Abstract
Vitamin C (l-ascorbate, AsA) is an essential nutrient required in key metabolic functions in humans and must be obtained from the diet, mainly from fruits and vegetables. Given its importance in human health and plant physiology we sought to examine the role of the ascorbate recycling enzymes monodehydroascorbate reductase (MDHAR) and dehydroascorbate reductase (DHAR) in tomato (Solanum lycopersicum), an economically important fruit crop. Cytosolic-targeted tomato genes Mdhar and Dhar were cloned and over-expressed under a constitutive promoter in tomato var. Micro-Tom. Lines with increased protein levels and enzymatic activity were identified and examined. Mature green and red ripe fruit from DHAR over-expressing lines had a 1.6 fold increase in AsA content in plants grown under relatively low light conditions (150 µmol m−2 s−1). Conversely, MDHAR over-expressers had significantly reduced AsA levels in mature green fruits by 0.7 fold. Neither over-expressing line had altered levels of AsA in foliar tissues. These results underscore a complex regulation of the AsA pool size in tomato.
1. Introduction
Ascorbate (AsA, vitamin C) is synthesized in a number of taxa in the plant kingdom including unicellular algae [1], macroalgae [2], and multicellular plants [3]. Within the plant cell, reactive oxygen species (ROS) are formed from internal biological reactions and triggered by external factors such as biotic and abiotic stresses. Regardless of their source, ROS can cause oxidative damages to proteins, nucleic acids, and lipid membranes, compromising cellular and organismal viability. The major antioxidant in plants poised to deal with ROS is AsA [4], being present at millimolar levels. In animals, vitamin C serves as a cofactor for the hydroxylation of proline and pro-collagen involved in the formation of normal structures of subcutaneous tissue, cartilage, bone and teeth and is implicated in proper immune system functioning, wound healing, allergic defenses, neurodegenerative and cardiovascular diseases, and even cancer [5–10]. In plants, AsA is also essential for the hydroxylation of proline as in the case of extensin and arabinogalactan proteins, and is also required for gibberellins and ethylene synthesis in reactions catalyzed by specific AsA-dependent dioxygenases [11, 12]. However, several lineages of invertebrates, insects, fish, some birds, flying mammals, and primates lack the capacity to synthesize AsA [13] which must be obtained from dietary sources. Thus, AsA is an important vitamin, especially in populations where antioxidant nutrients may be limiting. Understanding the synthesis and maintenance of vitamin C in plant sources would facilitate the development of nutrient rich foods by biotechnological or traditional breeding methods.
There are several published reports, as well as reviews, on the over-expression of AsA biosynthetic genes [14–25]. Of particular interest are the studies showing an increase in AsA or redox ratios via the over-expression of the enzymes that recycle oxidized forms of ascorbate, monodehydroascorbate reductase (NADH:monodehydroascorbate oxidoreductase; EC 1.6.5.4; MDHAR) and dehydroascorbate reductase (glutathione:dehydroascorbate oxidoreductase; EC 1.8.5.1; DHAR) (Table 1). Published literature on this topic has shown that AsA increases of 2–4 fold are possible in photosynthetic tissue and 1.5–6 fold can be attained in storage tissues such as potato tubers [26] and maize kernels [23], respectively. The majority of studies to date have focused on photosynthetically active tissues and mostly in model plants such as Arabidopsis thaliana [24, 25] and tobacco (Nicotiana tabacum) [14–16, 21]. To our knowledge, reports of over-expressing DHAR in a fleshy fruit like tomato are absent from the literature, as are over-expression of MDHAR in any storage organ. The effects of over-expressing these recycling genes in a nutritionally and economically important fruit crop, such as tomato, provide both fundamental and applied insights into strategies to modify AsA levels.
Table 1.
Summary of transgenic strategies to stably engineer elevated ascorbate levels in plants by overexpressing MDHAR or DHAR.
