Abstract
Background
Perseveration and sensorimotor gating deficits are core features of obsessive-compulsive disorder (OCD). Serotonin 1B receptor (5-HT1BR) agonists exacerbate OCD symptoms in patients, and induce perseveration and sensorimotor gating deficits in mice. Serotonin reuptake inhibitors (SRIs), but not noradrenaline reuptake inhibitors (NRIs), reduce OCD symptoms following 4–8 weeks of treatment. Using mice, we compared the effects of chronic SRI versus NRI treatment on 5-HT1BR-induced OCD-like behavior, and 5-HT1BR sensitivity in orbitofrontal-subcortical “OCD circuits”. Furthermore, we localized the 5-HT1BR population that mediates OCD-like behavior.
Methods
Mice chronically received the SRI clomipramine or the NRI desipramine and were examined for 5-HT1BR-induced OCD-like behavior, or 5-HT1BR binding and G-protein-coupling in caudate-putamen, nucleus accumbens, and orbitofrontal cortex. Separate mice were tested for OCD- or depression-like behavior following 4, 14, 21, 28 or 56 days of SRI treatment. Finally, OCD-like behavior was assessed following intra-orbitofrontal 5-HT1BR agonist infusion, or intra-orbitofrontal 5-HT1BR antagonist infusion coupled with systemic 5-HT1BR agonist treatment.
Results
Effective, but not ineffective, OCD treatments reduced OCD-like behavior in mice with a time-course that parallels the delayed therapeutic onset in OCD patients, and downregulated 5-HT1BR expression in the orbitofrontal cortex. Intra-orbitofrontal 5-HT1BR agonist infusion induced OCD-like behavior, and intra-orbitofrontal 5-HT1BR antagonist infusion blocked OCD-like effects of systemic 5-HT1BR agonist treatment.
Conclusions
These results indicate that orbitofrontal 5-HT1BRs are necessary and sufficient to induce OCD-like behavior in mice, and that SRI pharmacotherapy reduces OCD-like behavior by desensitizing orbitofrontal 5-HT1BRs. Our findings suggest an essential role for orbitofrontal 5-HT1BRs in OCD pathophysiology and treatment.
Keywords: serotonin 1B receptor, obsessive-compulsive disorder, orbitofrontal cortex, serotonin transporter, serotonin reuptake inhibitor, mouse model
INTRODUCTION
Obsessive-Compulsive Disorder (OCD) is characterized by intrusive thoughts, images, or impulses, and/or repetitive compulsive behaviors. Abnormalities in brain serotonergic systems have been implicated in the pathophysiology of OCD due in part to the efficacy of serotonin reuptake inhibitors (SRIs) in OCD treatment (1–3). SRIs include selective serotonin reuptake inhibitors (SSRIs) and clomipramine, a tricyclic antidepressant that potently blocks the serotonin transporter. Other classes of antidepressants, including norepinepherine reuptake inhibitors (NRIs) and monoamine oxidase inhibitors (MAOIs), are ineffective in OCD (1, 4). OCD is the only anxiety disorder showing selective response to SRI antidepressants (5). Furthermore, the mechanisms by which SRIs produce therapeutic effects in OCD remain poorly understood.
Several putative biomarkers have been identified in OCD. First, OCD is associated with deficits in sensorimotor gating (6, 7), a neural mechanism that inhibits extraneous sensory, cognitive and motor information to permit mental integration. Prepulse inhibition (PPI) is an operational measure of sensorimotor gating and refers to the reduction in startle magnitude that occurs when an abrupt startling stimulus is preceded 30–500 msec by a barely detectable stimulus (8), and is reduced in OCD (6, 7). Second, pharmacological challenge with serotonin 1B receptor (5-HT1BR) agonists exacerbates symptoms in OCD patients (9–11) (however, see (12)). Third, OCD patients exhibit overactivity of the orbitofrontal cortex (OFC) at rest (13–15) and following symptom provocation (16, 17) that is reversed by successful treatment (18–20).
Interestingly, 5-HT1BR agonist treatment produces several OCD-like behaviors in rodents, including PPI deficits (21, 22) and highly perseverative hyperlocomotion (23–25) characterized by repetitive circling of the perimeter of the open field to the near exclusion of other species-typical behaviors. These 5-HT1BR-induced behaviors are blocked by chronic treatment with the SRI fluoxetine (26), and therefore could model aspects of OCD. However, behaviors reduced by SRIs might not be OCD-like, since SRIs also effectively treat additional psychiatric conditions including depression and other anxiety disorders. To determine whether 5-HT1BR-induced behavior specifically models aspects of OCD in mice, we assessed the ability of effective (SRI) versus ineffective (NRI) treatments for OCD to block 5-HT1BR-induced behavior. We also compared the time-course for SRIs to reduce OCD-like versus depression-like behavior in mice, since a longer course of SRI treatment is required for therapeutic effects to emerge in OCD patients (4–8 weeks) (27) compared to depressed patients (2–4 weeks) (28).
We then examined the mechanisms underlying OCD-like behavior in mice and the attenuation of this behavior by SRIs. We hypothesized that discrete populations of 5-HT1BRs within the orbitofrontal-subcortical circuits implicated in OCD (13–20) mediate OCD-like behavior. Furthermore, we posited that SRIs desensitize these 5-HT1BR populations. We examined the effects of chronic SRI or NRI treatments on 5-HT1BR expression and functional coupling in the dorsal striatum, nucleus accumbens and orbitofrontal cortex. Finally, we performed local infusion studies to determine whether orbitofrontal 5-HT1BRs are necessary and sufficient to produce OCD-like behavior in mice.
METHODS AND MATERIALS
Animals
Female Balb/cJ mice (Jackson Laboratories, Bar Harbor, Maine) 8–12 weeks of age, weighing 20–30 g, were used for all experiments. Balb/cJ mice were used because this strain has been previously reported to respond behaviorally to chronic antidepressant treatment (26, 29). Female mice were used because Balb/cJ males exhibit excessive home-cage fighting. Prevalence rates have been reported to be approximately equal among males and females, with females beings slightly more likely to have OCD; the female/male sex ratio reported in OCD is 1 – 1.5 (30–32). Mice were housed in a temperature-controlled colony room on a 12-hour light/dark schedule with food and water available ad libitum. Behavioral testing occurred during the light phase. Animal testing was conducted in accord with the National Institutes of Health laboratory animal care guidelines and with Institutional Animal Care and Use Committee approval.
Chemicals
Clomipramine, fluoxetine, and desipramine were administered in the drinking water. Drug concentrations required for delivery of desired doses were as follows: 80 mg/L for 10 mg/kg/day fluoxetine; 90, 175, or 345 mg/L for 10, 20, or 40 mg/kg/day clomipramine, and 110, 215, or 385 mg/L for 10, 20, or 40 mg/kg/day desipramine (29). RU24969 and 8-OH-DPAT were dissolved in 0.9% saline as salt doses and injected IP. GR127935 was dissolved in distilled water and injected SC. Systemic drug injections were given at a volume of 5 mL/kg bodyweight with 1-cc syringes and 27 gauge needles. Drug doses were selected based on results of previous dose response studies (21, 29) and local infusion dose response studies (data not shown).
Details regarding drugs and radiochemicals are described in Supplement 1.
Behavioral Experiments and Apparatus
Open Field Test
Mice were placed in a corner of the open field and activity was monitored for 20 minutes. Locomotor activity was quantified using 42 × 42 × 30 cm Plexiglas activity chambers (Accuscan, Columbus, OH). Chambers were equipped with infrared beams (2.5 cm apart) along each wall. Paths taken by mice were recorded by breaks in the infrared beams and stored as x-y coordinate sequences.
Prepulse Inhibition
Immediately following open field testing, mice were transferred to PPI chambers. Sixty-five consecutive 1-ms readings were recorded beginning at startling stimulus onset to obtain the amplitude of the animals’ startle response to each stimulus (33). Sound levels were measured as described elsewhere using the A weighting scale (34). In each test session, mice were exposed to five different types of discrete stimuli or “trials”: a 40-msec broadband 120 dB burst (Pulse Alone trial); three different Prepulse + Pulse trials in which either 20-msec long 3 dB, 6 dB, or 12 dB above background stimuli preceded the 120 dB pulse by 100 msec (onset to onset); and a No Stimulus trial, in which only background noise (65 dB) was presented. Trials were presented in a pseudo-random order and separated by an average of 15 s (range: 9–20 s). The test session began with a 5-min acclimation period, followed by four consecutive blocks of test trials. Blocks one and four consisted of six consecutive Pulse Alone trials, while blocks two and three each contained six Pulse Alone trials, five of each kind of Prepulse + Pulse trial, and four No Stimulus trials. Startle chambers were nonrestrictive Plexiglas cylinders 5 cm in diameter resting on a Plexiglas platform in a ventilated chamber (San Diego Instruments, San Diego, CA) as described elsewhere (26).
