tube. Drug solutions (0.5 μl) were delivered to the cannula at the rate of 0.1 μl/min over a 5-min interval (unless otherwise stated) via PE-10 tubing attached to a 10-μ1 microsyringe (Precision Instruments, Baton Rouge, LA). The flow rate was controlled with a dual channel infusion pump (Sage Instrument Co., Cambridge, MA) and was monitored by following a small bubble inserted into the fluid column of the PE tubing. After the infusion, injection cannulas were left in place for an additional 60 sec.
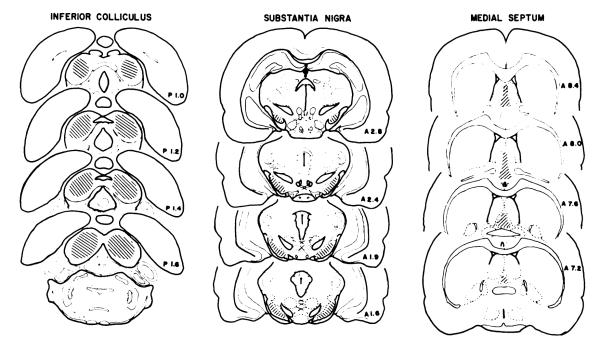
At the conclusion of each experiment, the microinjected animals were anesthetized with ethyl ether vapor, sacrificed, the brain gently removed, placed on a cryostat chuck and frozen on Dry Ice. Each brain was sectioned to allow visual identification of the cannula tip placement by a trained individual with no knowledge of the individual treatments. Data from animals with microinjector tip tracts outside the desired target area of the inferior colliculus, substantia nigra or medial septum (see fig. 1 for schematic representation of intended injection site) were excluded from the analysis.
Fig. 1.
Graphic representation of the sites considered acceptable for microinjections into the inferior colliculus, substantia nigra and medial septum. Drawings of the inferior colliculus and medial septum were based on the atlas of Pellegiino et al., (1979), whereas the drawing of the substantia nigra was based on the atlas of Konig and Kippel (1963).
Estimation of 1,3-butanediol distribution
In order to estimate the potential concentration of 1,3-butanediol within the inferior colliculus after a microinjection of 50 μg/0.5 μ1/5 min, the following assumptions were made. From previous work it was determined that an i.p. injection of 1,3-butanediol (67 mmol/kg) resulted in brain concentrations of 13.4 ± 1.0 μmol/g of brain tissue. Based on data from studies of dye diffusion after microinjection of 0.5 μl volumes into brain tissue (Myers, 1966), it was estimated that 1,3-butanediol might have been distributed within an area with a diameter of 1 mm around the cannula tip. Thus, a 50-μg microinjection of 1,3-butanediol might yield an approximate tissue concentration of 135 μmol/g within this sphere of tissue.
Results
Muscimol injection in the inferior colliculus
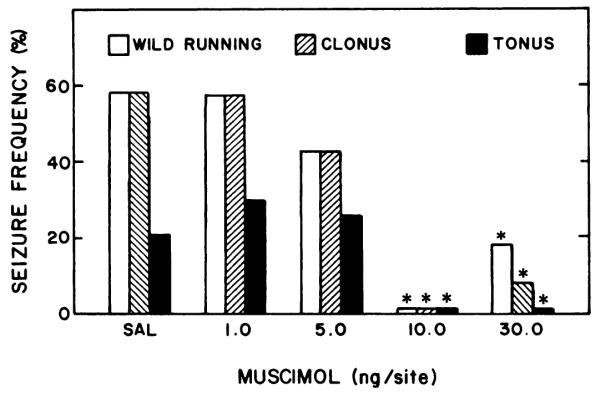
Microinjection of muscimol (10–30 ng) directly into the inferior colliculus 15 min before sound testing suppressed all components of audiogenic seizures in rats experiencing ethanol withdrawal (fig. 2). Muscimol (30 ng) completely prevented hindlimb tonus, whereas 10 ng totally suppressed wild running and clonus as well as tonus. Smaller doses of muscimol (1–5 ng) administered 15 min before the sound test failed to reduce audiogenic seizure susceptibility (fig. 2). In addition to the anticonvulsant action of muscimol 15 min after microinjection into the inferior colliculus, a significant anticonvulsant action was observed in this nucleus at 30, 60, 120 and 180 min after muscimol (30 ng) treatment (fig. 3). However, an anticonvulsant action was observed no longer in animals tested 300 min after microinjection of this dose of muscimol.
Fig. 2.