| Target | Plant engineered |
Enzyme | Gene source |
Promoter used |
Fold Increase in total AsA |
Other effects of modification / comments |
|---|---|---|---|---|---|---|
| Leaf Tissue | ||||||
| Tobacco[42] | Chl-DHAR | Human | NR | 0.84 | Increased GR activity 2X increase in AsA redox ratio Paraquat, cold and NaCl tolerance |
|
| Tobacco[21] | Chl-DHAR | Human | NR | 1.1 | Increased SOD and APX activities in conjunction via triple gene construct Enhanced GSH levels Paraquat, and NaCl tolerance |
|
| Tobacco[15] | DHAR | Arabidopsis | CaMV35S | 1.9–2.1 | Ozone, PEG, NaCl, and drought tolerance | |
| Arabidopsis[27] | DHAR | Rice | CaMV35S | 1.1–1.2 | NaCl tolerance | |
| Tobacco[29] | DHAR | Arabidopsis | CaMV35S | 1.2–1.3 | Aluminum tolerance in roots | |
| Arabidopsis[24] | DHAR | Arabidopsis | CaMV35S | 3.3 | Increased GSH level Paraquat, high light and temperature tolerance |
|
| Arabidopsis[25] | Chl-MDHAR | Tomato | CaMV35S | 1.2 | Paraquat, low and high temperature stress tolerance | |
| Tobacco[29] | MDHAR | Arabidopsis | CaMV35S | 1.1 | No significant responses | |
| Tobacco[29] | MDHAR | Arabidopsis | CaMV35S | 1.1 | No significant responses | |
| Tobacco[16] | MDHAR | Arabidopsis | CaMV35S | 2.0–2.2 | NaCl and PEG stress tolerance | |
| Storage organ | ||||||
| Maize[23] | DHAR | Rice | D-horderin | 6.2 | Increased folate and β-carotene levels engineered in conjunction via triple gene construct | |
| Leaf & storage organ | ||||||
| Potato[28] | Chl-DHAR, DHAR | Potato | CaMV35S | 1.1–1.2 | Increased AsA in leaves and tubers of cytosolic targeted Increased AsA only in leaves of chloroplast targeted |
|
| Potato[26] | DHAR | Sesame | Patatin CaMV35S |
1.1–1.3 1.5–1.6 |
Increased AsA in tuber only with patatin promoter Increased AsA in leaves and tuber with CaMV35S promoter |
|
| Tomato* | DHAR | Tomato | FMV34S | 1.5–1.6 | No increase in foliar AsA Increased AsA in mature green and red-ripe fruit |
|
| Tobacco and maize[14] | DHAR | Wheat | Shruken2 Ubiquitin CaMV35S |
1.8–3.9 | Increased GSH in maize kernel and leaves Increased GSH in tobacco leaves MDHAR activity reduced ~2X |
|
| Tomato* | MDHAR | Tomato | FMV34S | 0.6–1.6 | No increase in foliar or red-ripe fruit AsA Decreased AsA in mature green fruit |
|
Studies are grouped by targeted organ (leaf tissue, storage organ, or both); current study is indicated by asterisks.
Abbreviations: APX, ascorbate peroxidase; CaMV35S, 35S promoter of the Cauliflower mosaic virus; FMV34S, 34S promoter of the Figwort mosaic virus; Chl, chloroplastic enzyme; GR, glutathione reductase; GSH, glutathione; NR, not reported; PEG, polyethylene glycol; SOD, superoxide dismutase.
2. Results and Discussion
2.1 Generation of MDHAR and DHAR over-expressing lines
In order to better understand the contribution of the recycling enzymes DHAR and MDHAR to overall levels of AsA in tomato, we generated transgenic lines that over-expressed tomato genes encoding these two enzymes. Cytosolic Dhar and Mdhar coding sequences were isolated from Ailsa-Craig tomato and cloned under the regulation of the constitutive FMV34S promoter. The resulting constructs were then transformed into tomato (Solanum lycopersicum, cv. Micro-Tom) plants. Homozygous lines were selected in the T2 generation by screening on kanamycin-containing media and confirmed by PCR.
2.2 Identification of over-expressing transgenic lines
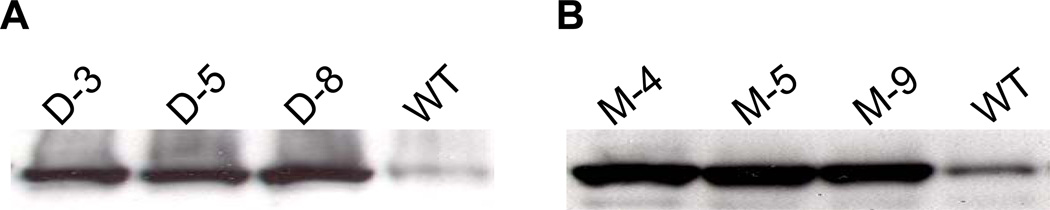
To investigate the levels of DHAR and MDHAR protein expression, these two enzymes were examined by Western Blot using enzyme-specific polyclonal antibodies. Preliminary data showed consistent trends in protein levels between fruit and leaf tissues and leaves were used to screen all of the transgenic lines. Total protein extracts of leaf tissue from homozygous lines (T2) showed elevated levels of DHAR and MDHAR in transgenic lines relative to WT (Fig. 1). Earlier experiments using chitin-column purified recombinant DHAR and MDHAR enzymes expressed in bacteria confirmed that the cDNAs selected to transform tomato plants were indeed enzymatically active against their respective targets in vitro (data not shown). However, since activities from heterologous expression systems might not mirror activities in planta, nor do increased protein levels necessarily translate to increased activity, we analyzed extracts from leaf tissues for enzymatic activity.
Fig. 1.
Western blot analysis of AsA recycling enzymes in leaf total protein extracts from WT and three independent (A) DHAR and (B) MDHAR transgenic lines. Ten µg of total protein was loaded per lane, resolved on a 4–20% gradient SDS-PAGE, transferred to PVDF and probed with respective antibodies.