Chronic Forced Swim Test
In the chronic forced swim test (cFST), Balb/cJ and several other mouse strains show reduced immobility following chronic, but not short-term, treatment with antidepressants via the drinking water (29, 35). Mice were place in plastic buckets 24 cm high and 19 cm in diameter filled 19 cm high with 23–25°C tap water positioned directly below a tripod-mounted camera and videotaped for 6 min. Only the last 4 min were scored for four measures: swimming, immobility, climbing, or other (36). The predominant behavior was recorded every 5 s. Mice were pre-exposed to the forced swim test for 6 min 24 h before test day (37).
Quantitative Autoradiographic Binding Experiments
Tissue Preparation
Following 4 weeks of antidepressant treatment, mice were sacrificed rapidly by decapitation. Brains were excised, frozen in dry ice, and stored at −20°C. Twenty micron coronal sections were collected onto Superfrost slides (Fisher, Pittsburgh, PA) and stored at −20°C. Before use, slides with brain sections were thawed sequentially to 4°C and then to room temperature (RT) over 1 hour.
[125I]-CYP
Receptor autoradiography using [125I]-iodocyanopindolol ([125I]-CYP) was based on the protocol by Waeber and Palacios (38). 5-HT1B receptor binding was evaluated by assessing [125I]-CYP in the presence of 8-OH-DPAT to block 5-HT1A receptors, and isoproterenol to block β adrenoreceptors. Nonspecific binding was determined in the presence of the 5-HT1B/1D antagonist GR127935.
[35S]-GTPγS
Functional coupling was evaluated by assessing [35S]-GTPγS binding in the presence or absence of the 5-HT1B agonist CP93129. Nonspecific binding was determined in the presence of GR127935.
Image Analysis
Autoradiograms were analyzed using ImageJ software (Bethesda, MD). Optical densities of images were converted to nCi/mg tissue with a calibration curve generated from coexposed 3H microscale standards (American Radiolabeled Chemicals, St. Louis, MO). For details regarding binding studies see Supplement 1.
Local Infusion Experiments
Surgery
Mice were anesthetized (100 mg/kg ketamine and 6 mg/kg xylazine, IP), placed in a stereotaxic apparatus (Stoelting, Wood Dale, IL), and implanted with 22-gauge bilateral guide cannulae (Plastics One, Roanoke, VA). Cannulae were aimed at the orbitofrontal cortex (AP, +2.5mm from bregma; ML, ±1.0mm from midsaggital line; DV, −2.5mm from dura mater) or the infralimbic cortex (AP, +1.6mm from bregma; ML, ±0.5mm from midsaggital line; DV, −2.5mm from dura mater). Internal cannulae (Plastics One) extending 0.5mm beyond guide cannulae tips were inserted after surgery.
Behavioral Experiments
Following 1 week recovery from surgery, internal cannulae were removed and injection cannulae extending 0.5mm beyond guide cannulae tips were inserted. Mice were infused bilaterally and tested 10 minutes later for locomotion, perseveration and PPI as described above. Drug solutions were infused at a rate of 0.5 μl/min using a 5 μL syringe (Hamilton, Reno, NV) connected by polyethylene tubing (Plastics One, Roanoke, VA) to the injection needle (Hamilton) which stayed in situ for 1 min following the injection.
Cannula placement verification
At the end of each experiment, mice received an overdose of ketamine/xylazine. Brains were excised, fixed with 4% paraformaldehyde overnight, dehydrated with 30% sucrose, and snap frozen. Brains were stored at −20°C and later cut into 80μm coronal sections using a cryostat. The slices were stained with Cresyl violet, and placements of cannula tracks were examined by light microscopy.
Statistical Analysis
For all experiments, dependent measures were analyzed using ANOVA. Significant interactions were resolved using post-hoc ANOVAs for within-subjects factors and/or Newman Keuls post-hoc tests for between-subjects factors. Significance was set at P<0.05, and P values from post-hoc ANOVAs were adjusted using the Bonferroni correction.
Details of statistical analysis are described in Supplement 1.
RESULTS
Reduction of OCD-like behavior with chronic SRI, but not NRI, treatment
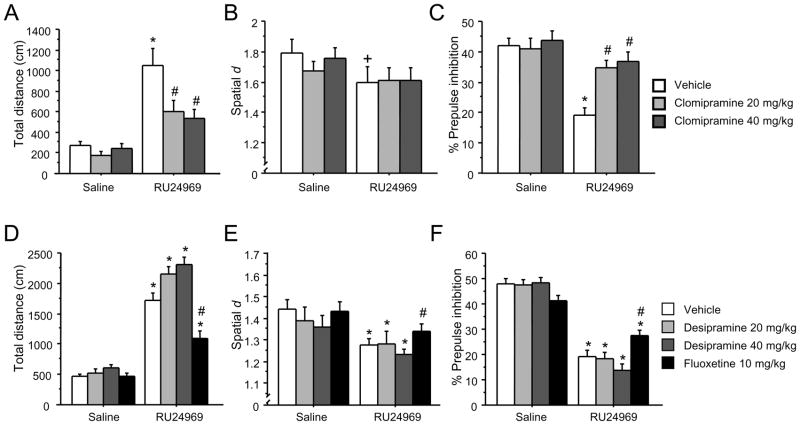
SRIs, but not NRIs provide effective treatment for OCD. We assessed whether chronic treatment with the SRI clomipramine or the NRI desipramine attenuates OCD-like behaviors in mice. Clomipramine attenuated 5-HT1BR-induced behavior following 4 weeks (Figure 1A – 1C), but not 1 week (Figure S1 in Supplement 1) of treatment. Planned comparisons showed that the 5-HT1BR agonist RU24969 induced hyperlocomotion in vehicle- [F(1,37)=8.35; P<0.05] but not clomipramine-pretreated mice (Figure 1A). Following RU24969, controls exhibited higher locomotion than clomipramine-pretreated mice. We quantified 5-HT1BR-induced perseverative hyperlocomotion using the total distance traveled and the spatial scaling exponent d. Spatial d quantifies the extent to which a sequence of movements falls along a straight line (d ≈ 1) or is characterized by many directional changes (d ≈ 2), and is independent of total distance traveled (39). 5-HT1BR agonist treatment induces highly perseverative locomotor paths reflected by reductions in spatial d (26, 40). A strong trend for RU24969 to induce perseveration in controls [F(1,10)=6.07; P=0.068] was found, whereas no effect of RU24969 was seen in clomipramine-pretreated mice (Figure 1B). Finally, a significant drug × pretreatment interaction [F(2,27)=4.07; P<0.05] and post hoc analyses revealed that RU24969 decreased PPI in vehicle- [F(1,12)=36.54; P<0.001] but not clompiramine-pretreated mice. Furthermore, Newman-Keuls post hoc tests revealed that the clomipramine-pretreated groups exhibited higher PPI than controls following RU24969 treatment (Figure 1C).
Figure 1.
Alleviation of OCD-like behavior with chronic clomipramine and fluoxetine, but not desipramine, treatment. Following 4 weeks of treatment with 0 mg/kg/d (n=14), 20 mg/kg/d (n=14), or 40 mg/kg/d (n=14) clomipramine, mice received injections of the 5-HT1BR agonist RU24969 (0 or 10 mg/kg) on separate test days. Following injections, mice were tested for total distance traveled (A) and perseveration (spatial d) (B) for 20 minutes in the open field, and prepulse inhibition (C). Following 4 weeks of treatment with 0 mg/kg/d (n=13), 20 mg/kg/d (n=15), or 40 mg/kg/d (n=15) desipramine or 10 mg/kg/d fluoxetine (n=15), mice received injections of RU24969 (0 or 10 mg/kg) and were tested for total distance traveled (D), perseveration (spatial d) (E), and prepulse inhibition (F) as above. Results are presented as means ± s.e.m. Asterisk (*) indicates P<0.05 compared to saline. Pound sign (#) indicates P<0.05 compared to chronic vehicle treatment. Plus sign (+) indicates a strong trend (P =0.068).