Effect of different doses of muscimol microinjected into the inferior colliculus, bilaterally, on susceptibility to audiogenic seizures during ethanol withdrawal. After 6.5 to 8.5 hr of withdrawal, ethanol-dependent rats were microinjected with muscimol or saline (SAL) solutions 15 min before being tested for susceptibility to audiogenic seizures. The total number of animals tested in each group are from left to right: 19,7, 8, 6 and 12. *P < .05 when compared with rats receiving microinjections of SAL.
Fig. 3.
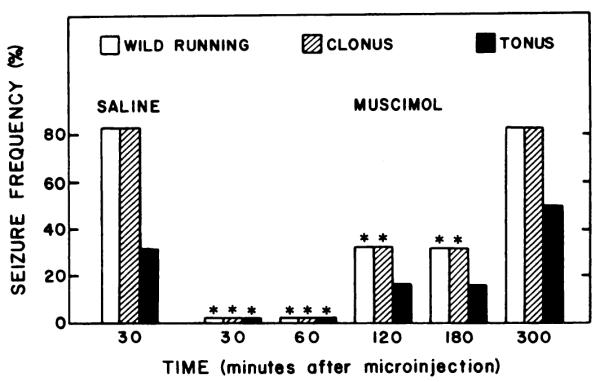
Effect of time on suppression of susceptibility to audiogenic seizures during ethanol withdrawal of muscimol (30 ng) microinjected into the inferior colliculus. After 3.5 to 8.5 hr of withdrawal, ethanol-dependent rats were microinjected with muscimol or saline solutions at various times before being tested for susceptibility to audiogenic seizures. The total number of animals tested in each group are from left to right: 6, 5, 5, 6, 6 and 6. * P < .05 when compared with rats receiving microinjections of saline.
Muscimol treatments which prevented audiogenic seizures did not appear to block the animals ability to detect sound when the electric bell was turned on or off during the sound test. In the presence of the ringing bell, muscimol-treated animals exhibited marked increases in exploratory activity (sniffing, rearing, etc.) characteristic of the behavior of control rats during similar sound tests. All rats consistently increased exploratory activities as soon as the hell was rung and terminated these responses immediately after the bell was shut off. Thus, muscimol treatments applied to the inferior colliculus probably did not block hearing completely at the time of testing.
The frequency of wild running and clonus for control groups which received bilateral microinjections of saline into the inferior colliculus 15 min before audiogenic seizure testing (fig. 2) was consistently lower (P < .05) than those of groups tested 30 min after saline treatment (fig. 3). The frequency of wild running, clonus and tonus in saline-injected controls pretreated 30 min earlier was not different from that of rats implanted with guide tubes but sound-tested without receiving microinjection (data not shown). The reason for differences between the 15- and 30-min saline groups was not clear, but may have been the result of transient anticonvulsant actions of substances released by cannula tract damage or abnormal ion flux during the microinfitsion into the inferior colliculus.
Previous experiments have shown that intracisternal administration of GABA, THIP or muscimol did not reduce the severity of forelimb tremors in the same animals in which susceptibility to audiogenic seizures was blocked (Frye et al, 1983). In the present study, microinjections of muscimol into the inferior colliculus at doses that suppressed audiogenic seizure susceptibility (figs. 2 and 3) similarly failed to change the severity of forelimb tremor (data not shown).
Muscimol injection into the substantia nigra
The effect of microinjections of muscimol, bilaterally, into the substantia nigra on audiogenic seizure susceptibility during ethanol withdrawal was examined next. Recent reports suggest that the substantia nigra might play an important role in the anticonvulsant action of GABAmimetic drugs like muscimol (Iadarola and Gale, 1981a, b, 1982; McNamara et al., 1982, 1983). Figure 3 and table 1 show the effect of microinjecting muscimol, bilaterally, into the substantia nigra on the incidence and severity of audiogenic seizures evoked 15 or 30 min later. The frequency of wild running and clonus was unaltered by muscimol injection (30 and 100 ng) into the substantia nigra at either time, in contrast to marked suppression of these measures by similar injections into the inferior colliculus (figs. 2 and 3).
TABLE 1. Effect of muscimol microinjected into the substantia nigra or medial septum on susceptibility to ethanol-withdrawal audiogenic seizures.
