Abstract
Background
Whether fish or the fatty acids they contain are independently associated with risk for incident heart failure (HF) among postmenopausal women is unclear.
Methods and Results
The baseline Women’s Health Initiative Observational Study (WHI-OS) cohort consisted of 93,676 women aged 50–79 of diverse ethnicity and background of which 84,493 were eligible for analyses. Intakes of baked/broiled fish, fried fish and omega-3 fatty acid (eicosapentaenoic acid (EPA) + docosahexaenoic acid (DHA), α-linolenic acid (ALA)), and trans fatty acid (TFA) were determined from the WHI food frequency questionnaire. Baked/broiled fish consumption was divided into 5 frequency categories: <1/mo (referent), 1–3/mo, 1–2/wk, 3–4/wk, ≥5/wk. Fried fish intake was grouped into 3 frequency categories: <1/mo (referent), 2) 1–3/mo, and 3) ≥1/wk. Associations between fish or fatty acid intake and incident HF were determined using Cox models adjusting for HF risk factors and dietary factors. Baked/broiled fish consumption (≥5 servings/wk at baseline) was associated with a hazard ratio (HR) of 0.70 (95% CI: 0.51, 0.95) for incident HF. In contrast, fried fish consumption (≥1 serving/wk at baseline) was associated with a HR of 1.48 (95% CI: 1.19, 1.84) for incident HF. No significant associations were found between EPA+DHA, ALA, or TFA intake and incident HF.
Conclusions
Increased baked/broiled fish intake may lower HF risk, while increased fried fish intake may increase HF risk in postmenopausal women.
Keywords: heart failure, epidemiology, nutrition, women
Heart failure (HF) is a major public health problem that currently affects greater than 5 million Americans.1 Recent studies suggest that 1 in 5 adults will develop HF over the course of their lifetime.2 Of note, the elderly, postmenopausal women, and racial/ethnic minority groups experience a greater burden of HF.3,4 Furthermore, it is predicted that these populations will make up a greater proportion of future HF cases in the US.1,3,4 Modification of dietary factors represents a potential means for mitigating risk for incident HF in postmenopausal women, however studies assessing diet and nutrient intakes and their role in the risk for HF in postmenopausal women are lacking.
Considerable evidence indicates that marine omega-3 fatty acids (e.g. eicosapentaenoic acid (EPA) + docosahexaenoic acid (DHA)), found in high amounts in baked and broiled fish, and vegetable sources (e.g. α-linolenic acid, ALA) of omega-3 fatty acids exert effects on cardiovascular physiology that may lower cardiovascular disease risk.5 Specifically, EPA, DHA and ALA decrease inflammation, oxidative stress, and blood pressure, and improve cardiac and endothelial function.5 In contrast, trans fatty acids (TFA) increase inflammation, oxidative stress, insulin resistance, and negatively affect endothelial function.6 Despite the emerging evidence that intake of fish and unsaturated fatty acid intake may favorably affect the pathogenesis of HF by influencing several pathophysiologic mechanisms5, it is unclear whether an independent association exists between these dietary measures and the risk for incident HF. The Women’s Health Initiative Observational Study (WHI-OS) provides the opportunity to evaluate these relationships in an ethnically diverse population of aging, postmenopausal women with a long period of follow-up, comprehensive baseline dietary assessment, and prospective ascertainment of HF events.7,8 This study was undertaken to determine whether the method of fish preparation and whether dietary estimates of EPA + DHA, ALA, or TFA are involved in increasing or decreasing the risk for incident HF.
Methods
Study Sample
The WHI-OS consists of a national sample of postmenopausal women (ages 50–79 at baseline) in overall good health that were either ineligible or unwilling to be randomized in the WHI clinical trials.8 Of the 93,676 women in the initial baseline cohort, the following exclusions were made: 893 had HF at baseline, 3,597 for missing fish intake data, and 4,693 for missing covariate data, leaving 84,493 women in the final analyses. The average follow-up period was 10.0 years through August 2008.
Dietary assessment
Fish and fatty acid intake were determined using the calculated values of fish, DHA+EPA, ALA, and TFA intake based on the WHI-food frequency questionnaire (WHI-FFQ) administered at the baseline screening visit.9 To account for variation in daily caloric intake, fatty acid intake was standardized to total energy intake (fatty acid intake in g/1000 kcal). In separate analyses, we also examined the association between fatty acid intake and HF in non-caloric adjusted models to determine whether a dose dependent increase in the amount of fatty acids consumed per day is associated with future HF risk (fatty acid intake in g/d) (data supplement). Fish intake was assessed from the WHI FFQ based on the following five questions asking about: 1) fried fish, fish sandwich, fried shellfish (3 ounces/1 sandwich), 2) shellfish, not fried (shrimp, lobster, crab, and oysters) (3 ounces or ½ cup), 3) canned tuna, tuna salad, and tuna casserole (1/2 cup tuna or 1 cup casserole), 4) white fish (broiled or baked) (sole, snapper, cod) (3 ounces), and 5) dark fish (broiled or baked) (salmon, mackerel, bluefish) (3 ounces). For analyses, two food groups were defined: baked/broiled fish or fried fish. The baked/broiled fish group consisted of canned tuna, tuna salad, tuna casserole, white fish (broiled or baked), dark fish (broiled or baked), and shellfish (not fried). The fried fish group consisted of fried fish, fish sandwich, and fried shellfish. Fried fish contributed TFA to the diet standardized as 3.05g per medium serving. Baked/broiled fish consumption was divided into 5 frequency categories with category 1 serving as the referent based on frequency of fish consumption (units are servings) as follows: 1) <1/mo, 2) 1–3/mo, 3) 1–2/wk, 4) 3–4/wk, and 5) ≥5/wk. For fried fish, only three consumption frequencies were defined because few women consumed 3 or more servings per week of fried fish. Accordingly, fried fish intake was grouped as follows with category 1 serving as the referent: 1) <1/mo, 2) 1–3/mo, and 3) ≥1/wk. Total omega-3 fat intake was dichotomized into omega-3 fat intake derived from vegetable oil (ALA) or marine oil (DHA+EPA) sources. Because the intakes of DHA+EPA and ALA tend to be correlated10, we examined the independent associations between DHA+EPA and HF risk as well as ALA and HF risk, while controlling for ALA and DHA+EPA intake, respectively. Total TFA intake consisted of the sum of: i) 7-trans-16:1 palmitoleic acid, ii) 12-trans-18:1 oleic acid, and iii) 9-trans-18:2 linoleic acid. All fatty acid intake was categorized into quartiles with quartile 1 serving as the referent. Finally, total fried food intake consisted of french fries, fried potatoes, fried rice, fried cassava, fritters, and fried chicken intake.
Ascertainment of Hospitalized Heart Failure
Adjudicated (hospitalized) HF in the WHI was determined as described previously.7 Adjudication of HF was done by local clinical center physician adjudicators, who were centrally trained and reviewed hospitalized events and made an initial diagnosis of HF.