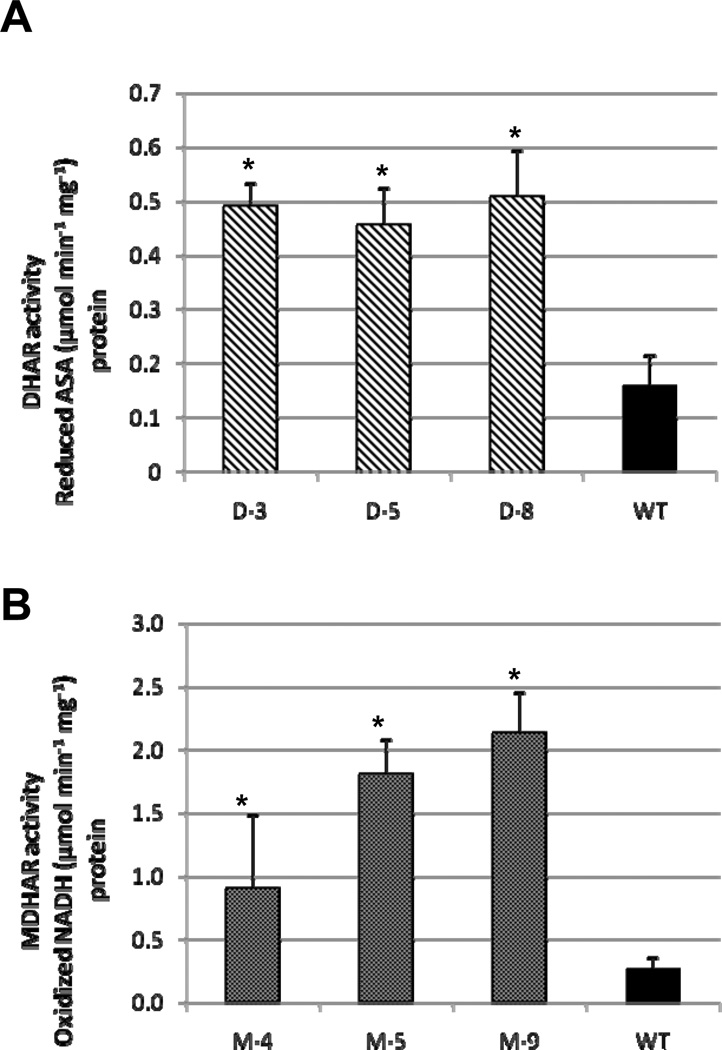
Enzymatic activities from leaf extracts of DHAR and MDHAR over-expressing tomato lines showed increases compared to WT counterparts (Fig. 2). In DHAR lines, approximately 3 fold increases in enzyme activity were observed (p<2.5×10−9), and in MDHAR lines, significant increases from 3–8 fold (p<0.0004) were also noted. The results in leaves were similar to data reported for DHAR [14, 15, 27] and MDHAR [16] in transgenic tobacco and Arabidopsis.
Fig. 2.
In vitro enzymatic activity of (A) DHAR and (B) MDHAR in leaf protein extracts. Activity was normalized for total protein input as determined by Bradford quantification. Data represent the mean ± standard deviation (n=12). Significance at P<0.05 is indicated by asterisks. Diagonal fill=DHAR, dotted fill=MDHAR, solid fill=WT.
2.3 Analysis of ascorbate levels in transgenic lines
Total vitamin C content was subsequently measured in both foliar and fruit tissues of lines identified as having increased enzymatic activity for the two AsA recycling enzymes, DHAR and MDHAR. We had previously observed that DHAR and MDHAR over-expressing plants grown under greenhouse conditions had higher levels of total AsA compared to plants grown in growth chambers. However, differences in AsA content among transgenic lines compared to WT plants were not statistically significant in leaf tissue or red ripe fruit for greenhouse grown plants (data not shown). To investigate if this was the case under different lighting conditions, plants were grown under relatively low light (150 µmol m−2 s−1) and samples were taken during the first hours of the light period in order to minimize the contribution of de novo synthesized AsA. Samples taken from foliar tissue of Micro-Tom plants at the 5-leaf stage showed no significant differences between transgenic and WT lines (p≥0.05) in spite of the increased DHAR and MDHAR enzyme activities observed in these transgenic lines (Table 2). In addition, nested one-way ANOVA indicated that differences between WT, MDHAR and DHAR over-expressers as a group were not significant (p=0.79). The experimental results complied in Table 1 showed that in a handful of studies, over-expressing cytoplasmic-targeted DHAR [27, 28] or MDHAR [29], did not result in significant changes in AsA. Yet, the majority of previously reported studies indicate that over-expression of DHAR tended to increase AsA levels at least 20% relative to WT controls.
Table 2.
Total ascorbate content in leaves of DHAR and MDHAR over-expressing tomato lines compared to WT controls. The content of oxidized AsA was less than 5% for all genotypes. Values represent the mean ± standard deviation, and are reported as µmol.g−1 fresh weight. P-values comparing WT to transgenic lines are indicated below.