Rats withdrawn from ethanol 6.5 to 8.5 hr received bilateral or unilateral microinjection of muscimol as appropriate. Susceptibility to audiogenic seizures was determined at the designated time after the start of the muscimol injection. N represents the total number of animals tested.
| Treatment |
Seizure Frequency |
||||
|---|---|---|---|---|---|
| Dose | Time | Wild running |
Clonus | Tonus | N |
| ng | min | % | % | % | |
| Substantia Nigra | |||||
| Saline | 15 | 88.8 | 77.7 | 11.1 | 9 |
| 30 | 87.5 | 75.0 | 37.5* | 8 | |
| 100 | 71.4 | 71.4 | 42.9* | 7 | |
| Saline | 30 | 80.0 | 70.0 | 50.0 | 10 |
| 30 | 90.0 | 90.0 | 10.0* | 10 | |
| 100 | 66.7 | 66.7 | 11.1* | 9 | |
| Medial Septum | |||||
| Saline | 30 | 81.8 | 81.8 | 45.5 | 11 |
| 30 | 67.6 | 67.6 | 25.0* | 12 | |
| 100 | 63.6 | 63.6 | 45.5 | 11 | |
P < .05 when compared with appropriate saline control.
The frequency of tonus also was reduced significantly by injection of muscimol (30 or 100 ng) into the substantia nigra when compared with the frequency of tonus for rats similarly treated with saline 30 min before testing (table 1). However, the effect of muscimol treatment on tonus after the shorter 15-min treatment interval was difficult to interpret. The frequency of tonus 15 min after saline was less than that observed 30 min (P < .05; table 1) and much lower than the 30 to 50% incidence of tonus observed in other 30-min post-treatment control groups (see figs. 3, 4 and 5). Thus, substances released by needle tract damage again may have transiently influenced the exhibition of tonus. However, there was no indication that wild running or clonus were altered differentially by either saline or muscimol injections into the substantia nigra at either test time (fig. 4; table 1).
Fig. 4.
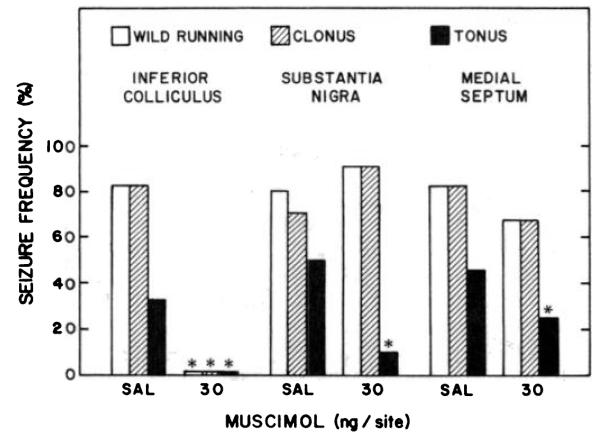
Effect of muscimol microinjected into the inferior colliculus, medial septum or substantia nigra on susceptibility to audiogenic seizures during ethanol withdrawal. After 6.5 to 8.5 hr of withdrawal, ethanol-dependent rats were microinjected with muscimol or saline (SAL) solutions 15 or 30 min before being tested for susceptibility to audiogenic seizures. The total number of animals tested in each group are from left to right: 6, 5, 10, 10, 11 and 12, respectively. *P < .05 when compared with rats receiving microinjections of SAL.
Fig. 5.
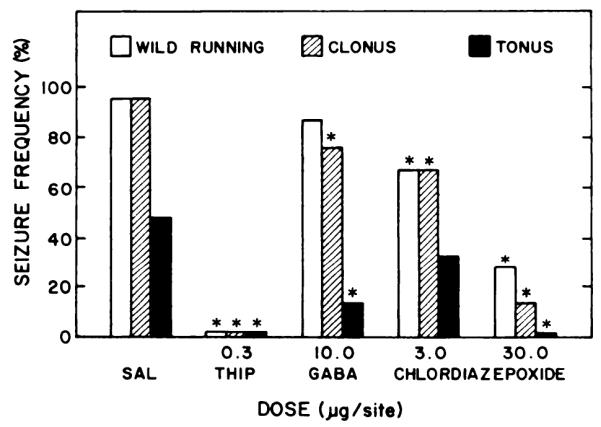
Effect of chlordiazepoxide, GABA and THIP microinjected into the inferior colliculus on susceptibility to audiogenic seizures during ethanol withdrawal. After 6.5 to 8.5 hr of withdrawal, ethanol-dependent rats were microinjected with muscimol or saline (SAL) solutions 15 min before being tested for susceptibility to audiogenic seizures. The total number of animals tested in each group are from left to right: 15, 6, 7,6 and 7. * P < .05 when compared with SAL microinjected controls.
Microinjections of muscimol into the substantia nigra of ethanol-dependent rats also failed to alter forelimb tremors measured just before sound testing. There was no evidence of any significant change in the severity of forelimb tremors after any of the muscimol treatments described in figure 4 or table 1 (data not shown).