Statistical Analyses
Potential confounders of the association between fish and fatty acid intake and risk for incident HF are summarized in Tables 1 and 2 and the data supplement (Supplemental Tables 1–3). Comparison of baseline covariates was done using t-tests from a linear model for continuous variables and χ2 tests for categorical variables. Cox-proportional hazards models with 95% confidence intervals were constructed to examine the associations between baseline baked/broiled fish, fried fish, DHA+EPA, ALA, and TFA intake and risk for incident HF. Exposure variables were treated categorically. Categorical strata were treated as numerical values such that each stratum received a numerical value ranging from 1–5. Hazard ratios were computed as the rate in a specific category of baked/broiled fish or fried fish divided by the rate in the lowest category. To adjust for potential confounders, a series of 3 Cox models were generated: model 1 (age, race, education, physical activity, and traditional HF risk factors: smoking status, alcohol consumption, diabetes mellitus, atrial fibrillation, coronary artery disease (CAD; defined as myocardial infarction/coronary artery bypass graft (CABG)/percutaneous transluminal coronary angioplasty (PTCA), hypertension, body mass index, and time-dependent MI), model 2 (model 1 + intake of fiber, fruits and vegetables, saturated fat, TFA, ALA, and -linoleic acid + alternate type of fish preparation, i.e. fried fish intake for baked/broiled fish analyses and baked/broiled fish intake for fried fish analyses), and model 3 (model 2 + fried food consumption + sodium intake). Given the strength of the association between MI and HF1, we also adjusted for interim MI during follow-up in model 2. For clarity, we only present results of a composite model 1, which adjusted for traditional HF risk factors, dietary factors, and interim MI. Results of the 3 models can be found in the data supplement (Supplemental Tables 4 and 5). For fatty acid intake, hazard ratios were computed as the rate in a specific quartile of ALA, DHA+EPA, or TFA intake divided by the rate in quartile 1. Three Cox models were generated: models 1 and 2 (as described above), model 3 (for DHA + EPA intake analyses) (model 2 + intake of fiber, fruits and vegetables, saturated fat, TFA, ALA, and α-linoleic acid), model 3 (for ALA intake analyses) (model 2 + intake of fiber, fruits and vegetables, saturated fat, TFA, DHA+EPA, and α-linoleic acid). The data were adjusted for α-linoleic acid because previous studies have indicated that its intake is tightly correlated with that of ALA.11 Moreover, ALA and α-linoleic acid compete as substrates for the same desaturase enzymes and thus high levels of α-linoleic acid may decrease the bioavailability of ALA and/or its metabolites (e.g. DHA/EPA).12 For TFA intake analyses, 3 Cox models were generated: models 1 and 2 (as described above), model 3 (model 2 + intake of fiber, fruits and vegetables, saturated fat, DHA+EPA, ALA, and α-linoleic acid). All analyses were person-time based in which women contributed follow-up time until adjudicated HF, death, lost to follow up, or end of the follow-up period. Once women developed HF, they were excluded from further follow-up analyses. Incidence rates were calculated as the number of incident HF events divided by the total person time of observation (risk). Cumulative incidence of HF based on baked/broiled fish, fried fish, DHA+EPA, ALA, or TFA intake categories was determined by the Kaplan-Meier method to evaluate HF-free probability of survival based on specific fish and fatty acid categories as defined above. Differences between categories were examined using the log-rank test for trend with α-level=0.05 defined as statistically significant. All analyses were performed in SAS for Windows Version 9.1 (The SAS Institute, Cary, NC).
Table 1.
Baseline Features of the WHI-OS Cohort Based on Frequency of Baked/Broiled Fish Intake.
| <1/mo. | 1–3/mo. | 1–2/wk. | 3–4/wk. | ≥5/wk. | p- value |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Participants, n (%) | 11700 (14) | 26344 (31) | 35034 (41) | 8109 (10) | 3306 (4) | ||||||
| Age (years) | 11700 | 64 (8) | 26344 | 64 (8) | 35034 | 63 (7) | 8109 | 63 (7) | 3306 | 63 (7) | <0.001 |
| Ethnicity (%) | <0.001 | ||||||||||
| White | 9349 | 80 | 22050 | 84 | 30438 | 87 | 7013 | 87 | 2695 | 82 | |
| African American | 1246 | 11 | 2088 | 8 | 2051 | 5.9 | 487 | 6.0 | 281 | 8.5 | |
| Hispanic | 659 | 6 | 1091 | 4 | 898 | 3 | 160 | 2 | 89 | 3 | |
| American Indian / AK Native | 87 | 0.7 | 118 | 0.4 | 97 | 0.3 | 31 | 0.4 | 7 | 0.2 | |
| Asian / Pacific Islander | 183 | 2 | 671 | 3 | 1088 | 3 | 313 | 4 | 158 | 5 | |
| Unknown | 176 | 2 | 326 | 1 | 462 | 1 | 105 | 1 | 76 | 2 | |
| Diabetes (%yes) | 518 | 4 | 1085 | 4 | 1219 | 4 | 283 | 4 | 137 | 4 | <0.001 |
| CAD (%yes) | 402 | 3 | 740 | 3 | 1040 | 3 | 220 | 3 | 109 | 3 | 0.005 |
| Atrial fibrillation (%yes) | 565 | 5 | 1180 | 5 | 1492 | 4 | 327 | 4 | 134 | 4 | 0.028 |
| Body mass index (kg/m2) | 11700 | 28 (6) | 26344 | 27 (6) | 35034 | 27 (6) | 8109 | 27 (6) | 3306 | 28 (6) | <0.001 |
| HDL-cholesterol (mg/dl)* | 151 | 62 (17) | 312 | 64 (16) | 403 | 64 (17) | 72 | 65 (19) | 17 | 65 (20) | 0.581 |