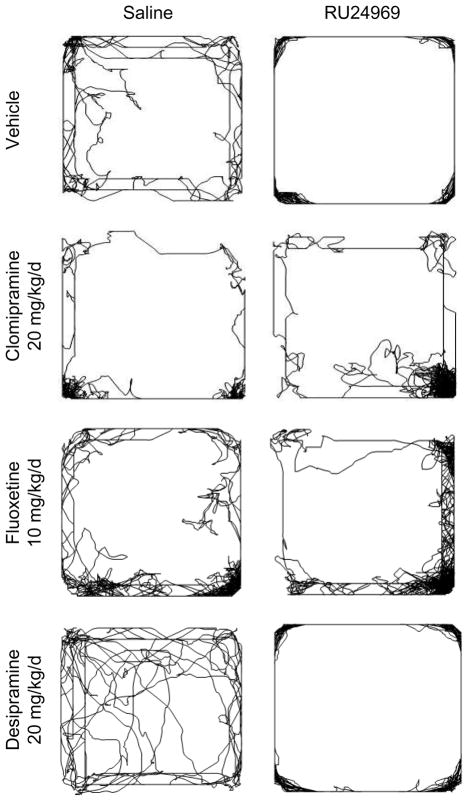
Desipramine did not affect any OCD-like behaviors (Figure 1D – 1F), but fluoxetine attenuated these behaviors. RU24969 increased locomotion across vehicle- and desipramine-pretreated groups [F(1,42)=154.62; P<0.001]. A significant drug × pretreatment interaction [F(1,28)=4.70; P<0.05] and post hoc tests showed that RU24969 increased locomotion in vehicle- [F(1,14)=33.61; P<0.001] and fluoxetine-pretreated mice [F(1,14)=10.45; P<0.01], although fluoxetine-pretreated mice had reduced locomotion compared to controls after RU24969 treatment (Figure 1D). RU24969 reduced spatial d scores across vehicle- and desiprmaine-pretreated groups [F(1,41)=20.39; P<0.001]. RU24969 also decreased spatial d across vehicle- and fluoxetine-pretreated groups [F(1,28)=11.43; P<0.01]. However, fluoxetine-pretreated mice exhibited higher spatial d scores than controls following RU24969 treatment (Figure 1E). The perseverative locomotor paths induced by RU24969 treatment which were blocked by clomipramine and fluoxetine, but not desipramine, are shown in Figure 2. RU24969 decreased PPI across vehicle- and desipramine-pretreated groups [F(1,42)=182.46; P<0.001]. A significant drug × pretreatment interaction [F(1,28)=6.48; P<0.05] and post hoc tests indicated that RU24969 decreased PPI in vehicle- [F(1,14)=62.63; P<0.001] and fluoxetine-pretreated mice [F(1,14)=8.58; P<0.05], although fluoxetine-pretreated mice exhibited higher PPI than controls following RU24969 treatment (Figure 1F). In sum, 5-HT1BR-induced OCD-like behavior is attenuated by effective, but not ineffective, pharmacological treatments for OCD.
Figure 2.
Representative paths taken by mice in the open field following 4 weeks of treatment with control, 20 mg/kg/d clomipramine, 10 mg/kg/d fluoxetine, or 20 mg/kg desipramine, and acute injection with 0 mg/kg (left column) or 10 mg/kg (right column) RU24969.
Time-course for fluoxetine to reduce OCD-like behavior versus depression-like behavior
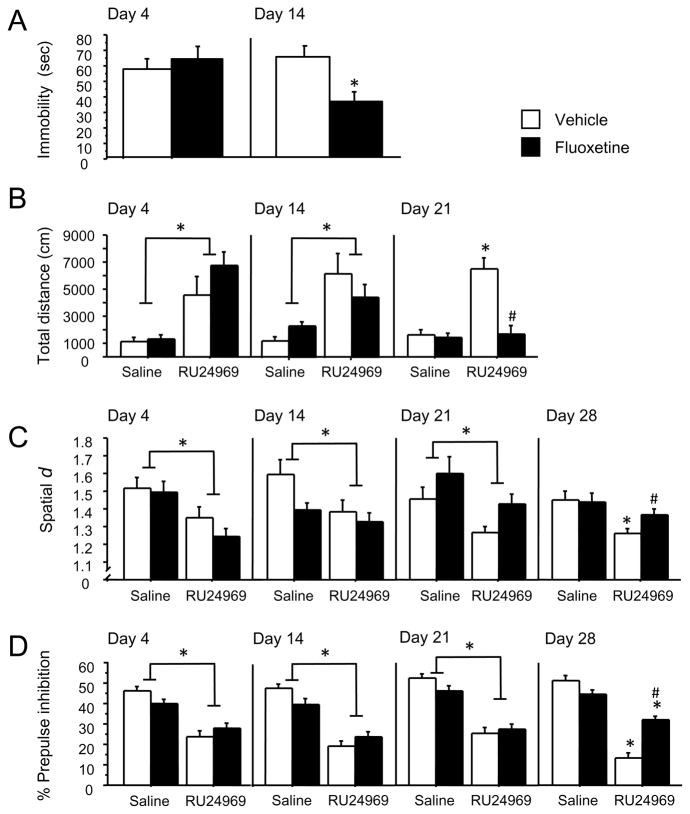
A longer course of SRI treatment is required for therapeutic effects to emerge in OCD patients (4–8 weeks) (27) compared to depressed patients (2–4 weeks) (28). We assessed the effect of 4, 14, 21, 28, or 56 days of fluoxetine treatment on 5-HT1BR-induced OCD-like behavior or on depression-like behavior in separate groups of mice.
Only 2 weeks of fluoxetine pretreatment were required to reduce depression-related behavior in the chronic forced swim test (cFST). Thus, fluoxetine had no effect in the cFST on day 4. However, by day 14, fluoxetine reduced immobility in the cFST [F(1,24)=7.86; P<0.01] (Figure 3A).
Figure 3.
Time-course for fluoxetine to reduce OCD-like behavior versus depression-like behavior. Mice receiving fluoxetine treatment (0 or 10 mg/kg/d) and were tested for immobility in the FST (A). Mice receiving the same fluoxetine treatment were injected with the 5-HT1BR agonist RU24969 (0 or 10 mg/kg), and tested for total distance traveled (B) and perseveration (C) in the open field, and prepulse inhibition (D). Separate groups of mice (n=15/group for all experiments) were used for each time point (day 4, 14, 21, 28 and 56 of fluoxetine treatment) and behavioral paradigm (FST, open field, PPI). Data are shown through the first time-point at which SRI effects were observed. All effects remained through day 56 (data not shown). Results are presented as means ± s.e.m. Asterisk (*) indicates P <0.05 compared to saline. Pound sign (#) indicates P <0.05 compared to chronic vehicle treatment.
In contrast, three weeks of fluoxetine pretreatment were required to prevent 5-HT1BR-induced hyperlocomotion. Thus, RU24969 induced hyperlocomotion across pretreatment groups on days 4 [F(1,22)=31.23; P<0.001] and 14 [F(1,24)=23.10; P<0.001]. However, on day 21 of fluoxetine pretreatment, a significant drug × pretreatment interaction [F(1,23)=19.76; P<0.001] and post hoc analyses revealed that RU24969 increased locomotion in vehicle- [F(1,12)=45.70; P<0.001] but not fluoxetine-pretreated mice. Furthermore, fluoxetine-pretreated mice exhibited less locomotion compared to controls following RU24969 treatment (Figure 3B).
Four weeks of fluoxetine pretreatment were required to reduce 5-HT1BR-induced perseverative behavior. RU24969 reduced spatial d across pretreatment groups on days 4 [F(1,22)=25.59; P<0.001], 14 [F(1,23)=9.39; P<0.01] and 21 [F(1,22)=12.55; P<0.01]. However, on day 28 of fluoxetine pretreatment, planned comparisons revealed that RU24969 decreased spatial d scores in vehicle- [F(1,12)=9.01; P<0.05] but not fluoxetine-pretreated mice [F(1,12)=1.72; P=0.42]. In addition, fluoxetine-pretreated animals exhibited higher spatial d values than controls following RU24969 treatment (Figure 3C).
Four weeks of fluoxetine pretreatment were also required to reduce 5-HT1BR-induced PPI deficits. RU24969 reduced PPI across pretreatment groups on days 4 [F(1,23)=26.79; P<0.001], 14 [F(1,24)=33.79; P<0.001] and 21 [F(1,24)=58.50; P<0.001]. However, on day 28 a significant drug × pretreatment interaction [F(24)=20.47; P<0.001] and post hoc analyses revealed that RU24969 decreased PPI in vehicle- [F(1,12)=66.07; P<0.001] and fluoxetine-pretreated mice [F(1,12)=15.39; P<0.01], although fluoxetine-pretreated mice exhibited higher PPI than controls following RU24969 treatment (Figure 3D). Therefore, approximately twice the duration of SRI treatment is required to reduce 5-HT1BR-induced OCD-like behavior than depression-like behavior.