There was evidence that injection of muscimol into the substantia nigra did activate GABA receptors as intended. Intense rotation and stereotypic behaviors such as head bobbing, gnawing, self-mutilation and sniffing were observed in some of the rats just before the 15- and 30-min tests. Circling behavior after bilateral microinjections appeared to be due to small differences of 0.1 to 0.2 mm in rostral-caudal placement of the right and left cannula. Circling and stereotypy did not appear to alter the audiogenic seizure responses. Wild running was easily distinguished from rotational behavior due to the rapid acceleration which characterized this measure.
Muscimol injection into the medial septum
Because lesions of the medial septum reduce the severity of audiogenic seizures in rodents with genetic susceptibility to audiogenic seizures (Kesner, 1966), the effect of muscimol infusion into this nucleus on audiogenic seizures during ethanol withdrawal was examined. Microinjection of muscimol (30–100 ng) into the medial septum failed to alter the frequency of wild running or clonus 30 min after treatment (fig. 4; table 1). After treatment with the 30-ng dose of muscimol, tonus was reduced slightly but significantly (fig. 4). However, the larger dose of muscimol (100 ng) did not alter significantly the frequency of tonus (table 1).
Muscimol injections into the medial septum, like those into the inferior colliculus and substantia nigra, failed to change the severity of forelimb tremor in the test animals (data not shown). Also, there were no obvious behavioral changes that distinguished animals treated with muscimol in the medial septum from saline microinjected controls, like the stereotypies observed after injections were made into the substantia nigra.
Effect of other drugs in the inferior colliculus on audiogenic seizures
In addition to the anticonvulsant actions of muscimol, injection of other GABAmimetics (GABA, 10 μg; THIP, 0.3 μg; or chlordiazepoxide, 3–30 μg) into the inferior colliculus caused a significant reduction in the frequency of audiogenic seizures during ethanol withdrawal (fig. 5). Each of these agents reduced the frequency of clonus and tonus. All except GABA also reduced the frequency of wild running. In contrast to the anticonvulsant actions of these agents, bilateral microinjection of 1,3-butanediol (50 μg), a glycol with properties similar to ethanol (Frye et al., 1981), had no effect on any of the components of the audiogenic seizure 5 min after infusion (data not shown). This dose of 1,3-butanediol (50 μg/0.5 μl) represented a concentration of 10% w/v and exceeded a conservative estimate (see “Materials and Methods") of the concentration of the glycol that would be present in the inferior colliculus after an anticonvulsant i.p. injection of 1,3-butane-diol (44–88 mmol/kg) which completely suppresses seizures (Frye et al, 1981).
Effect of bicuculline methiodide microinfusions into brain nuclei
Because activation of GABA receptors in the inferior colliculus with muscimol reduced audiogenic seizure susceptibility, the effect of blocking GABA receptors in the inferior colliculus, substantia nigra or medial septum was examined next. A solution of the GABA receptor antagonist, bicuculline methiodide, was slowly infused bilaterally into the inferior colliculus of ethanol-naive rats. During initial pilot work with bicuculline methiodide at a concentration of 1 μg/0.1 μl infused at the rate of 0.1 μl/min, it became clear that spontaneous running seizures were produced consistently after a brief infusion. Rats, lightly restrained by hand, became excited and restless within 20 sec after the start of the infusion. Between 40 and 60 sec all animals broke free from restraint and ran wildly about the room. Once the rats broke free from restraint, the sequence of events was very similar to that displayed by ethanol-dependent rats exhibiting audiogenic seizures outside the confines of the wire test chamber. After a period of wild running that included running directly into walls and other barriers in their paths, rats injected with bicuculline methiodide exhibited a loss of balance followed by forelimb and hindlimb clonus and tonus. Running seizures of this type were generally lethal despite i.p. injection of sodium pentobarbital (35 mg/kg) immediately after the running fit.
During a series of experiments, the concentration of bicuculline methiodide in the infusate was sequentially reduced to determine the threshold dose just sufficient to evoke a running seizure (table 2). In one group of seven rats, bicuculline methiodide was infused at the rate of 1.0 ng/0.1 μl/min. During the 6-min infusion or within 4 min after the termination of the infusion, all rats exhibited running seizures which included forelimb clonus. A concentration of 0.3 ng/0.1 μl of bicuculline methiodide was infused over 6 min into the inferior colliculi of seven other animals who failed to evoke any seizure activity within 4 min after the infusion, although the animals did become restless. Thus, the threshold dose of bicuculline methiodide for induction of running seizures under this 6-min infusion paradigm was between 1.8 and 6.0 ng (table 2).
TABLE 2. Frequency of forelimb clonus after microinjection of bicuculline methiodide into the inferior colliculus, substantia nigra and medial septum.