| WT | D-3 | D-5 | D-8 | M-4 | M-5 | M-9 |
|---|---|---|---|---|---|---|
| 3.22 | 2.22 | 2.80 | 3.35 | 2.43 | 2.58 | 2.61 |
| 3.30 | 2.14 | 2.92 | 3.38 | 3.24 | 2.44 | 2.41 |
| 2.79 | 4.22 | 4.01 | 3.42 | 3.99 | 4.43 | 3.10 |
| 3.09 | 3.93 | 3.95 | 3.57 | 5.50 | 3.43 | |
| 3.10 ± 0.22 | 3.13 ± 1.10 | 3.42 ± 0.65 | 3.43 ± 0.10 | 3.22 ± 0.78 | 3.74 ± 1.48 | 2.89 ± 0.46 |
| p-value | 0.96 | 0.40 | 0.05 | 0.82 | 0.46 | 0.46 |
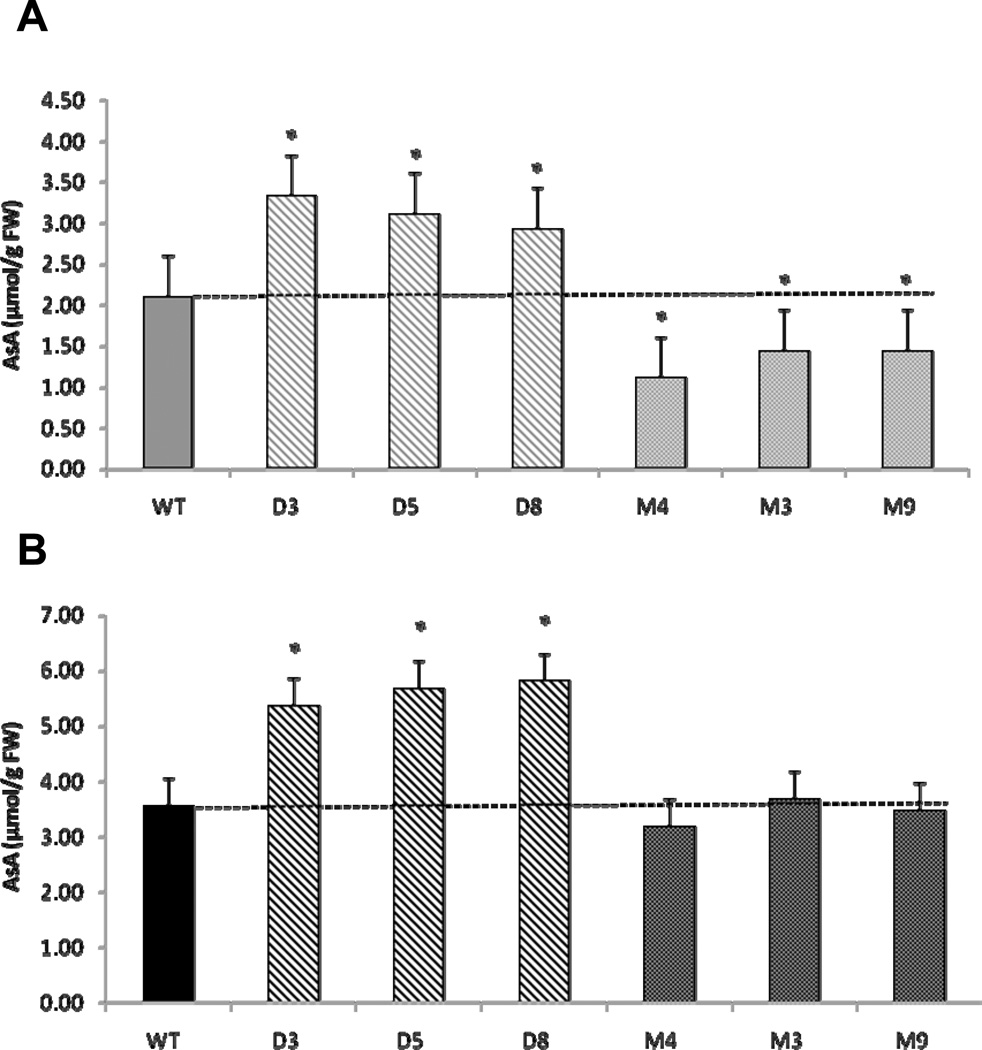
AsA content was also examined in red ripe and mature green whole fruits from DHAR and MDHAR over-expressing lines (Fig. 3). At the mature green (MG) stage, tomatoes are firm enough to be picked and transported in bulk, yet are developed enough to be ethylene responsive, allowing for post-harvest ripening. Red ripe (RR) fruit is the stage most commonly associated with market produce, thus understanding AsA recycling in both stages is important from a commercial perspective. When red ripe fruit were tested, 1.5–1.6 fold increases were evident in DHAR lines compared to controls (p<0.018). Similar differences were also observed in mature green fruit (p<0.002). The AsA content of red ripe MDHAR fruits was not significantly different from WT, however, mature green fruits of this genotype were significantly lower in total AsA by approximately 30–50% (p<0.014). The differences between lines over-expressing DHAR and MDHAR may indicate differential regulation of these two enzymes. In agreement with this concept, Jimenez and colleagues [30] showed an inverse relationship in DHAR and MDHAR activities in tomato fruit as it transitioned from mature green to breaker stages and then again during the over-ripe stage. Systematic expression profiling in tomato fruits revealed that transcript levels from two Mdhar isoforms were negatively correlated to rising AsA levels during fruit ripening [31]. Conversely, in an ascorbate-QTL mapping study [32], Stevens and colleagues showed that MDHAR activity had a modest positive effect on tomato AsA levels under field-grown conditions, but under chilling stress MDHAR activity strongly correlated with AsA levels, and explained 84% of the variation of AsA levels in tomato fruit. Furthermore, an earlier study showed MDHAR activity negatively correlated with AsA content, but Mdhar transcripts were over-expressed when tomato fruit was wounded [33]. Thus, although these correlations do not provide direct evidence for differential regulation of MDHAR and DHAR, they suggest that MDHAR may become an important determinant of AsA levels under stress-inducing conditions.
Fig. 3.