RU24969-induced behavior is mediated by 5-HT1BRs
5-HT1BR agonist-induced PPI deficits and perseverative hyperlocomotion are mediated by 5-HT1BRs and not 5-HT1ARs, for which the agonist used here, RU24969, has some affinity (22, 26, 41, 42). We assessed the effects of 5-HT1AR agonist 8-OH-DPAT on behavior. 8-OH-DPAT treatment produced effects that were opposite to those of RU24969 (Figures S2 and S3 in Supplement 1). Furthermore, effects of pretreatments on RU24969-induced behavior were not confounded by effects of pretreatments on 5-HT1AR sensitivity.
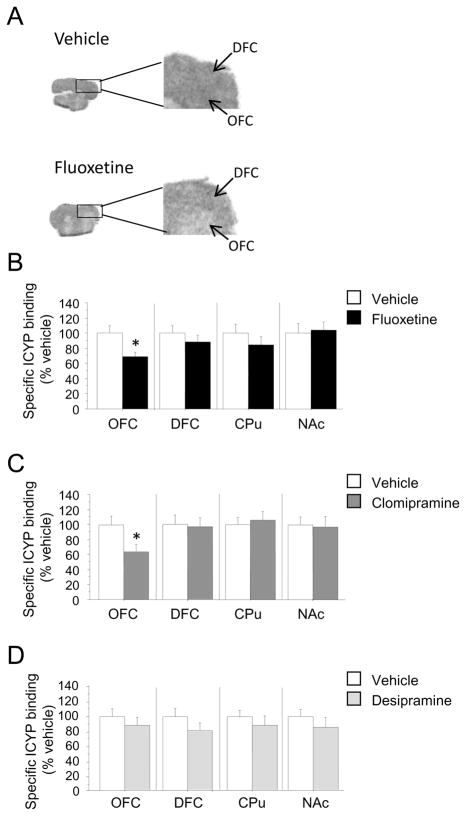
Chronic SRI, but not NRI, treatment downregulates 5-HT1BR expression in the orbitofrontal cortex
Next, we investigated the mechanisms by which chronic SRI treatment reduces 5-HT1BR-induced OCD-like behavior. We assessed 5-HT1BR expression and G-protein coupling in brain regions within the orbitofronto-subcortical circuits implicated in OCD (13–20) in mice receiving 4 weeks of treatment with fluoxetine, clomipramine, or desipramine. Fluoxetine treatment resulted in decreased 5-HT1BR expression in the OFC [F(1,24)=6.04; P<0.05], but not in the dorsofrontal cortex (DFC), caudate-putamen (CPu) or nucleus accumbens (NAc) (Figures 4A and 4B). Treatment with clomipramine also reduced 5-HT1BR expression in the OFC [F(1,22)=5.59; P<0.05], but not the DFC, CPu or NAc (Figure 4C). However, desipramine treatment had no effect on 5-HT1BR expression in any brain region studied (Figure 4D). No treatment altered 5-HT1BR-induced G-protein coupling in any brain region studied (Figure S4 in Supplement 1). In sum, SRIs, but not NRIs, selectively downregulate orbitofrontal 5-HT1BRs, the brain region most consistently implicated in OCD by neuroimaging studies(43).
Figure 4.
Downregulation of 5-HT1BRs in the OFC following chronic treatment with SRIs. 5-HT1B receptor autoradiography using [125I]-CYP was performed on coronal brain slices from mice treated for 4 weeks with control (n= 13), 10 mg/kg/d fluoxetine (n=13) (B), 20 mg/kg/d clomipramine (n=12) (C), or 20 mg/kg/d desipramine (n=12) (D). Representative autoradiograms showing [125I]-CYP binding in OFC and DFC of control- and fluoxetine-treated mice (A). Nonspecific [125I]-CYP binding was determined in the presence of the 5-HT1B/1DR antagonist GR127935 (20 μm) and was subtracted from total binding to give specific binding. All data are expressed as percent of control-treated specific binding. OFC, orbitofrontal cortex; DFC, dorsofrontal cortex; CPu, caudate-putamen; NAc, nucleus accumbens. Results are presented as means ± s.e.m. Asterisk (*) indicates P <0.05 compared to saline.
Orbitofrontal 5-HT1BR activation is necessary and sufficient for the expression of OCD-like behaviors
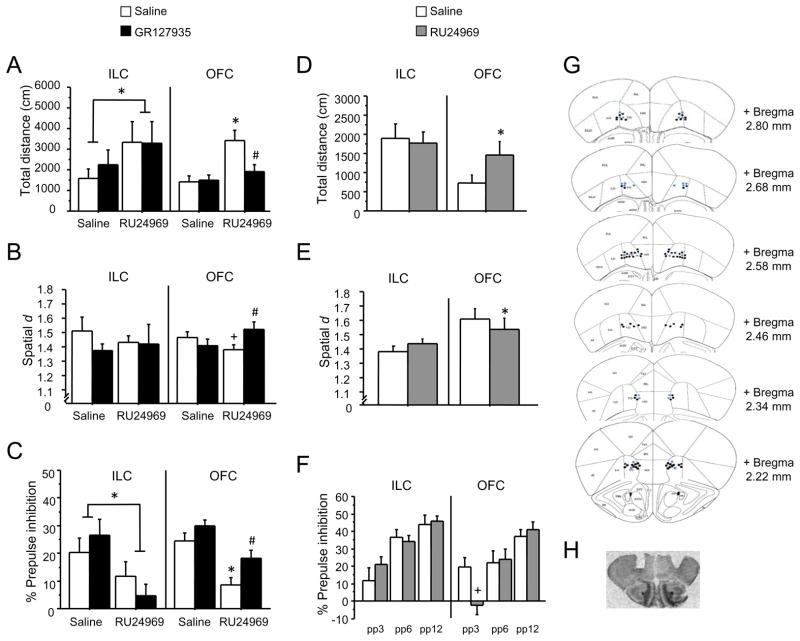
To determine whether activating orbitofrontal 5-HT1BRs is necessary for generating OCD-like behavior, we antagonized orbitofrontal 5-HT1BRs and administered 5-HT1BR agonist systemically in mice. Local infusion of the 5-HT1BR antagonist GR127935 into the OFC blocked the hyperlocomotion, perseveration, and PPI deficits induced by systemic injection of RU24969 (Figure 5). A significant drug × pretreatment interaction [F(1,36)=5.31; P<0.05] and post hoc analyses revealed that RU24969 increased locomotion in vehicle- [F(1,20)=13.24; P<0.01] but not GR127935-pretreated mice. Furthermore, controls showed more locomotion than GR127935-pretreated mice following RU24969 treatment (Figure 5A). Likewise, a significant drug × pretreatment interaction [F(1,35)=8.64; P<0.01] and post hoc analyses revealed a trend for RU24969 to decrease spatial d scores in vehicle- [F(1,18)=2.86; P=0.1] but not GR127935-pretreated animals. Furthermore, GR127935-pretreated mice exhibited higher spatial d scores than controls following RU24969 treatment (Figure 5B). Finally, RU24969 decreased PPI across groups [F(1,38)=14.96; P<0.001]. However, planned comparisons showed that RU24969 decreased PPI in vehicle- [F(1,20)=12.44; P<0.01] but not GR127935-pretreated mice. Additionally, GR127935-pretreated mice displayed higher PPI than controls following RU24969 treatment (Figure 5C).
Figure 5.
5-HT1BRs in the OFC are necessary and sufficient for the generation of OCD-like behaviors. Mice received bilateral local infusions of the 5-HT1B/1DR antagonist GR127935 (0 or 20 μg/side) into the ILC (n=7/group) or OFC (n=20/group) 30 min prior to a systemic (i.p.) injection of the 5-HT1BR agonist RU24969 (0 or 10 mg/kg). Mice were then tested for total distance traveled (A) and perseveration (spatial d) (B) in the open field, and prepulse inhibition (C). Separate groups of mice received bilateral local infusions of RU24969 (0 or 5 μg/side) into the ILC (n=10) or OFC (n=12) and were tested for total distance traveled (D) and perseveration (spatial d) (E) in the open field, and prepulse inhibition (F). Schematic representation of successive coronal sections (rostral to caudal) of the mouse brain (G) (adapted and reproduced with permission from (81)) and representative pictomicrograph (H) showing the histological verification of cannula placements in the orbitofrontal cortex. Black dots indicate cannula placement for animals infused with antagonist, and blue dots indicate cannula placement for animals infused with agonist. For ILC cannula placement, see Figure S3 in Supplement 1. Results are presented as means ± s.e.m. Asterisk (*) indicates P <0.05 compared to saline. Pound sign (#) indicates P <0.05 compared to chronic vehicle treatment. Plus sign (+) indicates a strong trend and P-values (in order of appearance) are: P =0.1, P =0.06.