Bicuculline methiodide (0.3–1000 ng/0.1 μl) was microinjected at the rate of 0.1 μl/min into the specified brain area of experimentally naive rats for up to 6 min or until the infusion was interrupted by seizure activity. The development of forelimb clonus was recorded during a 10-min interval that began with the start of bicuculline methiodide infusion.
| Treatment | Injection-Related Seizure Frequency |
||
|---|---|---|---|
| Inferior colliculus |
Substantia nigra |
Medial septum |
|
| mg/min | (No. exhibiting clonus/total no. tested) | ||
| Dose | |||
| 1000 | 10/10 | 4/4 | |
| 300 | 4/4 | 0/6† | |
| 125 | 5/5 | ||
| 100 | 7/7* | ||
| 30 | 5/5 | 0/7† | |
| 1 | 7/7* | ||
| 0.3 | 0/7 | ||
P < .05 when compared with the next smaller dose of bicuculline methiodide at the same brain site.
P < .05 when compared with the same dose of bicuculline methiodide placed in a different brain site.
Bilateral microinjection of bicuculline methiodide into the substantia nigra of ethanol-naive rats also induced seizure activity. Continuous infusion of bicuculline methiodide (300–000 ng/0.1 μl/min) resulted in seizure activity within 3 to 5 min (table 2). Seizure responses were initially limited to clonic jerks of the forelimbs but rapidly progressed to clonic hindlimb spasms, occasionally accompanied by tonus. A smaller total dose of bicuculline methiodide (600 ng) infused into the substantia nigra at the rate of 100 ng/0.1 μl/min over 6 min also consistently caused seizures. Myoclonus of the face and clonus of the forelimbs was the most frequently observed response at this dose, occurring within 4 min after the infusion in all seven animals tested in this treatment group. A more severe form of generalized clonus also occurred in three of these seven animals. None of the 15 rats injected into the substantia nigra with bicuculline methiodide (100–1000 ng/0.1 μl/min) exhibited the same type of explosive wild running that was observed when similar injections were made into the inferior colliculus or when ethanol-withdrawn rats were tested audiogenically, as described above.
Infusion of a smaller total dose of bicuculline methiodide (180 ng) at the rate of 30 ng/0.1 μl/min did not result in any sign of facial myoclonus or forelimb clonus (table 2). Thus, the threshold dose of bicuculline methiodide which caused clonic forelimb seizures during the 6-min infusion or within 4 min after the infusion was between 180 and 600 ng.
Microinjection of bicuculline methiodide (1.8 μg) at the rate of 300 ng/0.1 μl/min over 6 min into the medial septum failed to cause any wild running, facial myoclonus or generalized clonic response in a group of six rats. Larger doses of bicuculline methiodide were not evaluated as a slightly larger dose (10 μg) of the drug given intracisternally was found to be sufficient to induce forelimb clonus.
Discussion
Activation of GABA receptors in the inferior colliculus appears to play an important role in the suppression of audiogenic seizure susceptibility during ethanol withdrawal in laboratory rats. After a bilateral microinjection of muscimol (30 ng), seizure suppression was complete within 15 min and remained detectable for up to 3 hr. As little as 10 ng of this GABA agonist completely suppressed seizures 30 min later. Administration of GABA and its agonist, THIP, into the inferior colliculus also effectively reduced seizure susceptibility during ethanol withdrawal. Although a relatively large dose of chlordiazepoxide reduced the frequency of audiogenic seizures, the relationship of this anticonvulsant effect to an action on benzodiazepine receptors associated with GABAergic neurotransmission (Olsen, 1982) remains to be determined. The ability of GABAmimetics like muscimol to suppress audiogenic seizures when administered bilaterally into the inferior colliculus, may represent a unique neuroanatomical interaction of these drugs, as similar microinjections into the substantia nigra and medial septal nucleus exhibited only limited ability to modify the severity of audiogenic seizures during ethanol withdrawal (fig. 4; table 1).
Recent reports indicate that microinjection of muscimol into the substantia nigra can suppress selectively certain motor seizures induced by pentylenetetrazol, bicuculline, maximal electro-shock (Iadarola and Gale, 1981a,b, 1982) or electrical kindling of the amygdala (McNamara et al., 1982, 1983). The present findings and those of others indicate that the substantia nigra is not the only site of GABAmimetic anticonvulsant action. For example, audiogenic seizures evoked during ethanol withdrawal are only slightly sensitive to GABAmimetics in the substantia nigra. Only the incidence of tonus but not that of wild running and clonus was reduced slightly 30 min but not 15 min after muscimol injection (table 1). This action of muscimol in the substantia nigra does not seem to be related to the anticonvulsant action of intracisternal muscimol injections which completely suppress audiogenic seizures within 10 min (Frye et al., 1983). In addition, pentylenetetrazol-induced seizures are blocked by microinjection of diazepam into the amygdala (Nagy and Decsi, 1979; Nagy, 1981), whereas susceptibility to audiogenic seizures in rats which are genetically seizure-prone is blocked by injections of GABA into the inferior colliculus (Duplisse et al., 1974). Thus, the substantia nigra does not appear to be the only brain site at which GABA receptor activation can exert an anticonvulsant action.