| Triglycerides (mg/dl)* | 151 | 153 (80) | 312 | 148 (76) | 404 | 152 (107) | 72 | 134 (57) | 17 | 148 (118) | 0.629 |
| Systolic Blood Pressure (mmHg) | 11693 | 128 (18) | 26328 | 127 (18) | 35005 | 127 (18) | 8100 | 126 (18) | 3306 | 126 (18) | <0.001 |
| Alcohol Use (%) | <0.001 | ||||||||||
| Never drinker | 2226 | 19 | 3326 | 13 | 2729 | 8 | 564 | 7 | 289 | 9 | |
| Past drinker | 2938 | 25 | 5320 | 20 | 5424 | 16 | 1197 | 15 | 544 | 17 | |
| Current drinker | 6536 | 56 | 17698 | 67 | 26881 | 77 | 6348 | 78 | 2473 | 75 | |
| Smoking status (%) | <0.001 | ||||||||||
| Never | 6389 | 55 | 14099 | 54 | 17177 | 49 | 3710 | 46 | 1505 | 46 | |
| Former | 4441 | 38 | 10392 | 39 | 15923 | 46 | 4054 | 50 | 1636 | 50 | |
| Current | 870 | 7 | 1853 | 7 | 1934 | 6 | 345 | 4 | 165 | 5 | |
| Physical activity (METs/wk) | 11700 | 12 (14) | 26344 | 12 (14) | 35034 | 15 (14) | 8109 | 17 (14) | 3306 | 18 (16) | <0.001 |
| Education (%) | <0.001 | ||||||||||
| ≤ High school / GED | 3633 | 31 | 6364 | 24 | 5885 | 17 | 1099 | 14 | 448 | 14 | |
| Some college | 4461 | 38 | 10202 | 39 | 12364 | 35 | 2684 | 33 | 1029 | 31 | |
| College graduate | 3606 | 31 | 9778 | 37 | 16785 | 48 | 4326 | 53 | 1829 | 55 | |
| Energy intake (kcal/day) | 11700 | 1400 (564) | 26344 | 1463 (560) | 35034 | 1626 (577) | 8109 | 1783 (631) | 3306 | 1948 (731) | <0.001 |
| Fiber intake (g/1000 kcal) | 11700 | 11 (4) | 26344 | 11 (4) | 35034 | 11 (4) | 8109 | 11 (4) | 3306 | 11 (4) | <0.001 |
| Fruit and Vegetable intake (%kcal) | 11700 | 3.6 (2.2) | 26344 | 3.9 (2.1) | 35034 | 4.6 (2.1) | 8109 | 5.3 (2.2) | 3306 | 5.7 (2.5) | <0.001 |
| SFA intake (%kcal) | 11700 | 10.6 (3.6) | 26344 | 10.4 (3.4) | 35034 | 9.8 (3.2) | 8109 | 9.2 (3.0) | 3306 | 8.9 (3.1) | <0.001 |
| TFA intake (%kcal) | 11700 | 2.3 (1.2) | 26344 | 2.2 (1.1) | 35034 | 2.0 (1.0) | 8109 | 1.7 (0.9) | 3306 | 1.6 (0.9) | <0.001 |
| DHA+EPA intake (%kcal) | 11700 | 0.02 (0.02) | 26344 | 0.04 (0.03) | 35034 | 0.1 (0.1) | 8109 | 0.2 (0.1) | 3306 | 0.3 (0.2) | <0.001 |
| ALA intake (%kcal) | 11700 | 0.7 (0.3) | 26344 | 0.7 (0.3) | 35034 | 0.7 (0.3) | 8109 | 0.7 (0.3) | 3306 | 0.7 (0.3) | <0.001 |
| α -Linoleic Acid (%kcal) | 11700 | 5.7 (2.2) | 26344 | 5.6 (1.9) | 35034 | 5.4 (1.8) | 8109 | 5.4 (1.8) | 3306 | 5.6 (2.1) | <0.001 |
WHI blood subsample participants only (n=955). Means and SD's weighted by ethnicity.
Coronary artery disease (CAD), saturated fatty acids (SFA), trans fatty acids (TFA), eicosapentaenoic acid (EPA) + docosahexaenoic acid (DHA), alpha-linolenic acid (ALA).
Table 2.
Baseline Features of the WHI-OS Cohort Based on Frequency of Fried Fish/Fish Sandwich Intake
| <1/mo. | 1–3/mo. | ≥1/wk. | p-value | ||||
|---|---|---|---|---|---|---|---|
| Total Participants, n (%) | 63609 (75) | 18015 (21) | 2869 (3) | ||||
| Age (years) | 63609 | 64 (7) | 18015 | 63 (7) | 2869 | 63 (7) | <0.001 |
| Ethnicity (%) | <0.001 | ||||||
| White | 56002 | 88 | 13862 | 77 | 1681 | 59 | |
| African American | 3066 | 5 | 2320 | 13 | 767 | 27 | |
| Hispanic | 2076 | 3 | 690 | 4 | 131 | 5 | |
| American Indian / AK Native | 253 | 0.4 | 74 | 0.4 | 13 | 0.5 | |
| Asian / Pacific Islander | 1367 | 2 | 826 | 5 | 220 | 8 | |
| Unknown | 845 | 1 | 243 | 1 | 57 | 2 | |
| Diabetes (%yes) | 2047 | 3 | 964 | 5 | 231 | 8 | <0.001 |
| CAD (%yes) | 1842 | 3 | 585 | 3 | 84 | 3 | 0.049 |
| Atrial fibrillation (%yes) | 2707 | 4 | 841 | 5 | 150 | 5 | 0.004 |
| Body mass index (kg/m2 | 63609 | 27 (60 | 18015 | 28 (6) | 2869 | 30 (7) | <0.001 |
| HDL-cholesterol (mg/dl)* | 696 | 64 (17) | 222 | 63 (16) | 37 | 64 (23) | 0.892 |
| Triglycerides (mg/dl)* | 697 | 147 (78) | 222 | 153 (83) | 37 | 174 (124) | 0.281 |
| Systolic Blood Pressure (mmHg) | 63562 | 126 (18) | 18004 | 128 (18) | 2866 | 129 (18) | <0.001 |
| Alcohol use (%) | <0.001 | ||||||
| Never drinker | 6239 | 10 | 2405 | 13 | 490 | 17 | |
| Past drinker | 11156 | 17 | 3595 | 20 | 672 | 23 | |
| Current drinker | 46214 | 73 | 12015 | 67 | 1707 | 60 | |
| Smoking status (%) | <0.001 | ||||||
| Never | 31870 | 50 | 9475 | 53 | 1535 | 54 | |
| Former | 28210 | 44 | 7132 | 40 | 1104 | 39 | |
| Current | 3529 | 6 | 1408 | 8 | 230 | 8 | |
| Physical activity (METs/wk) | 63609 | 15 (15) | 18015 | 12 (13) | 2869 | 11 (14) | <0.001 |
| Education (%) | <0.001 | ||||||
| ≤ High school / GED | 11824 | 19 | 4755 | 26 | 850 | 30 | |
| Some college | 22669 | 36 | 6928 | 39 | 1143 | 40 | |
| College graduate | 29116 | 46 | 6332 | 35 | 876 | 31 | |
| Energy intake (kcal/day) | 63609 | 1503 (545) | 18015 | 1726 (652) | 2869 | 2134 (847) | <0.001 |
| Fiber intake (g/1000 kcal) | 63609 | 11 (4) | 18015 | 10 (3) | 2869 | 9 (3) | <0.001 |
| Fruit and Vegetable intake (%kcal) | 63609 | 4.5 (2.2) | 18015 | 4.0 (2.1) | 2869 | 4.1 (2.2) | <0.001 |
| SFA intake (%kcal) | 63609 | 9.6 (3.3) | 18015 | 11.2 (3.1) | 2869 | 11.6 (3.1) | <0.001 |
| TFA intake (%kcal) | 63609 | 1.9 (1.0) | 18015 | 2.5 (1.1) | 2869 | 2.9 (1.0) | <0.001 |
| DHA+EPA intake (%kcal) | 63609 | 0.1 (0.1) | 18015 | 0.1 (0.1) | 2869 | 0.1 (0.1) | <0.001 |
| ALA intake (%kcal) | 63609 | 0.7 (0.3) | 18015 | 0.7 (0.3) | 2869 | 0.8 (0.3) | <0.001 |
| α-Linoleic Acid (%kcal) | 63609 | 5.3 (1.9) | 18015 | 6.2 (1.9) | 2869 | 6.5 (1.9) | <0.001 |
WHI blood subsample participants only (n=955). Means and SD's weighted by ethnicity.