Total ascorbate levels in (A) mature green and (B) red ripe whole fruit extracts. Data represent the mean ± standard deviation (n>9). Significance at P<0.05 is indicated by asterisks. Diagonal fill=DHAR, dotted fill=MDHAR, solid fill=WT. Grey=mature green, black=red ripe.
At the tissue level, AsA content is determined by a number of factors including biosynthesis, turnover, long-distance transport, tissue oxidative burden, and developmental programs. We do not know which of these factors had the most influence in the strikingly different effects of DHAR and MDHAR over-expression between leaves and fruits we describe here. A report in which the actual contribution of AsA biosynthesis and recycling were evaluated in excised maize embryos by blocking biosynthesis, the authors found elevated DHAR activity [34]. These data suggest that all components of the AsA system are highly interconnected, and that DHAR activity may have an important role under conditions of reduced AsA biosynthesis.
Species-specific differences in biosynthetic and endogenous recycling capacities might also explain the differences between our results in tomato and other model plant systems where increases in AsA levels of up to 6 fold and 2 fold are reported for DHAR and MDHAR over-expressers, respectively (Table 1). Our results serve to illustrate that conclusions drawn from model plant systems do not necessarily translate to other crops. Attempts to increase vitamin C in tomato for applications in field-settings are not likely to be successful using constitutively over-expressed cytosolic Dhar and Mdhar cis-genes. In addition, results obtained in closely related tobacco and potato plants may not necessarily be mirrored when implemented in tomato. A very important redox and signaling molecule such as AsA is likely highly regulated. Strategies to increase AsA levels beyond current levels may require perturbations in entire regulatory networks using transcription factors or multi-gene approaches.
3. Conclusion
Contrary to previous reports in Arabidopsis, tobacco, and potato, over-expression of DHAR and MDHAR enzymes did not result in increases of AsA levels in photosynthetic tissues. However, under low lighting conditions, increases in AsA levels of DHAR over-expressing lines relative to WT were observed in red ripe and mature green fruit, while MDHAR lines had reduced AsA levels in mature green fruit only. This is the first report of over-expressing Dhar and Mdhar in a fleshy fruit like tomato. The roles of these two genes may be developmentally, and independently regulated as fruit matures and do not appear to be a significantly limiting factor in the maintenance of AsA in tomato leaves under normal growth conditions. However, they may be important in maintaining AsA levels in fruit when endogenous levels are low due to intrinsic or environmental factors.
Further research to determine how MDHAR over-expressing lines perform under cold conditions may be of interest, since MDHAR activity is closely correlated with AsA under chilling stress [32]. As a potential post-harvest application, MDHAR over-expressing tomatoes may serve to maintain AsA levels under low temperature storage conditions, a possibility that warrants examination in the near future.
4. Methods
4.1 Isolation of Dhar and Mdhar cDNA and Plant Transformation
Full-length Dhar (GenBank ID: AY971873.1) and Mdhar (GenBank ID: L41345.1) cDNAs were generated from reversed transcribed mRNA isolated from Ailsa-Craig tomato fruit. Resulting cDNAs were sequenced and in silico analysis confirmed the predicted open reading frames (ORFs) and respective amino acid sequences. ORFs from each gene were cloned into a plant binary vector, pCAMBIA 2301, having the polylinker replaced by the Figwort Mosaic Virus strong constitutive viral promoter (FMV34S) [35, 36] and a pea ribulose-1,5-bisphosphate carboxylase/oxygenase (RubisCO) small subunit terminator (rbcS-E9) [37]. Bioinformatic analyses using the TargetP [38] and SignalP [38, 39] algorithms indicated cytoplasmic targeting for both DHAR and MDHAR.
Plasmids were electroporated into Agrobacterium tumefaciens GV3101 strain and used to transform Micro-Tom tomato plants at the Ralph M. Parsons Plant Transformation Facility at the University of California, Davis. Transgenic tomatoes were allowed to self-pollinate and resulting T1 progeny were screened on 0.5X MS media supplemented with 1% sucrose (w/v), 1% agar (w/v), containing 50 µg mL−1 kanamycin. Surviving seedlings were transplanted and allowed to set seed for subsequent generations and homozygote selection.
4.2. MDHAR and DHAR Polyclonal Antibodies
Mdhar and Dhar cDNAs were sub-cloned into the IMPACT protein expression vector (New England Biolabs) and used to transform E. coli ER2566 cells (New England Biolabs). Recombinant protein expression was induced and purified as recommended using chitin-based affinity columns. Anti-MDHAR and anti-DHAR polyclonal rabbit antiserum was raised against column purified full-length recombinant protein (Antibodies Inc., Davis, CA). Rabbit sera were prescreened to avoid non-specific background reactivity with the target proteins. Competition immunoblots were performed to confirm antibody specificity against their respective targets.
4.3 Growth Conditions and Sampling
Growth chamber conditions (PAR=150 µmol m−2 s−1, 16 h day length, 25°C constant, and 65% humidity) were used in experiments for ascorbate quantification and sampling was performed immediately after lights were turned on (ASU, Jonesboro, AR).