The minimal anticonvulsant effects observed after microinjection of muscimol into the medial septum and substantia nigra, two areas known to play a role in the modulation of several different types of seizures (Kesner, 1966; Iadarola and Gale, 1981a,b, 1982; McNamara et al., 1982, 1983), suggests that the inferior colliculus may play a unique role in audiogenic seizures due to ethanol withdrawal. Whether audiogenic seizure blockade by muscimol in the inferior colliculus represents: 1) a selective GABAmimetic anticonvulsant action; 2) a specific reversal of an adaptive change evoked by ethanol; or 3) a simple blockade of auditory sensory input into the CNS is not yet known.
Preliminary work indicates that bilateral microinjections of muscimol into the inferior colliculus do not exert a generalized anticonvulsant action, as this treatment does not inhibit i.v. bicuculline-induced seizures (Frye et al., 1983). However, there is some evidence to suggest that GABAergic neurotransmission in the inferior colliculus may be important for the elaboration of audiogenic seizures in rodents which are sound susceptible for different reasons. For example, electrical stimulation (Duplisse et al., 1974; Laird and Huxtable, 1978) or microinjection of the GABA antagonists, bicuculline and bicuculline methiodide (Duplisse et al., 1974; present studies), into the inferior colliculus can evoke wild running, clonic-tonic seizures that are essentially identical to those evoked by sound. Neither the substantia nigra nor the medial septum are as sensitive to bicuculline infusion as the inferior colliculus. In addition, only lesions of the inferior colliculus cause a permanent loss of audiogenic seizure susceptibility (Kesner, 1966; Wada et al., 1970; Ward, 1971; Duplisse et al, 1974) in seizure-prone rodents.
Whether ethanol acts specifically on the inferior colliculus to increase audiogenic seizure susceptibility is unknown, but acute ethanol treatment does appear to facilitate GABAergic transmission in the CNS and repeated administration may lead to an adaptive reduction in central GABA receptor function (Hunt, 1983). The present results demonstrate that the inferior colliculus is uniquely sensitive to the GABA antagonist bicuculline methiodide, which can evoke seizure activity that closely resembles audiogenic seizures evoked during ethanol withdrawal. Reductions in GABAergic inhibition in the inferior colliculus possibly could be responsible for an increased audiogenic seizure susceptibility during ethanol withdrawal.
The possibility that GABAmimetics reduce audiogenic seizures by impairing audition cannot be ruled out. There is some evidence, however, that suggests that this may not be an important factor. First, in the present study, rats injected with muscimol responded to the electric bell with an increase in exploratory activity similar to that exhibited by saline-injected or untreated control animals. Other findings indicate that the medial geniculate body or auditory cortex, which are important in higher level integration of auditory input, do not have to be intact in order for the successful induction of audiogenic seizures in genetically seizure susceptible rats (Kesner, 1966). Thus, even if GABAmimetic drugs injected into the inferior colliculus blocked auditory input to the medial geniculate body or auditory cortex, this alone should not alter susceptibility to audiogenic seizures.
In conclusion, GABAmimetic drugs injected into the inferior colliculus can completely block audiogenic seizures during ethanol withdrawal. This action may be relatively selective for the inferior colliculus because similar treatments are relatively ineffective in the substantia nigra and medial septum, two brain areas also associated with modulation of some seizures. Induction of audiogenic-like seizures by microinjection of bicuculline methiodide suggests that reduced GABAergic inhibition in the inferior colliculus during ethanol withdrawal could be responsible for susceptibility to audiogenic seizures at this time.
Acknowledgments
The authors wish to recognize the expert technical assistance of Susan G. Emrick and Margret A. Horner in the completion of these studies.
ABBREVIATIONS
- CNS
central nervous system
- GABA
γ-aminobutyric acid
- THIP
4,5,6,7-tetrahydroisoxazole[5,40c]pyridine-3-ol
Footnotes
This work was supported by Grants AA-05713 and AA-02334 from the U.S. Public Health Service and Grants 8019 and 8207 from the North Carolina Alcoholism Research Authority.