Infusion of GR127935 into a nearby but anatomically (44) and functionally (45) distinct brain region, the infralimic cortex (ILC), had no effect on 5-HT1BR-induced behaviors. RU24969 induced hyperlocomotion across groups [F(1,11)=4.86; P<0.05] (Figure 5A), reduced spatial d overall, although this effect was not significant (Figure 5B), and reduced PPI across groups [F(1,11)=5.76; P<0.05] (Figure 5C). GR137935 did not affect RU24969-induced behavior.
We then tested whether activating orbitofrontal 5-HT1BRs is sufficient for generating OCD-like behavior. We assessed OCD-like behavior following local infusion of 5-HT1BR agonist into the OFC. Infusion of RU24969 into the OFC increased locomotion [F(1,20)=7.26; P<0.05] (Figure 5D) and decreased spatial d [F(1,11)=5.11; P<0.05] (Figure 5E). Furthermore, a significant drug × intensity interaction [F(2,22)=6.76; P<0.01] and post hoc analyses revealed a trend for RU24969 to decrease PPI at the 3 dB prepulse intensity level [F(1,11)=6.00; P=0.06] (Figure 5F). In contrast, infusion of 5-HT1BR agonist into the ILC had no effect on these behaviors (Figure 5D – 5F). Overall, activation of orbitofrontal 5-HT1BRs is both necessary and sufficient to produce perseverative hyperlocomotion and PPI deficits in mice.
DISCUSSION
Our present findings identify orbitofrontal 5-HT1BRs as a critical substrate for modulating OCD-like behavior in mice. Activating 5-HT1BRs induces PPI deficits and perseverative hyperlocomotion, which can be prevented by effective (SRI), but not ineffective (NRI), treatments for OCD. The time-course for SRIs to prevent OCD-like behavior closely parallels the time-course of the human therapeutic response to SRIs in OCD, and has not been reported in any previous mouse model of OCD. Additionally, chronic treatment with SRIs, but not NRIs, reduces the expression of 5-HT1BRs specifically in the OFC, the brain region most consistently implicated in OCD by neuroimaging studies (13–20, 43). Finally, we show that orbitofrontal 5-HT1BRs are both necessary and sufficient for the expression of OCD-like behavior. In summary, our findings indicate that orbitofrontal 5-HT1BRs may play a critical role in both the pathophysiology and SRI pharmacotherapy of OCD.
Predictive validity is the ability of an animal model to make accurate predictions about the human phenomenon being modeled, and is the only necessary and sufficient criterion to justify the use of a model (46). We used 5-HT1BR challenge to induce OCD-like behavior in mice since this manipulation exacerbates symptoms in OCD patients. The sensorimotor gating deficits and perseverative hyperlocomotion induced by the 5-HT1BR agonist RU24969 show face validity for OCD, since OCD patients report intrusive thoughts, impulses, and images, and exhibit repetitive stereotypical behaviors. But more importantly, our mouse model of these aspects of OCD exhibits strong predictive validity for effective treatments and their time-course of action. To our knowledge, the deer mouse model of stereotypic behavior (Korff et al., 2008 (47)), and nest-building behavior in selectively-bred house mice (Greene-Schloesser et al., 2011 (48)) are the only other models in which SRI, but not NRI, treatment reduces OCD-like behavior. However, no other behavioral, pharmacological, or genetic rodent model of OCD to date requires four or more weeks of SRI treatment for reduction of OCD-like behavior (47–51). Moreover, our results show that a longer period of SRI treatment (4 weeks) is required to reduce 5-HT1BR-induced OCD-like behavior compared to depression-like behavior (2 weeks), which parallels the human therapeutic response. Finally, we found that 5-HT1BRs modulate OCD-like behavior in the OFC, the brain region most consistently implicated in OCD by neuroimaging studies.
Our drug-induced model of OCD has several advantages and disadvantages compared to genetic (49, 51, 52) and spontaneous behavioral (48, 53, 54) animal models of OCD. Genetic and spontaneous models have the advantage of inducing permanent behavioral phenotypes. Indeed, OCD is typically a life-long illness. Our mouse model induces OCD-like behavior temporarily through a known neural substrate, 5-HT1BRs in the orbitofrontal cortex. This phenotype is likely to be mechanistically similar to 5-HT1BR-induced worsening of symptoms in OCD patients. In contrast, the neural substrates underlying behavioral phenotypes are often unknown in spontaneous models, and can be difficult to identify. Induced models acting at known substrates can lead more readily to the identification of underlying mechanisms, as shown in the present studies.
OCD and autism spectrum disorder (ASD) exhibit considerable comorbidity (55, 56) and may share some common genetic and neural substrates (57). The first-degree relatives of individuals with ASD are significantly more likely to have OCD (58, 59), and ASD is more common in first-degree relatives of individuals with OCD (60). Furthermore, OCD and ASD share some common features, such as PPI deficits (61, 62) and symptom exacerbation following 5-HT1BR agonist challenge (63–65). A further similarity is that only chronic treatment with SRIs reduces perseverative motor symptoms in ASD (66). Lastly, abnormalities of orbitofrontal-subcortical circuits have also been implicated in ASD (67–69). Therefore, our present findings may also have relevance to the pathophysiology and treatment of ASD.
Our findings suggest that SRIs treat aspects of OCD by desensitizing orbitofrontal 5-HT1BRs. Indeed, we show that SRIs, but not NRIs, reverse OCD-like behavior in mice while downregulating orbitofrontal 5-HT1BRs. Interestingly, SRI treatment did not alter 5-HT1BR G-protein coupling in the orbitofrontal cortex. Thus, SRI desensitization of orbitofrontal 5-HT1BRs occured through downregulation and not decoupling of these receptors from their G-proteins.
The specific population of orbitofrontal 5-HT1BRs that influence OCD-like behavior remains to be identified. 5-HT1BRs are located presynaptically on the axon terminals of serotonergic neurons (70, 71), and postsynaptically on the terminals of neurons containing other neurotransmitters (72, 73). Presynaptic orbitofrontal 5-HT1B autoreceptors could modulate OCD-like behavior, since these receptors require more than 3 weeks of SRI treatment to desensitize according to electrophysiological and microdialysis studies (74, 75). Indeed, SRI-induced enhancement of orbitofrontal serotonin release in rodents temporally correlates with the onset of therapeutic effects in OCD (76, 77). However, orbitofrontal 5-HT1B heteroreceptors on GABAergic, glutamatergic or other neuronal terminals might be responsible for modulating OCD-like behavior. For example, activation of 5-HT1BRs on GABAergic terminals could reduce inhibitory tone and underlie orbitofrontal overactivity in OCD patients. Indeed, activation of 5-HT1BRs on GABAergic neurons in other brain regions has been shown to reduce local levels of GABA and result in more excitable postsynaptic neurons (78, 79). Furthermore, the ability of SRIs to reduce orbitofrontal overactivity in OCD could result from a reduction in the expression of orbitofrontal GABAergic 5-HT1BRs.
Our findings identify the 5-HT1B receptor pathway as a potential therapeutic target for novel OCD treatments. Novel treatments are greatly needed because SRIs do not reduce symptoms in approximately 65% of patients, and reduce symptoms only by 40% in responders (80). Compounds that either block or downregulate 5-HT1BRs might be effective treatments for OCD. Furthermore, such compounds might relieve OCD symptoms faster than SRIs, which require weeks to induce therapeutic effects and downregulate orbitofrontal 5-HT1BRs. Our present findings also suggest that OCD pathophysiology might involve an upregulation of orbitofrontal 5-HT1BRs. Neuroimaging studies of orbitofrontal 5-HT1BRs in OCD patients are needed to address this question.
The present findings indicate that orbitofrontal 5-HT1BRs are both necessary and sufficient for the expression of OCD-like behavior in mice. These findings link orbitofrontal 5-HT1BRs to core features of OCD including perseverative behaviors and sensorimotor gating deficits. These data also suggest that SRIs may induce therapeutic effects in OCD by desensitizing orbitofrontal 5-HT1BRs. Lastly, our findings implicate the 5-HT1B receptor pathway as a potential target for novel treatments for OCD.
Supplementary Material
Acknowledgments
We thank Christian Waeber for advice regarding receptor binding studies and Helen Blair Simpson for her insights regarding OCD. This work was funded by NIH grants MH079424 and MH071555 and a generous gift from Geraldi Norton Foundation.