4.4 Western Blot and Enzyme Assays
Total tomato protein was extracted using Extraction Buffer A [1.3 M NaCl, 0.2 M CaCl2, 13 mM CyDTA, 50 mM Tris-HCl pH 8.0, 0.1% (v/v) Igepal CA-630, 0.75% (w/v) PVPP, 0.25% (w/v) PVP, 5 mM TCEP, 1X Protease Inhibitor Cocktail (Sigma)]. Ten µg of total protein per lane was separated by electrophoresis in a precast 4–20% SDS-PAGE (Bio-Rad). Protein was immobilized by semi-dry transfer to Immuno-Blot PVDF (Bio-Rad) membranes and blocked in 1% (w/v) ECL Advance Blocking Reagent (GE Healthcare) in TBS-T Buffer [50 mM Tris pH 7.5, 150 mM NaCl, 0.025% (v/v) Tween-20]. Three 5 min washes with TBS-T were performed after each antibody incubation. MDHAR and DHAR antisera were used at a 1:3,000 and 1:5,000 dilutions respectively in 0.5% (w/v) blocking reagent TBS-T for 1 h. Secondary antibody incubation of 1:20,000 goat anti-rabbit conjugated with horseradish peroxidase for 1 h was performed. Chemi-luminescent detection with ECL-Lightening (Perkin Elmer) was carried out as per manufacturer’s recommendations.
Active protein from leaves was extracted using Extraction Buffer B [50 mM MES pH 7.5, 40 mM KCl, 2 mM CaCl2, 13 mM CyDTA, 1 mM AsA, 0.1% (v/v) Igepal CA-630, 0.75% (w/v) PVPP, 0.25% (w/v) PVP, 1X Protease Inhibitor Cocktail (Sigma)]. DHAR, MDHAR activity were quantified as previously described [40] with the exception that volumes were modified for use in disposable 1.0 mL acrylic cuvettes and 20 µL of protein extract was used. Activity was calculated using extinction coefficients as described [40], and total protein was measured using the method of Bradford [41]; activity was normalized for total protein input.
4.5 Ascorbate Assay
Foliar and fruit ascorbate content were determined by the ascorbate oxidase assay adapted to a 96-well plate format and setup essentially as described [22]. Leaf samples were collected during the first hours of the light period and frozen immediately in liquid nitrogen. Whole fruits were collected at the proper ripening stage during the first hours of the light period and immediately used for AsA measurements. Tissues were ground in fresh 6% (w/v) meta-phosphoric acid and centrifuged at 15,000 g for 5 min. Reduced AsA was determined by measuring the decline in A265 after addition of 0.5 U of ascorbate oxidase (Sigma, St. Louis, MO) to 300 µL of the reaction mix including tissue extract and 100 mM potassium phosphate pH 6.9. Oxidized AsA was determined in a 300 µL reaction mixture including 40 µM dithiothreitol and incubating at room temperature in the dark for 30 min. Total AsA was the sum of reduced and oxidized AsA. Calculations were based on a standard curve with pure AsA run in parallel.
4.6 Statistics
Statistics were performed in Excel using the 2-tailed Student’s T-Test function, assuming unequal variances. Nested one-way ANOVA was also performed for leaf ascorbate content. An asterisk in figures denotes statistically significant values. Results were reported to be statistically significant if P-values were <0.05.
Acknowledgements
A Plant Sciences Departmental GSR award and the Rockefeller Foundation supported work performed at UC Davis. AL thanks support to her laboratory provided by a seed grant from the Plant Powered Production (P3) Center through the RII Arkansas ASSET Initiative (AR EPSCoR) by the NSF (grant # EPS-0701890), by the Arkansas Biosciences Institute, the major research component of the Arkansas Tobacco Settlement Proceeds Act and by a sub-award from the NIH Grant # P20- RR-016460 from the IDeA Networks of Biomedical Research Excellence (INBRE) program of the National Center for Research Resources.
Abbreviations
- AsA
ascorbate
- BSA
bovine serum albumin
- CyDTA
1,2-cyclohexylenedinitrolotetraacetic acid
- DHA
dehydroascorbate
- DHAR
DHA reductase
- Igepal CA-630
(octylphenoxy)polyethoxyethanol
- MDHA
monodehydroascorbate
- MDHAR
MDHA reductase
- MES
2-(N-morpholino)ethanesulfonic acid
- MS
Murashige and Skogg (1962)
- PVP
polyvinylpyrrolidone
- PVPP
polyvinylpolypyrrolidone
- TBS
tris-buffered saline
- TCEP
tris(2-carboxyethyl)phosphine
- WT
wild type
References
- 1.Running JA, Huss RJ, Olson PT. HETEROTROPHIC PRODUCTION OF ASCORBIC-ACID BY MICROALGAE. J. Appl. Phycol. 1994;6:99–104. [Google Scholar]
- 2.Dummermuth AL, Karsten U, Fisch KM, Konig GM, Wiencke C. Responses of marine macroalgae to hydrogen-peroxide stress. J. Exp. Mar. Biol. Ecol. 2003;289:103–121. [Google Scholar]
- 3.Smirnoff N. The function and metabolism of ascorbic acid in plants. Annals Of Botany. 1996;78:661–669. [Google Scholar]