Coronary artery disease (CAD), saturated fatty acids (SFA), trans fatty acids (TFA), eicosapentaenoic acid (EPA) + docosahexaenoic acid (DHA), alpha-linolenic acid (ALA).
Results
Baseline Characteristics
High baked/broiled fish consumption was associated with younger age, lower prevalence of diabetes, atrial fibrillation, coronary artery disease (CAD), lower systolic blood pressure (SBP), lower body mass index (BMI), and higher physical activity, higher education, higher consumption of fruits and vegetables, higher dietary intake of DHA/EPA, and lower dietary intake of SFA and TFA (Table 1). High consumption of fried fish/fish sandwich was associated with higher prevalence of diabetes, AF, CAD, higher SBP, higher BMI, higher prevalence of smoking, lower physical activity, lower education, lower fiber intake, lower fruit/vegetable intake, higher energy intake, and higher SFA, TFA, and ALA intake (Table 2). Baseline characteristics by categories of fatty acid intake are summarized in the data supplement (Supplemental Tables 1–3).
Fish Intake and Incident HF
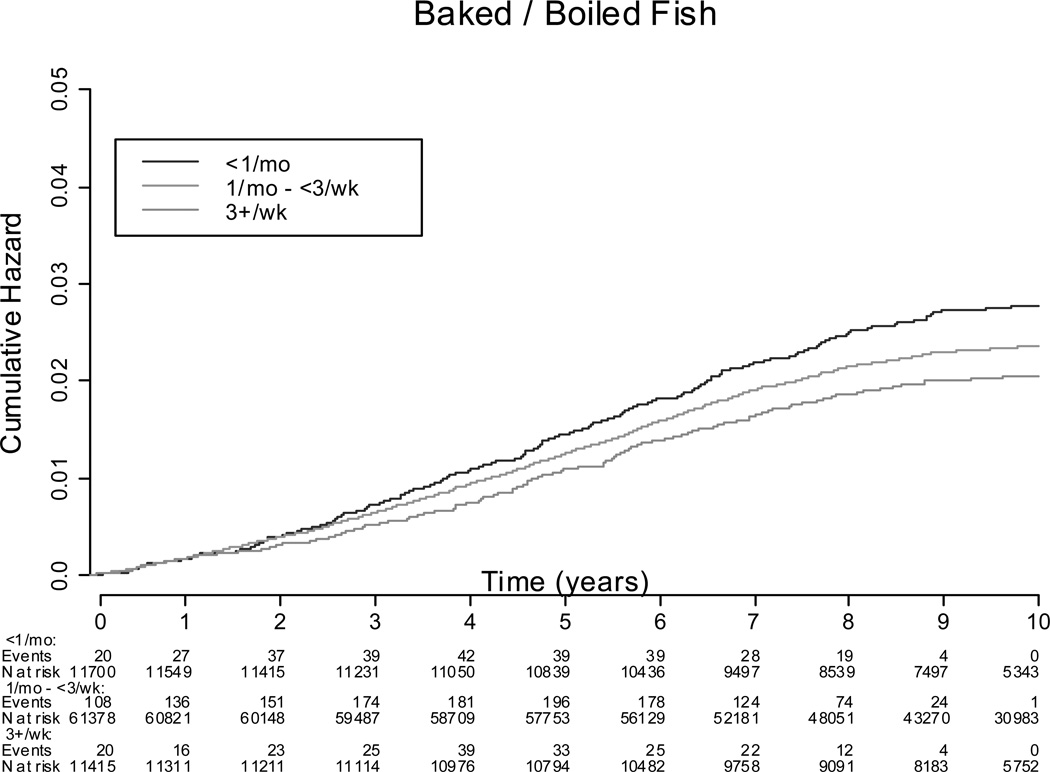
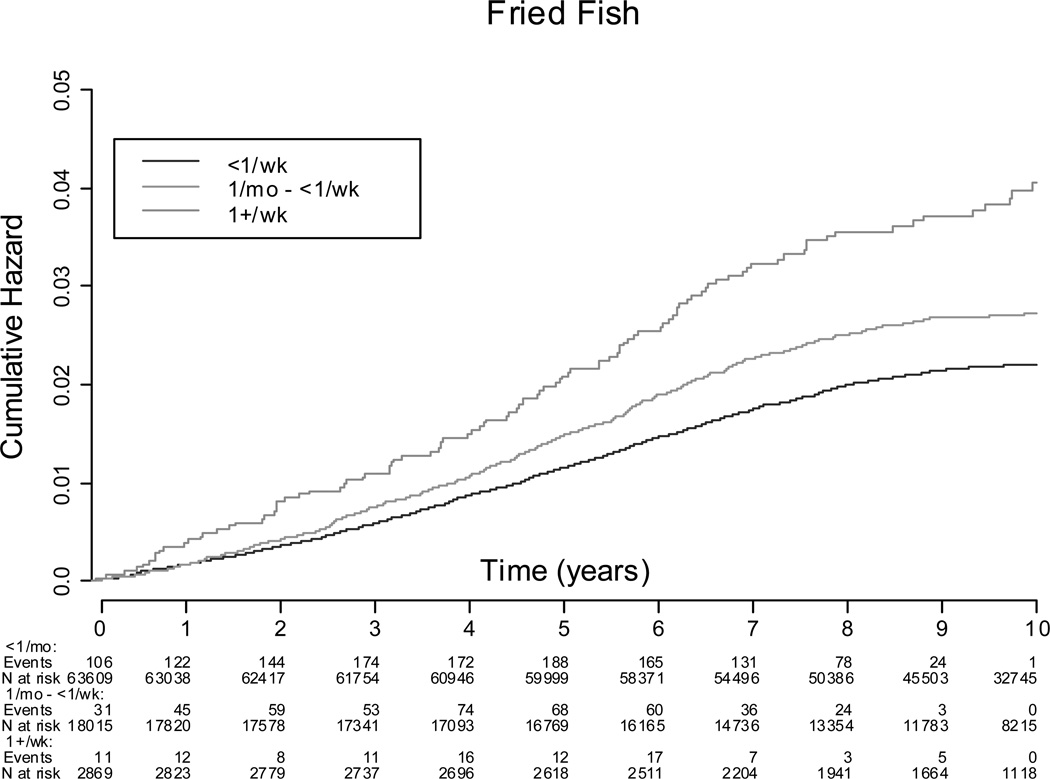
There were 1,858 incident cases (2.2% of cohort) of HF during an average of 10.0 years of follow up. The incidence of HF in women who consumed <1 servings/week of baked/broiled fish was 26.7 per 10,000 person-years, compared to 19.4 per 10,000 person-years in women who consumed ≥5 servings/week. The incidence of HF in women consuming fried fish ≤1 serving/month was 20.8 per 10,000 person-years compared to 39.4 per 10,000 person-years in women consuming fried fish ≥1 serving/week. Kaplan-Meier survival functions illustrating the probability of incident HF according to baked/broiled fish and fried fish intake categories is summarized in Figures 1 and 2. In the adjusted Cox models adjusting for traditional HF risk factors including interim MI during follow-up and dietary constituents (fiber, fruit and vegetable, fatty acid intake, fried food intake, and sodium intake) (model 1), a significant trend of increasing baked/broiled fish intake was associated with lower risk of HF, with the greatest reduction in risk associated with consumption of ≥5 servings of baked/broiled fish/wk (Table 3). In further analysis by type of baked/broiled fish, a significant trend of increasing baked/broiled dark fish intake (including salmon, mackerel, and bluefish) was associated with a lower HF risk, with the greatest reduction in risk associated with consumption of ≥1 servings/wk of dark baked/broiled fish/wk, while consumption of >1 serving/wk of baked/broiled white fish (e.g. sole, snapper, cod) or tuna fish was not (Table 4). Increasing consumption of fried fish increased HF risk in a dose-dependent manner, with consumption of ≥1 serving of fried fish/fish sandwich per week associated with the greatest increase in HF risk (Table 3). No significant differences in the hazard ratio were noted between models adjusting for HF risk factors and models adjusting for both HF risk factors and dietary factors (Supplement Tables 4 and 5).
