Abstract
Genetic risk factors for major psychiatric disorders play key roles in neurodevelopment. Thus, exploring the molecular pathways of risk genes is important not only for understanding the molecular mechanisms underlying brain development, but also to decipher how genetic disturbances affect brain maturation and functioning relevant to major mental illnesses. During the last decade, there has been significant progress in determining the mechanisms whereby risk genes impact brain development. Nonetheless, given that the majority of psychiatric disorders have etiological complexities encompassing multiple risk genes and environmental factors, the biological mechanisms of these diseases remain poorly understood. How can we move forward to our research for discovery of the biological markers and novel therapeutic targets for major mental disorders? Here we review recent progress in the neurobiology of disrupted in schizophrenia 1 (DISC1), a major risk gene for major mental disorders, with a particular focus on its roles in cerebral cortex development. Convergent findings implicate DISC1 as part of a large, multi-step pathway implicated in various cellular processes and signal transduction. We discuss links between the DISC1 pathway and environmental factors, such as immune/inflammatory responses, which may suggest novel therapeutic targets. Existing treatments for major mental disorders are hampered by a limited number of pharmacological targets. Consequently, elucidation of the DISC1 pathway, and its association with neuropsychiatric disorders, may offer hope for novel treatment interventions.
Keywords: DISC1, cerebral cortex development, genetic risk factors, major mental disorder, immune responses
Introduction
Disrupted in schizophrenia 1 (DISC1) was initially discovered at the breakpoint in a balanced chromosomal translocation t (1; 11) segregating with major mental conditions, such as schizophrenia, bipolar disorder, and major depression in a Scottish pedigree (Millar et al., 2000). Since then, accumulating evidence from genetic studies indicated that DISC1 is not only associated with schizophrenia and mood disorders, but also other psychiatric disorders of neurodevelopmental origin, such as autism, Asperger syndrome, and agenesis of the corpus callosum (Hennah et al., 2003; Hodgkinson et al., 2004; Callicott et al., 2005; Kilpinen et al., 2008; Song et al., 2008, 2010; Osbun et al., 2011). Although recent genome wide association studies (GWAS) have not found DISC1 as a key genetic risk factor for patients met the current diagnostic criteria for schizophrenia (Purcell et al., 2009; Stefansson et al., 2009; Mathieson et al., 2011), it is noted that variations of DISC1 influence anatomical and functional endophenotypes even in control subjects (Thomson et al., 2005; Di Giorgio et al., 2008; Prata et al., 2008; Tomppo et al., 2009b). Collectively, genetic variation of DISC1 may confer vulnerabilities to a wide range of neurodevelopmental psychiatric conditions by affecting brain maturation, thereby modifying brain function.
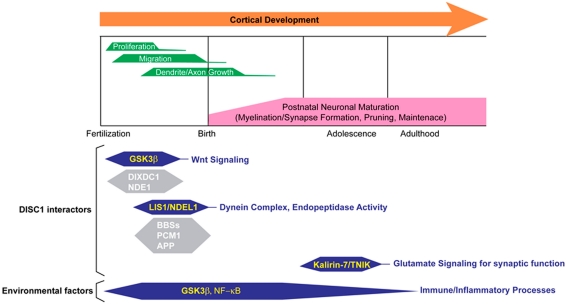
Consistently, extensive biological studies indicate that DISC1 plays a role in multiple cellular processes during and after brain development (Chubb et al., 2008; Brandon and Sawa, 2011). In fact, many protein binding partners of DISC1 are associated with various molecular pathways that regulate fundamental cellular processes for brain development and function (Table 1). Nonetheless, it is still unknown which functional aspects of DISC1 directly affect molecular mechanisms underlying disease susceptibility. How can we utilize accumulating biological data of DISC1 to discover novel therapeutic targets and biological markers for major mental conditions? Here, we will review DISC1-associated molecular pathways which have the potential to be novel therapeutic targets, with particular focus on well documented DISC1 pathways involved in cerebral cortex development and function (Figure 1). We will also discuss the potential link of DISC1 pathways and environmental factors, such as immune/inflammatory responses, to explore therapeutic interventions based on understanding disease mechanisms of genetic and environmental interaction.