- 4.Noctor G, Foyer CH. Ascorbate and glutathione: Keeping active oxygen under control. Annual Review Of Plant Physiology And Plant Molecular Biology. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- 5.Olson JA, Hodges RE. Recommended dietary intakes (RDI) of vitamin C in humans. Am J Clin Nutr. 1987;45:693–703. doi: 10.1093/ajcn/45.4.693. [DOI] [PubMed] [Google Scholar]
- 6.Bsoul SA, Terezhalmy GT. Vitamin C in health and disease. The Journal of Contemporary Dental Practice. 2004;5:1–13. [PubMed] [Google Scholar]
- 7.Mandl J, Szarka A, Banhegyi G. Vitamin C: update on physiology and pharmacology. Br J Pharmacol. 2009;157:1097–1110. doi: 10.1111/j.1476-5381.2009.00282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chu YF, Sun J, Wu X, Liu RH. Antioxidant and antiproliferative activities of common vegetables. J Agric Food Chem. 2002;50:6910–6916. doi: 10.1021/jf020665f. [DOI] [PubMed] [Google Scholar]
- 9.Fawzi W, Herrera MG, Nestel P. Tomato intake in relation to mortality and morbidity among sudanese children. Journal Of Nutrition. 2000;130:2537–2542. doi: 10.1093/jn/130.10.2537. [DOI] [PubMed] [Google Scholar]
- 10.Block G. VITAMIN-C AND CANCER PREVENTION - THE EPIDEMIOLOGIC EVIDENCE. American Journal of Clinical Nutrition. 1991;53:S270–S282. doi: 10.1093/ajcn/53.1.270S. [DOI] [PubMed] [Google Scholar]
- 11.De Tullio MC, Arrigoni O. Hopes, disillusions and more hopes from vitamin C. Cell Mol Life Sci. 2004;61:209–219. doi: 10.1007/s00018-003-3203-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arrigoni O, De Tullio MC. Ascorbic acid: much more than just an antioxidant. Bba-Gen Subjects. 2002;1569:1–9. doi: 10.1016/s0304-4165(01)00235-5. [DOI] [PubMed] [Google Scholar]
- 13.Gillespie DS. OVERVIEW OF SPECIES NEEDING DIETARY VITAMIN-C. Journal of Zoo Animal Medicine. 1980;11:88–91. [Google Scholar]
- 14.Chen Z, Young TE, Ling J, Chang SC, Gallie DR. Increasing vitamin C content of plants through enhanced ascorbate recycling. Proceedings Of The National Academy Of Sciences Of The United States Of America. 2003;100:3525–3530. doi: 10.1073/pnas.0635176100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eltayeb AE, Kawano N, Badawi GH, Kaminaka H, Sanekata T, Morishima I, Shibahara T, Inanaga S, Tanaka K. Enhanced tolerance to ozone and drought stresses in transgenic tobacco overexpressing dehydroascorbate reductase in cytosol. Physiologia Plantarum. 2006;127:57–65. [Google Scholar]
- 16.Eltayeb AE, Kawano N, Badawi GH, Kaminaka H, Sanekata T, Shibahara T, Inanaga S, Tanaka K. Overexpression of monodehydroascorbate reductase in transgenic tobacco confers enhanced tolerance to ozone, salt and polyethylene glycol stresses. Planta. 2007;225:1255–1264. doi: 10.1007/s00425-006-0417-7. [DOI] [PubMed] [Google Scholar]
- 17.Fotopoulos V, Sanmartin M, Kanellis AK. Effect of ascorbate oxidase over-expression on ascorbate recycling gene expression in response to agents imposing oxidative stress. Journal of Experimental Botany. 2006;57:3933–3943. doi: 10.1093/jxb/erl147. [DOI] [PubMed] [Google Scholar]
- 18.Giovannoni JJ. Completing a pathway to plant vitamin C synthesis. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:9109–9110. doi: 10.1073/pnas.0703222104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishikawa T, Dowdle J, Smirnoff N. Progress in manipulating ascorbic acid biosynthesis and accumulation in plants. Physiologia Plantarum. 2006;126:343–355. [Google Scholar]
- 20.Jain AK, Nessler CL. Metabolic engineering of an alternative pathway for ascorbic acid biosynthesis in plants. Molecular Breeding. 2000;6:73–78. [Google Scholar]
- 21.Lee YP, Kim SH, Bang JW, Lee HS, Kwak SS, Kwon SY. Enhanced tolerance to oxidative stress in transgenic tobacco plants expressing three antioxidant enzymes in chloroplasts. Plant Cell Rep. 2007;26:591–598. doi: 10.1007/s00299-006-0253-z. [DOI] [PubMed] [Google Scholar]
- 22.Lorence A, Chevone BI, Mendes P, Nessler CL. myo-inositol oxygenase offers a possible entry point into plant ascorbate biosynthesis. Plant Physiology. 2004;134:1200–1205. doi: 10.1104/pp.103.033936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naqvi S, Zhu C, Farre G, Ramessar K, Bassie L, Breitenbach J, Perez Conesa D, Ros G, Sandmann G, Capell T, Christou P. Transgenic multivitamin corn through biofortification of endosperm with three vitamins representing three distinct metabolic pathways. Proc Natl Acad Sci U S A. 2009;106:7762–7767. doi: 10.1073/pnas.0901412106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Z, Xiao Y, Chen W, Tang K, Zhang L. Increased vitamin C content accompanied by an enhanced recycling pathway confers oxidative stress tolerance in Arabidopsis. J Integr Plant Biol. 2010;52:400–409. doi: 10.1111/j.1744-7909.2010.00921.x. [DOI] [PubMed] [Google Scholar]