Figure 1.
Kaplan-Meier Survival Function Curves for Cumulative Hazard of Heart Failure by Fried Fish Intake.
Figure 2.
Kaplan-Meier Survival Function Curves for Cumulative Hazard of Heart Failure by Fried Fish Intake.
Table 3.
Multivariable hazard ratios for incident HF based on baked/broiled and fried fish categories
| <1/month | 1–3/month | 1–2/week | 3–4/week | ≥5/week | Trend P- value |
|
|---|---|---|---|---|---|---|
| Baked/broiled fish | ||||||
| Number of participants | 11700 | 26344 | 35034 | 8109 | 3306 | |
| Person-years | 110243 | 253778 | 346142 | 80016 | 32282 | |
| Number of HF events | 294 | 646 | 700 | 167 | 51 | |
| Incidence Rates (per 10,000 person-years) | 26.7 | 25.5 | 20.3 | 20.9 | 19.4 | |
| Model 1* | 1.00 | 1.03 (0.89, 1.18) | 0.89 (0.77, 1.02) | 0.99 (0.80, 1.21) | 0.70 (0.51, 0.95) | 0.022 |
| <1/month | 1–3/month | ≥1/week | ||||
| Fried Fish | ||||||
| Number of participants | 63609 | 18015 | 2869 | |||
| Person-years | 625924 | 170610 | 25928 | |||
| Number of events | 1305 | 451 | 102 | |||
| Incidence Rates (per 10,000 person-years) | 20.8 | 26.4 | 39.4 | |||
| Model 2† | 1.00 | 1.06 (0.95, 1.19) | 1.48 (1.19, 1.84) | 0.005 | ||
Adjusted for age, ethnicity, education, physical activity, smoking, alcohol, diabetes, hypertension, AF, MI/CABG/PTCA, BMI, time-dependent MI, fiber, fruit/vegetable servings, fried fish servings, saturated fat intake (%), DHA+EPA (%), linolenic acid (ALA, %), linoleic acid (%),fried food servings, sodium intake (mg)
Identical to model 1, except adjustment for non-fried fish servings
Table 4.
Multivariable hazard ratios for incident HF based on Subtype of Baked/Broiled Fish (White Fish, Dark Fish, Tuna Fish)
| <1/month | 1–3/month | ≥1/week | Trend P-value | |
|---|---|---|---|---|
| White fish (sole, snapper, cod) | ||||
| Number of participants | 45161 | 32327 | 7005 | |
| Person-years | 433704 | 319729 | 69029 | |
| Number of HF events | 1064 | 653 | 141 | |
| Incidence Rates (per 10,000 person-years) | 24.5 | 20.4 | 20.4 | |
| Model 1* | 1.00 | 0.91 (0.82, 1.01) | 0.95 (0.79, 1.15) | 0.173 |
| <1/month | 1–3/month | ≥1/week | Trend P-value | |
| Dark fish (salmon, mackerel, bluefish) | ||||
| Number of participants | 59578 | 20817 | 4098 | |
| Person-years | 577541 | 205147 | 39773 | |
| Number of HF events | 1416 | 379 | 63 | |
| Incidence Rates (per 10,000 person-years) | 24.5 | 18.5 | 15.8 | |
| Model 1* | 1.00 | 0.89 (0.79, 1.00) | 0.78 (0.61, 1.02) | 0.012 |
| <1/month | 1–3/month | ≥1/week | Trend P-value | |
| Tuna fish | ||||
| Number of participants | 35842 | 39882 | 8769 | |
| Person-years | 346411 | 390854 | 85197 | |
| Number of HF events | 814 | 867 | 177 | |
| Incidence Rates (per 10,000 person-years) | 23.5 | 22.2 | 20.8 | |
| Model 1* | 1.00 | 0.95 (0.86, 1.05) | 0.92 (0.77, 1.09) | 0.233 |
Adjusted for age, ethnicity, education, physical activity, smoking, alcohol, diabetes, hypertension, AF, MI/CABG/PTCA, BMI, time-dependent MI, fiber, fruit/vegetable servings, fried fish servings, saturated fat intake (%), DHA+EPA (%), linolenic acid (ALA, %), linoleic acid (%), fried food servings, sodium intake (mg)
In race-stratified analyses, a significant trend toward lower risk of HF with increasing baked/broiled fish intake was found for white but not black participants (Table 5). Consumption of ≥5 servings of baked/broiled fish/wk was associated with a HR of 0.74 (95% CI: 0.53–1.03) in white participants and a HR 0.85 (95% CI: 0.34–2.08) in black participants. However, evidence of more similar trends between increasing fried fish/fish sandwich consumption and increased HF risk was found for white and black participants, although the p-value for the trend for black women did not quite achieve significance. Consumption of >1 serving of fried fish/fish sandwich per week was linked with an increased risk for HF in blacks and white participants (Table 5). In further analyses, evaluating consumption of both types of fish preparation, women consuming less than 1 serving of baked/broiled fish per month and more than 1 serving of fried fish per week had a 2.3 fold higher risk for incident HF relative to women consuming less than 1 serving of baked/broiled fish/wk and less than 1 serving of fried fish per month (HR 2.28, 95%CI: 1.38, 3.75) (Supplemental Figure 1, Supplemental Table 6).
Table 5.