Footnotes
FINANCIAL DISCLOSURES
The authors of this paper have no biomedical financial interests or potential conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cartwright C, Hollander E. SSRIs in the treatment of obsessive-compulsive disorder. Depress Anxiety. 1998;8( Suppl 1):105–13. doi: 10.1002/(sici)1520-6394(1998)8:1+<105::aid-da16>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 2.Fineberg NA, Gale TM. Evidence-based pharmacotherapy of obsessive-compulsive disorder. Int J Neuropsychopharmacol. 2005 Mar;8(1):107–29. doi: 10.1017/S1461145704004675. [DOI] [PubMed] [Google Scholar]
- 3.Mavissakalian M, Jones B, Olson S, Perel JM. The relationship of plasma clomipramine and N-desmethylclomipramine to response in obsessive-compulsive disorder. Psychopharmacol Bull. 1990;26(1):119–22. [PubMed] [Google Scholar]
- 4.Goodman WK, Price LH, Delgado PL, Palumbo J, Krystal JH, Nagy LM, et al. Specificity of serotonin reuptake inhibitors in the treatment of obsessive-compulsive disorder. Comparison of fluvoxamine and desipramine. Arch Gen Psychiatry. 1990 Jun;47(6):577–85. doi: 10.1001/archpsyc.1990.01810180077011. [DOI] [PubMed] [Google Scholar]
- 5.Bourin M, Lambert O. Pharmacotherapy of anxious disorders. Hum Psychopharmacol. 2002 Dec;17(8):383–400. doi: 10.1002/hup.435. [DOI] [PubMed] [Google Scholar]
- 6.Hoenig K, Hochrein A, Quednow BB, Maier W, Wagner M. Impaired prepulse inhibition of acoustic startle in obsessive-compulsive disorder. Biol Psychiatry. 2005 May 15;57(10):1153–8. doi: 10.1016/j.biopsych.2005.01.040. [DOI] [PubMed] [Google Scholar]
- 7.Swerdlow NR, Benbow CH, Zisook S, Geyer MA, Braff DL. A preliminary assessment of sensorimotor gating in patients with obsessive compulsive disorder. Biol Psychiatry. 1993 Feb 15;33(4):298–301. doi: 10.1016/0006-3223(93)90300-3. [DOI] [PubMed] [Google Scholar]
- 8.Graham FK. Presidential Address, 1974. The more or less startling effects of weak prestimulation. Psychophysiology. 1975 May;12(3):238–48. doi: 10.1111/j.1469-8986.1975.tb01284.x. [DOI] [PubMed] [Google Scholar]
- 9.Gross-Isseroff R, Cohen R, Sasson Y, Voet H, Zohar J. Serotonergic dissection of obsessive compulsive symptoms: a challenge study with m-chlorophenylpiperazine and sumatriptan. Neuropsychobiology. 2004;50(3):200–5. doi: 10.1159/000079970. [DOI] [PubMed] [Google Scholar]
- 10.Koran LM, Pallanti S, Quercioli L. Sumatriptan, 5-HT(1D) receptors and obsessive-compulsive disorder. Eur Neuropsychopharmacol. 2001 Apr;11(2):169–72. doi: 10.1016/s0924-977x(01)00082-7. [DOI] [PubMed] [Google Scholar]
- 11.Stein DJ, Van Heerden B, Wessels CJ, Van Kradenburg J, Warwick J, Wasserman HJ. Single photon emission computed tomography of the brain with Tc-99m HMPAO during sumatriptan challenge in obsessive-compulsive disorder: investigating the functional role of the serotonin auto-receptor. Prog Neuropsychopharmacol Biol Psychiatry. 1999 Aug;23(6):1079–99. doi: 10.1016/s0278-5846(99)00051-2. [DOI] [PubMed] [Google Scholar]
- 12.Boshuisen ML, den Boer JA. Zolmitriptan (a 5-HT1B/1D receptor agonist with central action) does not increase symptoms in obsessive compulsive disorder. Psychopharmacology (Berl) 2000 Sep;152(1):74–9. doi: 10.1007/s002130000529. [DOI] [PubMed] [Google Scholar]
- 13.Baxter LR, Jr, Schwartz JM, Mazziotta JC, Phelps ME, Pahl JJ, Guze BH, et al. Cerebral glucose metabolic rates in nondepressed patients with obsessive-compulsive disorder. Am J Psychiatry. 1988 Dec;145(12):1560–3. doi: 10.1176/ajp.145.12.1560. [DOI] [PubMed] [Google Scholar]
- 14.Nordahl TE, Benkelfat C, Semple WE, Gross M, King AC, Cohen RM. Cerebral glucose metabolic rates in obsessive compulsive disorder. Neuropsychopharmacology. 1989 Mar;2(1):23–8. doi: 10.1016/0893-133x(89)90003-1. [DOI] [PubMed] [Google Scholar]
- 15.Swedo SE, Schapiro MB, Grady CL, Cheslow DL, Leonard HL, Kumar A, et al. Cerebral glucose metabolism in childhood-onset obsessive-compulsive disorder. Arch Gen Psychiatry. 1989 Jun;46(6):518–23. doi: 10.1001/archpsyc.1989.01810060038007. [DOI] [PubMed] [Google Scholar]
- 16.Breiter HC, Rauch SL, Kwong KK, Baker JR, Weisskoff RM, Kennedy DN, et al. Functional magnetic resonance imaging of symptom provocation in obsessive-compulsive disorder. Arch Gen Psychiatry. 1996 Jul;53(7):595–606. doi: 10.1001/archpsyc.1996.01830070041008. [DOI] [PubMed] [Google Scholar]
- 17.Cottraux J, Gerard D, Cinotti L, Froment JC, Deiber MP, Le Bars D, et al. A controlled positron emission tomography study of obsessive and neutral auditory stimulation in obsessive-compulsive disorder with checking rituals. Psychiatry Res. 1996 Mar 29;60(2–3):101–12. doi: 10.1016/0165-1781(96)02697-2. [DOI] [PubMed] [Google Scholar]
- 18.Benkelfat C, Nordahl TE, Semple WE, King AC, Murphy DL, Cohen RM. Local cerebral glucose metabolic rates in obsessive-compulsive disorder. Patients treated with clomipramine. Arch Gen Psychiatry. 1990 Sep;47(9):840–8. doi: 10.1001/archpsyc.1990.01810210048007. [DOI] [PubMed] [Google Scholar]
- 19.Saxena S, Brody AL, Maidment KM, Dunkin JJ, Colgan M, Alborzian S, et al. Localized orbitofrontal and subcortical metabolic changes and predictors of response to paroxetine treatment in obsessive-compulsive disorder. Neuropsychopharmacology. 1999 Dec;21(6):683–93. doi: 10.1016/S0893-133X(99)00082-2. [DOI] [PubMed] [Google Scholar]
- 20.Swedo SE, Pietrini P, Leonard HL, Schapiro MB, Rettew DC, Goldberger EL, et al. Cerebral glucose metabolism in childhood-onset obsessive-compulsive disorder. Revisualization during pharmacotherapy. Arch Gen Psychiatry. 1992 Sep;49(9):690–4. doi: 10.1001/archpsyc.1992.01820090018003. [DOI] [PubMed] [Google Scholar]
- 21.Dulawa SC, Geyer MA. Effects of strain and serotonergic agents on prepulse inhibition and habituation in mice. Neuropharmacology. 2000 Aug 23;39(11):2170–9. doi: 10.1016/s0028-3908(00)00030-7. [DOI] [PubMed] [Google Scholar]
- 22.Dulawa SC, Hen R, Scearce-Levie K, Geyer MA. Serotonin1B receptor modulation of startle reactivity, habituation, and prepulse inhibition in wild-type and serotonin1B knockout mice. Psychopharmacology (Berl) 1997 Jul;132(2):125–34. doi: 10.1007/s002130050328. [DOI] [PubMed] [Google Scholar]
- 23.Chaouloff F, Courvoisier H, Moisan MP, Mormede P. GR 127935 reduces basal locomotor activity and prevents RU 24969-, but not D-amphetamine-induced hyperlocomotion, in the Wistar-Kyoto hyperactive (WKHA) rat. Psychopharmacology (Berl) 1999 Jan;141(3):326–31. doi: 10.1007/s002130050841. [DOI] [PubMed] [Google Scholar]
- 24.Oberlander C, Blaquiere B, Pujol JF. Distinct functions for dopamine and serotonin in locomotor behaviour: evidence using the 5-HT1 agonist RU 24969 in globus pallidus-lesioned rats. Neurosci Lett. 1986 Jun 18;67(2):113–8. doi: 10.1016/0304-3940(86)90382-4. [DOI] [PubMed] [Google Scholar]
- 25.Rempel NL, Callaway CW, Geyer MA. Serotonin1B receptor activation mimics behavioral effects of presynaptic serotonin release. Neuropsychopharmacology. 1993 May;8(3):201–11. doi: 10.1038/npp.1993.22. [DOI] [PubMed] [Google Scholar]