- 25.Li F, Wu Q-Y, Sun Y-L, Wang L-Y, Yang X-H, Meng Q-W. Overexpression of chloroplastic monodehydroascorbate reductase enhanced tolerance to temperature and methyl viologen-mediated oxidative stresses. Physiologia Plantarum. 2010;139:421–434. doi: 10.1111/j.1399-3054.2010.01369.x. [DOI] [PubMed] [Google Scholar]
- 26.Goo Y-M, Chun H, Kim T-W, Lee C-H, Ahn M-J, Bae S-C, Cho K-J, Chun J-A, Chung C-H, Lee S-W. Expressional characterization of dehydroascorbate reductase cDNA in transgenic potato plants. Journal of Plant Biology. 2008;51 35-41-41. [Google Scholar]
- 27.Ushimaru T, Nakagawa T, Fujioka Y, Daicho K, Naito M, Yamauchi Y, Nonaka H, Amako K, Yamawaki K, Murata N. Transgenic Arabidopsis plants expressing the rice dehydroascorbate reductase gene are resistant to salt stress. J Plant Physiol. 2006;163:1179–1184. doi: 10.1016/j.jplph.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Qin A, Shi Q, Yu X. Ascorbic acid contents in transgenic potato plants overexpressing two dehydroascorbate reductase genes. Mol Biol Rep. 2011;38:1557–1566. doi: 10.1007/s11033-010-0264-2. [DOI] [PubMed] [Google Scholar]
- 29.Yin L, Wang S, Eltayeb AE, Uddin MI, Yamamoto Y, Tsuji W, Takeuchi Y, Tanaka K. Overexpression of dehydroascorbate reductase, but not monodehydroascorbate reductase, confers tolerance to aluminum stress in transgenic tobacco. Planta. 2010;231:609–621. doi: 10.1007/s00425-009-1075-3. [DOI] [PubMed] [Google Scholar]
- 30.Jimenez A, Creissen G, Kular B, Firmin J, Robinson S, Verhoeyen M, Mullineaux P. Changes in oxidative processes and components of the antioxidant system during tomato fruit ripening. Planta. 2002;214:751–758. doi: 10.1007/s004250100667. [DOI] [PubMed] [Google Scholar]
- 31.Ioannidi E, Kalamaki MS, Engineer C, Pateraki I, Alexandrou D, Mellidou I, Giovannonni J, Kanellis AK. Expression profiling of ascorbic acid-related genes during tomato fruit development and ripening and in response to stress conditions. J Exp Bot. 2009 doi: 10.1093/jxb/ern322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stevens R, Page D, Gouble B, Garchery C, Zamir D, Causse M. Tomato fruit ascorbic acid content is linked with monodehydroascorbate reductase activity and tolerance to chilling stress. Plant Cell Environ. 2008;31:1086–1096. doi: 10.1111/j.1365-3040.2008.01824.x. [DOI] [PubMed] [Google Scholar]
- 33.Grantz A, Brummell DA, Bennett AB. Ascorbate Free-Radical Reductase Messenger-Rna Levels Are Induced By Wounding. Plant Physiology. 1995;108:411–418. doi: 10.1104/pp.108.1.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Tullio MC, De Gara L, Paciolla C, Arrigoni O. Dehydroascorbate-reducing proteins in maize are induced by the ascorbate biosynthesis inhibitor lycorine. Plant Physiology and Biochemistry. 1998;36:433–440. [Google Scholar]
- 35.Sanger M, Daubert S, Goodman RM. Characteristics of a strong promoter from figwort mosaic virus: comparison with the analogous 35S promoter from cauliflower mosaic virus and the regulated mannopine synthase promoter. Plant Mol Biol. 1990;14:433–443. doi: 10.1007/BF00028779. [DOI] [PubMed] [Google Scholar]
- 36.Maiti IB, Gowda S, Kiernan J, Ghosh SK, Shepherd RJ. Promoter/leader deletion analysis and plant expression vectors with the figwort mosaic virus (FMV) full length transcript (FLt) promoter containing single or double enhancer domains. Transgenic Res. 1997;6:143–156. doi: 10.1023/a:1018477705019. [DOI] [PubMed] [Google Scholar]
- 37.Coruzzi G, Broglie R, Edwards C, Chua NH. Tissue-specific and light-regulated expression of a pea nuclear gene encoding the small subunit of ribulose-1,5-bisphosphate carboxylase. EMBO J. 1984;3:1671–1679. doi: 10.1002/j.1460-2075.1984.tb02031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Emanuelsson O, Brunak S, von Heijne G, Nielsen H. Locating proteins in the cell using TargetP, SignalP and related tools. Nat Protoc. 2007;2:953–971. doi: 10.1038/nprot.2007.131. [DOI] [PubMed] [Google Scholar]
- 39.Bendtsen JD, Nielsen H, von Heijne G, Brunak S. Improved prediction of signal peptides: SignalP 3.0. J Mol Biol. 2004;340:783–795. doi: 10.1016/j.jmb.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 40.Murshed R, Lopez-Lauri F, Sallanon H. Microplate quantification of enzymes of the plant ascorbate-glutathione cycle. Anal Biochem. 2008;383:320–322. doi: 10.1016/j.ab.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 41.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]