Multivariable hazard ratios for incident HF based on baked/broiled and fried fish categories by ethnicity
|
Baked/Broiled Fish | ||||||
|---|---|---|---|---|---|---|
| <1/month | 1–3/month | 1–2/week | 3–4/week | ≥5/week | Trend P-value | |
| White participants | ||||||
| Number of participants | 9349 | 22050 | 30438 | 7013 | 2695 | |
| Person-years | 9038 | 216363 | 305209 | 70344 | 26898 | |
| Number of HF events | 247 | 572 | 614 | 147 | 44 | |
| Model 1* | 1.00 | 1.05 (0.90, 1.22) | 0.87 (0.74, 1.01) | 0.99 (0.80, 1.23) | 0.74 (0.53, 1.03) | 0.022 |
| Black participants | ||||||
| Number of participants | 1246 | 2088 | 2051 | 487 | 281 | |
| Person-years | 10596 | 18209 | 18018 | 4198 | 2516 | |
| Number of HF events | 33 | 49 | 55 | 12 | 6 | |
| Model 1* | 1.00 | 0.91 (0.58, 1.42) | 1.05 (0.67, 1.64) | 1.03 (0.52, 2.06) | 0.85 (0.34, 2.08) | 0.946 |
|
Fried Fish | ||||||
| <1/month | 1–3/month | ≥1/week | Trend P-value | |||
| White participants | ||||||
| Number of participants | 56002 | 13862 | 1681 | |||
| Person-years | 558228 | 134991 | 15976 | |||
| Number of HF events | 1187 | 373 | 64 | |||
| Model 2† | 1.00 | 1.07 (0.95, 1.21) | 1.41 (1.09, 1.82) | 0.018 | ||
| Black participants | ||||||
| Number of participants | 3066 | 2320 | 767 | |||
| Person-years | 27068 | 20053 | 6417 | |||
| Number of HF events | 70 | 55 | 30 | |||
| Model 2† | 1.00 | 1.03 (0.71, 1.50) | 1.75 (1.09, 2.83) | 0.080 | ||
Adjusted for age, ethnicity, education, physical activity, smoking, alcohol, diabetes, hypertension, AF, MI/CABG/PTCA, BMI, time-dependent MI, fiber, fruit/vegetable servings, fried fish servings, saturated fat intake (%), DHA+EPA (%), linolenic acid (ALA, %), linoleic acide (mg)
Similar to model 1, except adjustment for non-fried fish servings
Fatty Acid Intake and Incident HF
The risk of incident HF in women consuming the highest amounts of EPA + DHA was no longer significant after adjustment for HF risk factors and did not change after further adjustment for dietary constituents in caloric adjusted models (Supplemental Table 7) or unadjusted models (Supplemental Table 8). Similarly, no relationship was found between ALA or TFA consumption and HF in caloric adjusted or unadjusted models (Supplemental Tables 7–10).
Discussion
The goal of this paper was to determine whether fish as a complete food or its method of preparation is associated with the risk for incident HF. We found that increasing baked/broiled fish intake was associated with decreased HF risk, primarily due to the consumption of baked/broiled dark fish such as salmon, mackerel, and bluefish, while fried fish consumption once a week or more was associated with increased HF risk.
Fish Intake and Heart Failure
Clinical and epidemiologic data suggest that postmenopausal women experience a greater burden of HF and that, in the future, the prevalence of HF in this population will increase.3,4 Thus, current nutritional epidemiologic data elucidating dietary measures which may be associated with increased or decreased risks for HF in postmenopausal women are important. We found that consumption of ≥5 servings of baked/broiled fish per week was independently associated with lower risk for incident HF by 30%, while consumption of more than 1 serving of fried fish per week was independently associated with increased HF risk by 48% in postmenopausal women. Our results compare favorably to those in elderly, white and black subjects in the Cardiovascular Health Study (CHS) in which it was reported that subjects consuming baked/broiled fish ≥5 times per week had a 32% lower risk for incident HF, while consumption of fried fish at least once/week was associated with 35% higher risk for incident.13 Mozzaffarian and colleagues also found a significant 20% lower risk of HF in subjects consuming baked/broiled fish at least once per week, a finding which we did not observe.13 Similarly, a recent study illustrated that moderate consumption of fatty fish (1–2 times per week) was associated with lower HF risk, while more frequent servings were not in a Swedish cohort of women.14 These differences may relate to lack of adjustment for potential confounders such as intake of α-linolenic acid, α-linoleic acid, trans fatty acid, or intake of other fried foods (e.g. fried chicken) that could have exerted an effect particularly at moderate levels of fish intake in those studies.
Our data extend findings from the previous study in the CHS13 in several ways. First, we adjusted for incident MI during the follow-up period. Such adjustment is essential as it is known that MI is a strong predictor of future HF in many populations.1 Moreover, observational and clinical studies have shown that increased fish intake lowers the risk for coronary artery disease.15–17 Taken together, it can be concluded that the associations we observe between fish intake and heart failure are independent of incident MI occurring during the follow-up period. Additionally, we adjusted for consumption of other fried foods such as French fries, fried potatoes, and fried chicken. Also, we extend this previous study13 by finding that consumption of baked/broiled dark fish (e.g. salmon, mackerel, bluefish) was associated with a significant 22% lower risk for incident HF, while consumption of baked/broiled white fish (e.g. sole, snapper, cod) or tuna fish was not. This finding suggests that dark fish such as salmon may be a key fish meal that significantly lowers the risk for incident HF. Salmon seems the most likely candidate based on its omega-3 fatty acid content (1.1–1.9g/3oz serving), which is the highest among all fish meals considered and its likely higher availability and consequent consumption compared to mackerel or bluefish.18 This report also provides new information on fish intake and risk for HF based on race in postmenopausal women. Our findings suggest future studies should examine racial and ethnic differences in nutritional influences on the development of HF. Although the lack of a significant beneficial association between baked/broiled fish and HF in black women may have resulted from a smaller number of black women in our study, the relationship between fried fish consumption and increased HF risk in black women was of similar magnitude as in white women and approached statistical significance. This may be of particular importance since the risk of HF is highest in black individuals, largely related to hypertension and diabetes rather than to ischemic causes as in whites.1, 19
Several mechanisms have been proposed for the protective associations between fish consumption on cardiovascular disease. A 2006 study in the elderly found that increased consumption of baked and broiled fish, including tuna, is linked with lower blood pressure, systemic vascular resistance, markers of inflammation and increases arterial compliance, stroke volume, and diastolic filling (E/A ratio).20 In contrast, higher consumption of fried fish was associated with higher blood pressure, vascular resistance, wall motion abnormalities, and reduced stroke volume.20 These data provide the potential physiologic basis by which consumption of baked/broiled or fried fish affects risk for HF.