- 26.Shanahan NA, Holick Pierz KA, Masten VL, Waeber C, Ansorge M, Gingrich JA, et al. Chronic reductions in serotonin transporter function prevent 5-HT1B-induced behavioral effects in mice. Biol Psychiatry. 2009 Mar 1;65(5):401–8. doi: 10.1016/j.biopsych.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montgomery S. Pharmacological treatment of obsessive-compulsive disorder. In: Hollander EZJ, Marazziti D, Olivier B, editors. Current Insights in Obsessive Disorder. New York: Wiley; 1994. pp. 215–26. [Google Scholar]
- 28.Blier P. The pharmacology of putative early-onset antidepressant strategies. Eur Neuropsychopharmacol. 2003 Mar;13(2):57–66. doi: 10.1016/s0924-977x(02)00173-6. [DOI] [PubMed] [Google Scholar]
- 29.Dulawa SC, Holick KA, Gundersen B, Hen R. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology. 2004 Jul;29(7):1321–30. doi: 10.1038/sj.npp.1300433. [DOI] [PubMed] [Google Scholar]
- 30.Thomsen PH, Jensen J. Obsessive-compulsive disorder: admission patterns and diagnostic stability. A case-register study. Acta Psychiatr Scand. 1994 Jul;90(1):19–24. doi: 10.1111/j.1600-0447.1994.tb01550.x. [DOI] [PubMed] [Google Scholar]
- 31.Bebbington PE. Epidemiology of obsessive-compulsive disorder. Br J Psychiatry Suppl. 1998;(35):2–6. [PubMed] [Google Scholar]
- 32.Fireman B, Koran LM, Leventhal JL, Jacobson A. The prevalence of clinically recognized obsessive-compulsive disorder in a large health maintenance organization. Am J Psychiatry. 2001 Nov;158(11):1904–10. doi: 10.1176/appi.ajp.158.11.1904. [DOI] [PubMed] [Google Scholar]
- 33.Geyer MA, Dulawa SC. Assessment of murine startle response, prepulse inhibition, and habituation. In: Crawley J, Skolnick P, editors. Current protocols in neuroscience. New York: Jon Wiley & Sons; 2004. [Google Scholar]
- 34.Mansbach RS, Geyer MA, Braff DL. Dopaminergic stimulation disrupts sensorimotor gating in the rat. Psychopharmacology (Berl) 1988;94(4):507–14. doi: 10.1007/BF00212846. [DOI] [PubMed] [Google Scholar]
- 35.Holick KA, Lee DC, Hen R, Dulawa SC. Behavioral effects of chronic fluoxetine in BALB/cJ mice do not require adult hippocampal neurogenesis or the serotonin 1A receptor. Neuropsychopharmacology. 2008 Jan;33(2):406–17. doi: 10.1038/sj.npp.1301399. [DOI] [PubMed] [Google Scholar]
- 36.Cryan JF, Page ME, Lucki I. Noradrenergic lesions differentially alter the antidepressant-like effects of reboxetine in a modified forced swim test. Eur J Pharmacol. 2002 Feb 2;436(3):197–205. doi: 10.1016/s0014-2999(01)01628-4. [DOI] [PubMed] [Google Scholar]
- 37.Borsini F, Lecci A, Sessarego A, Frassine R, Meli A. Discovery of antidepressant activity by forced swimming test may depend on pre-exposure of rats to a stressful situation. Psychopharmacology (Berl) 1989;97(2):183–8. doi: 10.1007/BF00442247. [DOI] [PubMed] [Google Scholar]
- 38.Waeber C, Palacios JM. Non 5-HT1A/5-HT1C [3H]5-HT binding sites in the hamster, opossum, and rabbit brain show similar regional distribution but different sensitivity to beta-adrenoceptor antagonists. Synapse. 1992 Dec;12(4):261–70. doi: 10.1002/syn.890120403. [DOI] [PubMed] [Google Scholar]
- 39.Paulus MP, Geyer MA. Quantitative assessment of the microstructure of rat behavior: I, f(d), the extension of the scaling hypothesis. Psychopharmacology (Berl) 1993;113(2):177–86. doi: 10.1007/BF02245695. [DOI] [PubMed] [Google Scholar]
- 40.Martinez-Price DL, Geyer MA. Subthalamic 5-HT(1A) and 5-HT(1B) receptor modulation of RU 24969-induced behavioral profile in rats. Pharmacol Biochem Behav. 2002 Apr;71(4):569–80. doi: 10.1016/s0091-3057(01)00704-3. [DOI] [PubMed] [Google Scholar]
- 41.Cheetham SC, Heal DJ. Evidence that RU 24969-induced locomotor activity in C57/B1/6 mice is specifically mediated by the 5-HT1B receptor. Br J Pharmacol. 1993 Dec;110(4):1621–9. doi: 10.1111/j.1476-5381.1993.tb14010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peroutka SJ. Pharmacological differentiation and characterization of 5-HT1A, 5-HT1B, and 5-HT1C binding sites in rat frontal cortex. J Neurochem. 1986 Aug;47(2):529–40. doi: 10.1111/j.1471-4159.1986.tb04532.x. [DOI] [PubMed] [Google Scholar]
- 43.Saxena S, Rauch SL. Functional neuroimaging and the neuroanatomy of obsessive-compulsive disorder. Psychiatr Clin North Am. 2000 Sep;23(3):563–86. doi: 10.1016/s0193-953x(05)70181-7. [DOI] [PubMed] [Google Scholar]
- 44.Chudasama Y, Robbins TW. Dissociable contributions of the orbitofrontal and infralimbic cortex to pavlovian autoshaping and discrimination reversal learning: further evidence for the functional heterogeneity of the rodent frontal cortex. J Neurosci. 2003 Sep 24;23(25):8771–80. doi: 10.1523/JNEUROSCI.23-25-08771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bechara A, Tranel D, Damasio H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain. 2000 Nov;123( Pt 11):2189–202. doi: 10.1093/brain/123.11.2189. [DOI] [PubMed] [Google Scholar]
- 46.Geyer MA, Markou A. Animal models of psychiatric disorders. In: Bloom F, Kupfer D, editors. Psychopharmacology: the fourth generation of progress. New York: Raven Press; 1995. pp. 787–98. [Google Scholar]
- 47.Korff S, Stein DJ, Harvey BH. Stereotypic behaviour in the deer mouse: pharmacological validation and relevance for obsessive compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2008 Feb 15;32(2):348–55. doi: 10.1016/j.pnpbp.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 48.Greene-Schloesser DM, Van der Zee EA, Sheppard DK, Castillo MR, Gregg KA, Burrow T, et al. Predictive validity of a non-induced mouse model of compulsive-like behavior. Behav Brain Res. 2011 Aug 1;221(1):55–62. doi: 10.1016/j.bbr.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shmelkov SV, Hormigo A, Jing D, Proenca CC, Bath KG, Milde T, et al. Slitrk5 deficiency impairs corticostriatal circuitry and leads to obsessive-compulsive-like behaviors in mice. Nat Med. 2010 May;16(5):598–602. doi: 10.1038/nm.2125. 1p following. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sugimoto Y, Tagawa N, Kobayashi Y, Hotta Y, Yamada J. Effects of the serotonin and noradrenaline reuptake inhibitor (SNRI) milnacipran on marble burying behavior in mice. Biol Pharm Bull. 2007 Dec;30(12):2399–401. doi: 10.1248/bpb.30.2399. [DOI] [PubMed] [Google Scholar]
- 51.Welch JM, Lu J, Rodriguiz RM, Trotta NC, Peca J, Ding JD, et al. Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. Nature. 2007 Aug 23;448(7156):894–900. doi: 10.1038/nature06104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang L, Simpson HB, Dulawa SC. Assessing the validity of current mouse genetic models of obsessive-compulsive disorder. Behav Pharmacol. 2009 Mar;20(2):119–33. doi: 10.1097/FBP.0b013e32832a80ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoffman KL, Rueda Morales RI. Toward an understanding of the neurobiology of “just right” perceptions: nest building in the female rabbit as a possible model for compulsive behavior and the perception of task completion. Behav Brain Res. 2009 Dec 1;204(1):182–91. doi: 10.1016/j.bbr.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 54.Korff S, Harvey BH. Animal models of obsessive-compulsive disorder: rationale to understanding psychobiology and pharmacology. Psychiatr Clin North Am. 2006 Jun;29(2):371–90. doi: 10.1016/j.psc.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 55.Bejerot S. An autistic dimension: a proposed subtype of obsessive-compulsive disorder. Autism. 2007 Mar;11(2):101–10. doi: 10.1177/1362361307075699. [DOI] [PubMed] [Google Scholar]