Table 1.
DISC1 interacting proteins and functions.
| DISC1 interactor | Function | Risk gene | Reference |
|---|---|---|---|
| CENTROSOME/CYTOSKELETON | |||
| NDEL1 | Neurite extension, migration | + | Morris et al. (2003), Ozeki et al. (2003), Kamiya et al. (2005), Taya et al. (2007), Burdick et al. (2008) |
| NDE1 | Proliferation | + | Burdick et al. (2008), Bradshaw et al. (2009) |
| PCM1 | Microtubule organization | + | Kamiya et al. (2008) |
| BBS4 | Migration, primary cilia function | − | Kamiya et al. (2008), Ishizuka et al. (2011) |
| KIF5A | Neuronal transport | − | Taya et al. (2007) |
| 14-3-3ε | Migration axon growth | + | Taya et al. (2007) |
| FEZ1 | Neurite extension | + | Miyoshi et al. (2003) |
| Kendrin | Centrosome function | − | Miyoshi et al. (2004) |
| MAP1A | Microtubule associated | − | Morris et al. (2003) |
| MIPT3 | Microtubule associated | − | Morris et al. (2003) |
| SYNAPSE | |||
| Kalirin-7 | Dendritic spine/synapse function | − | Hayashi-Takagi et al. (2010) |
| TNIK | Dendritic spine/synapse function | + | Wang et al. (2011) |
| Citron | Rho signaling, synapse function | + | Ozeki et al. (2003) |
| NUCLEUS | |||
| ATF4 | Transcription factor | − | Morris et al. (2003), Sawamura et al. (2008) |
| N-CoR | Corepressor for gene transcription | − | Sawamura et al. (2008) |
| OTHER | |||
| PDE4B | cAMP signaling | + | Millar et al. (2005) |
| Girdin | AKT signaling | + | Enomoto et al. (2009), Kim et al. (2009) |
| Grb2 | Tyrosine kinase mediated signal transduction | − | Shinoda et al. (2007) |
| DBZ | PACAP signaling | − | Hattori et al. (2007) |
| Mitofilin | Mitochondrial function | − | Park et al. (2010) |
Many protein binding partners of DISC1 have been reported. DISC1 may function as an anchoring molecule to regulate various molecular pathways via interaction with said protein interactors in a context dependent manner.
Figure 1.
Multiple roles for DISC1 in cerebral cortex in the developmental trajectory. Various DISC1-mediated pathways with many binding partners and environmental factors synergistically affect proper cerebral cortex development and function. For reviews of the other DISC1 interactors, see Brandon and Sawa (2011), Porteous et al. (2011), Soares et al. (2011).
DISC1 in Cerebral Cortex Development
Disrupted in schizophrenia 1 plays a critical role for the regulation of cell proliferation in the developing cerebral cortex via the canonical Wnt signaling pathway (Mao et al., 2009). The data suggested that DISC1 inhibits the activity of glycogen synthase kinase 3 beta (GSK3β) via protein interaction, thereby stabilizing β-catenin which is required for proper progenitor proliferation through Wnt pathway. The same group later reported that DIX domain containing-1 (DIXDC1), a homolog of the Wnt signaling genes Disheveled axin, interacts with DISC1 to co-modulate GSK3β/β-catenin signaling for proper cell proliferation (Singh et al., 2010). Accumulating evidences have shown that GSK3β signaling may be involved in various neuropsychiatric disorders, such as schizophrenia, autism, and Alzheimer’s disease, suggesting that GSK3β appears as a prominent therapeutic target for mental disorders (Bachmann et al., 2005; Hur and Zhou, 2010). In fact, lithium, the mood stabilizer which is commonly used for the treatment of bipolar disorder, is known to inhibit GSK3 activity (Stambolic et al., 1996). The other psychoactive drugs, such as clozapine, risperidone, and valproic acid, have also been reported to affect GSK3β activity (Stambolic et al., 1996; Kang et al., 2004; Li et al., 2007; Rowe et al., 2007). Nonetheless, since GSK3β regulates various downstream effectors, which are not only implicated in the Wnt pathway, but also other signaling required for cellular development, such as sonic hedgehog and Notch signaling pathways (Hur and Zhou, 2010), it is important to examine specific GSK3β-mediated pathways relevant to disease mechanisms to find novel therapeutic strategies. In this regard, it may be ideal to focus on DISC1-mediated GSK3β pathways, especially those in association with other genetic risk factors, to explore disease-associated molecular mechanisms. For instance, collapsin response mediator protein-2 (CRMP-2)/dihydropyrimidinase-like-2 (DPYSL2), a susceptibility gene for schizophrenia (Nakata et al., 2003), is reported to be a potential protein interactor of DISC1 by yeast-two-hybrid screening (Camargo et al., 2007). Interestingly, CRMP-2/DPYSL2 is known to be phosphorylated by GSK3β for the regulation of axon outgrowth (Yoshimura et al., 2005).