Fatty Acid Intake and Heart Failure
Fatty fish (e.g. mackerel, tuna, herring, and salmon), are rich sources of the omega-3 fatty acids: EPA and DHA, both of which mediate the above mentioned cardioprotective effects of fish.5,15 In addition, increased intake of vegetable sources of omega-3 fat, specifically α-linolenic acid (ALA) has been shown to be inversely related to the risk for incident CHD.21 The risk reduction has been suggested to involve similar mechanisms as fish oils (e.g. lower inflammation, blood pressure, atherogenic blood lipids, and arrythmogenesis).5,22 Increased intake of ALA slightly increases DHA/EPA availability via the desaturation-chain elongation pathway due to inefficient conversion and is not the primary means of increasing DHA/EPA levels.23 ALA is found in high quantities in vegetable oils such as canola, soybean, and flaxseed oil and are consumed much more frequently than marine omega-3 fatty acid rich foods (i.e. fish).15 However, we did not find any independent associations between intake of EPA+DHA or ALA as estimated from the WHI-FFQ and the risk for incident HF, in contrast to the significant associations we found between consumption of fish foods and HF risk. Our findings contrast with studies in the CHS, which reported a 37% risk reduction in HF risk in elderly subjects consuming >487mg/day of EPA+DHA.13 Also, our results contrast with work by Levitan et al who reported a dose dependent reduction in HF risk with increasing consumption of marine omega-3 fatty acids.14 The difference between their study and ours may be related to several factors, including differences in populations examined, as well as geographic and cultural differences in type of fatty fish meal preferred. Of note, Levitan et al included data regarding marine fish oil intake in the form of fish oil supplements, data which were not available from the WHI-FFQ. In addition, observational cohort studies suggest that increased consumption of TFA is causally associated with an increased risk for incident CHD.6 Frying foods increases the trans fatty acid content, which may account for the increased HF risk with higher fried fish consumption in our study.24 However, we did not find a positive association between TFA and HF risk here.
How fish consumption could be associated with risk for heart failure, while omega-3 fatty acids or trans fatty acids are not, raises a challenging question. One plausible explanation for the differences between our study and others13 relates to the known over- and under- reporting of food and nutrient intake associated with the FFQ.9 Differences in statistical modeling represent another possible explanation. This investigation controlled for other potential confounders of the association between marine omega-3 fatty acid and HF such as TFA, ALA, and α-linoleic acid intake. We chose to adjust for α-linoleic acid because previous studies indicate that its intake is tightly correlated with that of ALA.11 Moreover, it is known that ALA and α-linoleic acid compete as substrates for the same desaturase enzymes and thus high levels of α-linoleic acid may decrease the bioavailability of ALA and/or its metabolites (e.g. DHA/EPA).12 Third, it may be that fish, as a complete food, exerts a more robust impact on HF risk due to synergism between fish food nutrients which collectively modulates cardiovascular physiology and thus significantly affects risk for HF. Indeed, studies by Hu and colleagues reported that fish intake was more strongly linked with lower risk of coronary artery disease (CAD) than intake of omega-3 fatty acids in the Nurses’ Health Study.25 It may be the case that the lack of association is due to changes in dietary patterns during the follow-up period. If women adopted healthier lifestyles during the follow up period such as consuming less trans fatty acid and more omega-3 fatty acids, an underestimation of the associations between fatty acid intake and HF might be expected. In the future, some serum biomarkers of nutrient status can help to address these problems. Indeed, serum and red blood cell measures of omega-3 fats and trans fatty acids have been reported to be strong risk predictors of future cardiovascular disease events.26
Limitations
The Food Frequency Questionnaire provides an estimated intake of foods that are grouped together in ways that do not allow specific energy or nutrient quantification. Specifically, we do not have information on the method of preparation of the fried fish. Though we adjusted for sodium intake, we did not have information on the amount of salt study participants added to meals. Also differences in brand names or unconventional preparation methods are not documented. We cannot discount error in the adjudication of HF as a potential source of error. Moreover, we were unable to discern the exact casual pathways linking dietary intake and incidence of HF in the WHI. While an association between omega-3 fatty acid intake and incident HF was not found, as had been reported in previous studies14, it is possible that the lack of such an association may relate, in part, to the lack of supplemental omega-3 fatty acid intake, which was not assessed in the WHI-FFQ. Finally, while high intake of baked/broiled fish was found to be associated with lower risk for HF, it may be that increased intake of baked fish or decreased intake of fried fish are surrogate markers for overall healthy lifestyle. This postulate is supported by our finding that greater intake of baked/broiled fish (or reduced intake of fried fish) is associated with lower BMI, lower SBP, lower current smoking, increased physical activity, increased consumption of fruits and vegetables, and decreased consumption of SFA and TFA and that residual confounding of the association between fish/fatty acid intake and HF exists.
Strengths
The large sample size and ethnic and socioeconomic diversity of the WHI-OS provides a representative study sample for analysis of these food and nutrient exposures and risk for HF among an understudied subgroup of the population, postmenopausal women. Second, the prospective design of our study reduces the likelihood of recall bias compared to case-control studies. Third, the focused and attentive follow-up and peripheral adjudication of HF events reduces the chances of outcome misclassification. Also, the WHI-FFQ was designed to be especially sensitive to fat intake and took into account regional and ethnic eating patterns in the US to a degree that other studies have not. The WHI-FFQ quantifies foods and fatty acid intake and differentiates the type (e.g. white vs. dark fish vs. tuna fish) and preparation of fish meal consumed which allows more detailed analyses of fats used in fish preparation9 thereby allowing the examination of the association between the type of baked/broiled fish meal consumed, the method of fish preparation (fried vs. baked/broiled), and risk for incident HF, which, until now had not been shown. Additionally, the WHI-FFQ provided data on other fried food servings (e.g. fried chicken, French fries, and fried potatoes). Standardized, longitudinal dietary assessment and food group analyses utilized in WHI as well as adjustment for interim MI during follow-up may have offered advantages over previous work in this area.13 Finally, to our knowledge, this is the first study to examine the association between ALA, DHA+EPA, TFA intake, and HF risk. While no associations were found, future studies are needed using serum and red blood cell biomarkers of omega-3 and TFA status to clearly elucidate whether and to what degree altered status of these fatty acids plays a key role in modulating cardiovascular pathophysiology and future risk for incident HF.
Summary
These findings indicate that, in a diverse population of postmenopausal women, a population known to be at increased risk for HF3–5, higher intake of baked/broiled fish is associated with a lower risk for incident heart failure, while higher intake of fried fish is linked with a higher risk for incident heart failure. These studies suggest the possibility that dietary modification through increased baked/broiled fish, specifically dark fish (salmon, mackarel, blue fish) and/or decreased fried fish consumption could plausibly lower the risk for HF in postmenopausal women. However, in the future, randomized clinical trials are needed to unequivocally clarify the link between fish/fatty acid intake and risk for incident.