- 56.Leyfer OT, Folstein SE, Bacalman S, Davis NO, Dinh E, Morgan J, et al. Comorbid psychiatric disorders in children with autism: interview development and rates of disorders. J Autism Dev Disord. 2006 Oct;36(7):849–61. doi: 10.1007/s10803-006-0123-0. [DOI] [PubMed] [Google Scholar]
- 57.Ozaki N, Goldman D, Kaye WH, Plotnicov K, Greenberg BD, Lappalainen J, et al. Serotonin transporter missense mutation associated with a complex neuropsychiatric phenotype. Mol Psychiatry. 2003 Nov;8(11):933–6. doi: 10.1038/sj.mp.4001365. [DOI] [PubMed] [Google Scholar]
- 58.Bolton PF, Pickles A, Murphy M, Rutter M. Autism, affective and other psychiatric disorders: patterns of familial aggregation. Psychol Med. 1998 Mar;28(2):385–95. doi: 10.1017/s0033291797006004. [DOI] [PubMed] [Google Scholar]
- 59.Micali N, Chakrabarti S, Fombonne E. The broad autism phenotype: findings from an epidemiological survey. Autism. 2004 Mar;8(1):21–37. doi: 10.1177/1362361304040636. [DOI] [PubMed] [Google Scholar]
- 60.Hollander E, King A, Delaney K, Smith CJ, Silverman JM. Obsessive-compulsive behaviors in parents of multiplex autism families. Psychiatry Res. 2003 Jan 25;117(1):11–6. doi: 10.1016/s0165-1781(02)00304-9. [DOI] [PubMed] [Google Scholar]
- 61.Frankland PW, Wang Y, Rosner B, Shimizu T, Balleine BW, Dykens EM, et al. Sensorimotor gating abnormalities in young males with fragile X syndrome and Fmr1-knockout mice. Mol Psychiatry. 2004 Apr;9(4):417–25. doi: 10.1038/sj.mp.4001432. [DOI] [PubMed] [Google Scholar]
- 62.McAlonan GM, Daly E, Kumari V, Critchley HD, van Amelsvoort T, Suckling J, et al. Brain anatomy and sensorimotor gating in Asperger’s syndrome. Brain. 2002 Jul;125(Pt 7):1594–606. doi: 10.1093/brain/awf150. [DOI] [PubMed] [Google Scholar]
- 63.Hollander E, Novotny S, Allen A, Aronowitz B, Cartwright C, DeCaria C. The relationship between repetitive behaviors and growth hormone response to sumatriptan challenge in adult autistic disorder. Neuropsychopharmacology. 2000 Feb;22(2):163–7. doi: 10.1016/S0893-133X(99)00121-9. [DOI] [PubMed] [Google Scholar]
- 64.Novotny S, Hollander E, Allen A, Mosovich S, Aronowitz B, Cartwright C, et al. Increased growth hormone response to sumatriptan challenge in adult autistic disorders. Psychiatry Res. 2000 May 15;94(2):173–7. doi: 10.1016/s0165-1781(00)00134-7. [DOI] [PubMed] [Google Scholar]
- 65.Novotny S, Hollander E, Phillips A, Allen A, Wasserman S, Iyengar R. Increased repetitive behaviours and prolactin responsivity to oral m-chlorophenylpiperazine in adults with autism spectrum disorders. Int J Neuropsychopharmacol. 2004 Sep;7(3):249–54. doi: 10.1017/S146114570400433X. [DOI] [PubMed] [Google Scholar]
- 66.Brodkin ES, McDougle CJ, Naylor ST, Cohen DJ, Price LH. Clomipramine in adults with pervasive developmental disorders: a prospective open-label investigation. J Child Adolesc Psychopharmacol. 1997 Summer;7(2):109–21. doi: 10.1089/cap.1997.7.109. [DOI] [PubMed] [Google Scholar]
- 67.Bachevalier J, Loveland KA. The orbitofrontal-amygdala circuit and self-regulation of social-emotional behavior in autism. Neurosci Biobehav Rev. 2006;30(1):97–117. doi: 10.1016/j.neubiorev.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 68.Dawson G, Munson J, Estes A, Osterling J, McPartland J, Toth K, et al. Neurocognitive function and joint attention ability in young children with autism spectrum disorder versus developmental delay. Child Dev. 2002 Mar-Apr;73(2):345–58. doi: 10.1111/1467-8624.00411. [DOI] [PubMed] [Google Scholar]
- 69.Salmond CH, de Haan M, Friston KJ, Gadian DG, Vargha-Khadem F. Investigating individual differences in brain abnormalities in autism. Philos Trans R Soc Lond B Biol Sci. 2003 Feb 28;358(1430):405–13. doi: 10.1098/rstb.2002.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Engel G, Gothert M, Hoyer D, Schlicker E, Hillenbrand K. Identity of inhibitory presynaptic 5-hydroxytryptamine (5-HT) autoreceptors in the rat brain cortex with 5-HT1B binding sites. Naunyn Schmiedebergs Arch Pharmacol. 1986 Jan;332(1):1–7. doi: 10.1007/BF00633189. [DOI] [PubMed] [Google Scholar]
- 71.Johanning H, Plenge P, Mellerup E. Serotonin receptors in the brain of rats treated chronically with imipramine or RU24969: support for the 5-HT1B receptor being a 5-HT autoreceptor. Pharmacol Toxicol. 1992 Feb;70(2):131–4. doi: 10.1111/j.1600-0773.1992.tb00442.x. [DOI] [PubMed] [Google Scholar]
- 72.Wu SY, Wang MY, Dun NJ. Serotonin via presynaptic 5-HT1 receptors attenuates synaptic transmission to immature rat motoneurons in vitro. Brain Res. 1991 Jul 19;554(1–2):111–21. doi: 10.1016/0006-8993(91)90178-x. [DOI] [PubMed] [Google Scholar]
- 73.Bennett-Clarke CA, Leslie MJ, Chiaia NL, Rhoades RW. Serotonin 1B receptors in the developing somatosensory and visual cortices are located on thalamocortical axons. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):153–7. doi: 10.1073/pnas.90.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Blier P, de Montigny C. Current advances and trends in the treatment of depression. Trends Pharmacol Sci. 1994 Jul;15(7):220–6. doi: 10.1016/0165-6147(94)90315-8. [DOI] [PubMed] [Google Scholar]
- 75.Briley M, Moret C. The possibility of 5-HT1B autoreceptors in the action of serotonergic antidepressant drugs. In: Briley M, Montgomery SA, editors. Antidepressant therapy: at the dawn of the third millenium. London: Martin Dunitz; 1998. pp. 37–54. [Google Scholar]
- 76.Bergqvist PB, Bouchard C, Blier P. Effect of long-term administration of antidepressant treatments on serotonin release in brain regions involved in obsessive-compulsive disorder. Biol Psychiatry. 1999 Jan 15;45(2):164–74. doi: 10.1016/s0006-3223(98)00154-1. [DOI] [PubMed] [Google Scholar]
- 77.el Mansari M, Bouchard C, Blier P. Alteration of serotonin release in the guinea pig orbito-frontal cortex by selective serotonin reuptake inhibitors. Relevance to treatment of obsessive-compulsive disorder. Neuropsychopharmacology. 1995 Oct;13(2):117–27. doi: 10.1016/0893-133X(95)00045-F. [DOI] [PubMed] [Google Scholar]
- 78.Bramley JR, Sollars PJ, Pickard GE, Dudek FE. 5-HT1B receptor-mediated presynaptic inhibition of GABA release in the suprachiasmatic nucleus. J Neurophysiol. 2005 Jun;93(6):3157–64. doi: 10.1152/jn.00770.2004. [DOI] [PubMed] [Google Scholar]
- 79.Matsuoka T, Hasuo H, Akasu T. 5-Hydroxytryptamine 1B receptors mediate presynaptic inhibition of monosynaptic IPSC in the rat dorsolateral septal nucleus. Neurosci Res. 2004 Mar;48(3):229–38. doi: 10.1016/j.neures.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 80.Simpson HB. Pharmacological treatment of obsessive-compulsive disorder. Curr Top Behav Neurosci. 2010;2:527–43. doi: 10.1007/7854_2009_12. [DOI] [PubMed] [Google Scholar]
- 81.Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. 3. San Diego, CA: Academic Press; 2007. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.