References
- Cooper BR, Viik K, Ferris RM, White HL. Antagonism of the enhanced susceptibility to audiogenic seizures during alcohol withdrawal in the rat by 7-aminobutyric acid (GABA) and “GABA-mimetic” agents. J. Pharmacol. Exp. Ther. 1979;209:396–403. [PubMed] [Google Scholar]
- DeLuca JR, editor. Fourth Special Report to the U.S. Congress on Alcohol and Health. U.S. Government Printing Office; Washington, D.C.: 1981. [Google Scholar]
- Duplisse BR, Picchoini AL, Chin L, Consroe PF. Relationship of the inferior colliculus and gamma-amino butyric acid (GABA) to audiogenic seizure in the rat. Fed. Proc. 1974;33:468. [Google Scholar]
- Freund G. Induction of physical dependence on alcohol in rodents. In: Majchrowicz E, editor. Biochemical Pharmacology of Ethanol. Plenum Press; New York: 1975. pp. 311–325. [DOI] [PubMed] [Google Scholar]
- Frye GD, Breese GR, Mailman RB, Vogel RA, Mueller RA. Similarities in the central actions of GABA-mimetic drugs and ethanol. Brain Res. Bull. 1979;4:706. [Google Scholar]
- Frye GD, Chapin RE, Vogel RA, Mailman RB, Kilts CD, Mueller RA, Breese GR. Effects of acute and chronic 1,3-butanediol treatment on central nervous system function: A comparison with ethanol. J. Pharmacol. Exp. Ther. 1981;216:306–314. [PubMed] [Google Scholar]
- Frye GD, Ellis FW. Audiogenic evaluation of withdrawal excitability in rats after chronic ethanol feeding. Pharmacologist. 1975;17:197. [Google Scholar]
- Frye GD, Ellis FW. Effects of 6-hydroxydopamine or 5,7-dihydroxy-tryptamine on the development of physical dependence on ethanol. Drug Alcohol Depend. 1977;2:349–359. doi: 10.1016/0376-8716(77)90037-0. [DOI] [PubMed] [Google Scholar]
- Frye GD, McCown TJ, Breese GR. Differential sensitivity of ethanol withdrawal signs in the rat to GABA-mimetics: Blockade of audiogenic seizures but not forelimb tremors. J. Pharmacol. Exp. Ther. 1983;226:720–725. [PubMed] [Google Scholar]
- Goldman GD, Volicer L, Gold BI, Roth RH. Cerebrospinal fluid GABA and cyclic nucleotides in alcoholics with and without seizures. Alcohol Clin. Exp. Res. 1981;5:431–434. doi: 10.1111/j.1530-0277.1981.tb04927.x. [DOI] [PubMed] [Google Scholar]
- Goldstein DB. An animal model for testing effects of drugs on alcohol withdrawal reactions. J. Pharmacol. Exp. Ther. 1972;183:14–23. [PubMed] [Google Scholar]
- Goldstein DB. Sodium bromide and sodium valproate: Effective suppressants of ethanol withdrawal reactions in mice. J. Pharmacol. Exp. Ther. 1979;208:223–227. [PubMed] [Google Scholar]
- Hunt WA. The effect of ethanol on GABAergic transmission. Neurosci. Biobehav. Rev. 1983;7:87–95. doi: 10.1016/0149-7634(83)90009-x. [DOI] [PubMed] [Google Scholar]
- Iadarola MJ, Gale K. Cellular compartments of GABA in brain and their relationship to anticonvulsant activity. Mol. Cell Biochem. 1981a;39:305–330. doi: 10.1007/BF00232582. [DOI] [PubMed] [Google Scholar]
- Iadarola MJ, Gale K. The substantia nigra: Site of GABA-mediated anticonvulsant activity in rats. Neurosci. Abstr. 1981b;7:591. [Google Scholar]
- Iadarola MJ, Gale K. Substantia nigra: Site of anticonvulsant activity mediated by gamma-aminobutyric acid. Science (Wash. DC) 1982;218:1237–1240. doi: 10.1126/science.7146907. [DOI] [PubMed] [Google Scholar]
- Kalant H. Direct effects of ethanol on the nervous system. Fed. Proc. 1975;34:1930–1941. [PubMed] [Google Scholar]
- Kesner RP. Subcortical mechanisms of audiogenic seizures. Exp. Neurol. 1966;15:192–205. doi: 10.1016/0014-4886(66)90045-8. [DOI] [PubMed] [Google Scholar]
- Konig FR, Kippel RA. The Rat Brain, a Stereotaxic Atlas of the Forebrain and Lower Parts of the Brain Stem. Williams and Wilkins Co.; Baltimore: 1963. [Google Scholar]