Supplementary Material
Acknowledgments
Sources of Funding
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221. Dr. Rashad Belin was supported by a National Heart, Lung, and Blood Institute training grant (T32 HL069771).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
References
- 1.Lloyd-Jones D, Adams R, Carnethon M. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Executive summary: heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:948–954. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones DM, Larson MG, Leip EP, Beiser A, D'Agostino RB, Kannel WB, Murabito JM, Vasan RS, Benjamin EJ, Levy D. Lifetime risk for developing chronic heart failure: the Framingham Heart Study. Circulation. 2002;106:3068–3072. doi: 10.1161/01.cir.0000039105.49749.6f. [DOI] [PubMed] [Google Scholar]
- 3.Ghali JK, Krause-Steinrauf HJ, Adams KF, Khan SS, Rosenberg YD, Yancy CW, Young JB, Goldman S, Peberdy MA, Lindenfeld J. Gender differences in advanced heart failure: insights from the BEST study. J Am Coll Cardiol. 2003;42:2128–2134. doi: 10.1016/j.jacc.2003.05.012. [DOI] [PubMed] [Google Scholar]
- 4.Yancy CW. Heart failure in African Americans. Am J Cardiol. 2005;96:3i–12i. doi: 10.1016/j.amjcard.2005.07.028. [DOI] [PubMed] [Google Scholar]
- 5.Wijendran V, Hayes KC. Dietary n-6 and n-3 fatty acid balance and cardiovascular health. Annu Rev Nutr. 2004;24:597–615. doi: 10.1146/annurev.nutr.24.012003.132106. [DOI] [PubMed] [Google Scholar]
- 6.Mozaffarian D, Katan MB, Ascherio A, Stampfer MJ, Willett WC. Trans fatty acids and cardiovascular disease. N Engl J Med. 2006;354:1601–1613. doi: 10.1056/NEJMra054035. [DOI] [PubMed] [Google Scholar]
- 7.Curb JD, McTiernan A, Heckbert SR, Kooperberg C, Stanford J, Nevitt M, Johnson KC, Proulx-Burns L, Pastore L, Criqui M, Daugherty S. Outcomes ascertainment and adjudication methods in the Women's Health Initiative. Ann Epidemiol. 2003;13:S122–S128. doi: 10.1016/s1047-2797(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 8.Design of the Women's Health Initiative clinical trial and observational study. The Women's Health Initiative Study Group. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 9.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women's Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9:178–187. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 10.Djousse L, Arnett DK, Pankow JS, Hopkins PN, Province MA, Ellison RC. Dietary linolenic acid is associated with a lower prevalence of hypertension in the NHLBI Family Heart Study. Hypertension. 2005;45:368–373. doi: 10.1161/01.HYP.0000154679.41568.e6. [DOI] [PubMed] [Google Scholar]
- 11.Hu FB, Stampfer MJ, Manson JE, Ascherio A, Colditz GA, Speizer FE, Hennekens CH, Willett WC. Dietary saturated fats and their food sources in relation to the risk of coronary heart disease in women. Am J Clin Nutr. 1999;70:1001–1008. doi: 10.1093/ajcn/70.6.1001. [DOI] [PubMed] [Google Scholar]
- 12.Calder PC. Conjugated linoleic acid in humans--reasons to be cheerful? Curr Opin Clin Nutr Metab Care. 2002;5:123–126. doi: 10.1097/00075197-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Mozaffarian D, Bryson CL, Lemaitre RN, Burke GL, Siscovick DS. Fish intake and risk of incident heart failure. J Am Coll Cardiol. 2005;45:2015–2021. doi: 10.1016/j.jacc.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 14.Levitan EB, Wolk A, Mittleman MA. Fatty fish, marine omega-3 fatty acids and incident heart failure. Eur J Clin Nutr. 2010;64:587–594. doi: 10.1038/ejcn.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish, oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–2757. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 16.Burr ML, Fehily AM, Gilbert JF, Rogers S, Holliday RM, Sweetnam PM, Elwood PC, Deadman NM. Effects of changes in fat fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART) Lancet. 1989;2:757–761. doi: 10.1016/s0140-6736(89)90828-3. [DOI] [PubMed] [Google Scholar]
- 17.Daviglus ML, Stamler J, Orencia AJ, Liu K, Greenland P, Walsh MK, Morris D, Shekelle RB. Fish consumption and the 30-year risk of fatal myocardial infarction. N Engl J Med. 1997;336:1046–1053. doi: 10.1056/NEJM199704103361502. [DOI] [PubMed] [Google Scholar]
- 18.Kris-Etherton PM, Grieger JA, Etherton TD. Dietary reference intakes for DHA and EPA. Prostaglandins Leukotrienes and Essential Fatty Acids. 2009;81:99–104. doi: 10.1016/j.plefa.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 19.Bahrami H, Kronmal R, Bluemke DA, Olson J, Shea S, Liu K, Burke GL, Lima JA. Differences in the Incidence of Congestive Heart Failure by Ethnicity: The Multi-Ethnic Study of Atherosclerosis. Arch Intern Med. 2008;168:2138–2145. doi: 10.1001/archinte.168.19.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mozaffarian D, Gottdiener JS, Siscovick DS. Intake of tuna or other broiled or baked fish versus fried fish and cardiac structure, function, and hemodynamics. Am J Cardiol. 2006;97:216–222. doi: 10.1016/j.amjcard.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 21.Baylin A, Kabagambe EK, Ascherio A, Spiegelman D, Campos H. Adipose tissue alpha-linolenic acid and nonfatal acute myocardial infarction in Costa Rica. Circulation. 2003;107:1586–1591. doi: 10.1161/01.CIR.0000058165.81208.C6. [DOI] [PubMed] [Google Scholar]
- 22.Rallidis LS, Paschos G, Liakos GK, Velissaridou AH, Anastasiadis G, Zampelas A. Dietary alpha-linolenic acid decreases C-reactive protein, serum amyloid A and interleukin-6 in dyslipidaemic patients. Atherosclerosis. 2003;167:237–242. doi: 10.1016/s0021-9150(02)00427-6. [DOI] [PubMed] [Google Scholar]
- 23.Emken EA, Adlof RO, Gulley RM. Dietary linoleic acid influences desaturation and acylation of deuterium-labeled linoleic and linolenic acids in young adult males. Biochim Biophys Acta. 1994;1213:277–288. doi: 10.1016/0005-2760(94)00054-9. [DOI] [PubMed] [Google Scholar]
- 24.Ammu K, Raghunath MR, Sankar TV, Lalitha KV, Devadasan K. Repeated use of oil for frying fish. Effects of feeding the fried fish to rats. Nahrung. 2000;44:368–372. doi: 10.1002/1521-3803(20001001)44:5<368::AID-FOOD368>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 25.Hu FB, Cho E, Rexrode KM, Albert CM, Manson JE. Fish and long chain omega-3 fatty acid intake and risk of coronary heart disease and total mortality in diabetic women. Circulation. 2003;107:1852–1857. doi: 10.1161/01.CIR.0000062644.42133.5F. [DOI] [PubMed] [Google Scholar]
- 26.Lemaitre RN, King IB, Raghunathan TE, Pearce RM, Weinmann S, Knopp RH, Copass MK, Cobb LA, Siscovick DS. Cell membrane trans-fatty acids and the risk of primary cardiac arrest. Circulation. 2002;105:697–701. doi: 10.1161/hc0602.103583. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.