- Laird HE, Huxtable RJ. Taurine and audiogenic epilepsy. In: Barbeau A, Huxtable RJ, editors. Taurine and Neurological Disorders. Raven Press; New York: 1978. pp. 339–357. [Google Scholar]
- Majchrowicz E. Induction of physical dependence on alcohol and the associated behavioral changes in rats. Psychopharmacology. 1975;43:245–254. doi: 10.1007/BF00429258. [DOI] [PubMed] [Google Scholar]
- McNamara J, Iadarola MJ, Rigsbee L, Galloway M. Kindled motor seizures are abolished by microinjection of muscimol into the ventral midbrain tegmentum. Neurosci. Abstr. 1982;8:86. [Google Scholar]
- McNamara JO, Rigsbee LC, Galloway MT. Evidence that substantia nigra is crucial to neural network of kindled seizures. Eur. J. Pharmacol. 1983;86:485–486. doi: 10.1016/0014-2999(83)90202-9. [DOI] [PubMed] [Google Scholar]
- Myers RD. Injection of solutions into cerebral tissue: Relation between volume and diffusion. Physiol. Behav. 1966;1:171–174. [Google Scholar]
- Nagy J. An endogenous substance from porcine brain antagonizes the anticonvulsant effect of diazepam. Neuropharmacology. 1981;20:529–533. doi: 10.1016/0028-3908(81)90190-8. [DOI] [PubMed] [Google Scholar]
- Nagy J, Decsi L. Further studies on the site of action of diazepam: Anticonvulsant effect in the rabbit. Neuropharmacology. 1979;18:39–45. doi: 10.1016/0028-3908(79)90007-8. [DOI] [PubMed] [Google Scholar]
- National Research Council . Nutrient Requirements of Laboratory Animals. 2nd ed National Academy of Sciences; Washington, DC: 1972. Nutrient requirements of the rat; pp. 56–117. [Google Scholar]
- Olsen RW. Drug interactions at the GABA receptor-ionophore complex. Annu. Rev. Pharmacol. Toxicol. 1982;22:245–277. doi: 10.1146/annurev.pa.22.040182.001333. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; New York: 1982. [Google Scholar]
- Pelligrino LJ, Pelligrina AS, Cushman AJ. A Stereotaxic Atlas of the Rat Brain. Plenum Press; New York: 1979. [Google Scholar]
- Peng TC, Garner SC, Frye GD, Crenshaw MA. Evidence of toxic effects of ethanol on bone in rats. Alcohol Clin. Exp. Res. 1982;6:96–99. doi: 10.1111/j.1530-0277.1982.tb05386.x. [DOI] [PubMed] [Google Scholar]
- Tarika JS, Winger G. The effects of ethanol, phenobarbital and baclofen on ethanol withdrawal in the rhesus monkey. Psychopharmacology. 1980;70:201–208. doi: 10.1007/BF00435315. [DOI] [PubMed] [Google Scholar]
- Thompson JA, Reitz RC. Effects of ethanol ingestion and dietary fat levels on mitochondrial lipids in male and female rats. Lipids. 1978;13:540–550. doi: 10.1007/BF02533593. [DOI] [PubMed] [Google Scholar]
- Ticku MK. The effect of acute and chronic ethanol administration and its withdrawal on gamma-aminobutyric acid receptor binding in rat brain. Br. J. Pharmacol. 1980;70:403–410. doi: 10.1111/j.1476-5381.1980.tb08716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ticku MK, Burch T. Alterations in gamma-aminobutyric acid receptor sensitivity following acute and chronic ethanol treatment. J. Neurochem. 1980;34:417–423. doi: 10.1111/j.1471-4159.1980.tb06612.x. [DOI] [PubMed] [Google Scholar]
- Volicer L. GABA levels and receptor binding after acute and chronic ethanol administration. Brain Res. Bull. 1980;5(suppl.):809–813. [Google Scholar]
- Wada JA, Teras A, White B, Jung E. Inferior colliculus lesions and audiogenic seizure susceptibility. Exp. Neurol. 1970;28:326–332. doi: 10.1016/0014-4886(70)90240-2. [DOI] [PubMed] [Google Scholar]
- Ward R. Unilateral susceptibility to audiogenic seizure impaired by contralateral lesions in the inferior colliculus. Exp. Neurol. 1971;32:313–316. doi: 10.1016/0014-4886(71)90074-4. [DOI] [PubMed] [Google Scholar]
- Winer BJ. Statistical Principals in Experimental Design. McGraw-Hill Co. Inc.; New York: 1971. [Google Scholar]