Neuronal migration is a fundamental cellular process that is required for proper cortical organization. Many groups have consistently reported that knockdown of DISC1 using RNA interference (RNAi) impaired radial neuronal migration in the developing cerebral cortex (Kamiya et al., 2005, 2008; Kubo et al., 2010; Singh et al., 2010; Young-Pearse et al., 2010; Ishizuka et al., 2011). Findings from these studies suggest that DISC1, along with many protein binding partners, regulate neuronal migration via centrosome and microtubule-dependent mechanisms. Of note, some of these binding partners are known as risk or causative genes for various neuropsychiatric disorders. These include nuclear distribution element-like (NDEL1) and pericentriolar material 1 (PCM1), risk genes for schizophrenia, and BBS4, a causative gene for Bardet–Biedl syndrome that frequently accompanies impaired cognition, mental retardation, and psychosis (Burdick et al., 2008; Kamiya et al., 2008; Tomppo et al., 2009a). Amyloid precursor protein (APP) also interacts with DISC1 to recruit DISC1 to the centrosome for regulation of neuronal migration (Young-Pearse et al., 2010). Furthermore, DISC1 is a component of the LIS1/dynein motor complex (Kamiya et al., 2005). Mutations in human LIS1 gene cause classical lissencephaly resulting in mental retardation (Pilz et al., 1998). Consistently, LIS1 heterozygous knockout mice in which LIS1 expression is reduced, display disorganization of proper cortical layer formation and behavioral abnormalities, such as impaired spatial learning and motor function, indicating that this is a good animal model for human lissencephaly caused by LIS1 haploinsufficiency (Hirotsune et al., 1998). Interestingly, the prenatal administration of ALLN, a calpain inhibitor which prevents the degradation of LIS1, is effective to ameliorate neuronal migration defect and improve motor coordination in this animal model (Yamada et al., 2009).
Although mental disorders undoubtedly have genetic complexities and could not be explained by the simple “haploinsufficiency” model as the case of lissencephaly, elucidation of risk genes, and/or molecules in their interactome, specifically ones with enzymatic activity, may offer hope for novel treatment interventions for neuropsychiatric disorders. In this regard, endo-oligopeptidase activity of NDEL1 is quite interesting from a drug discovery viewpoint (Hayashi et al., 2005). As a matter of fact, inhibitors of angiotensin-converting enzyme (ACE), an exopeptidase, are currently being used to treat hypertension and renal disease (Izzo and Weir, 2011), making peptidase activity an attractive drug target. Although endogenous substrates for NDEL1-oligopeptidase in brain development remain unknown, in vitro experiments identified several oligopeptides, such as neurotensin and bradykinin, as potential targets for NDEL1 (Camargo et al., 1983). Interestingly, neurotensin has a modulatory effect on neurotransmitter systems, including dopaminergic neurons, which may be involved in the pathophysiologies of schizophrenia (Boules et al., 2007).
Posttranslational modifications, which affect the functional diversity of target proteins, could also have potential as novel drug targets and biological markers in the DISC1 pathways. We have recently reported that phosphorylation of DISC1 at Serine 710 is a molecular switch signaling from cell proliferation to neuronal migration in the developing cerebral cortex (Ishizuka et al., 2011). By utilizing in utero electroporation, this study has shown that a phosphor-dead mutant DISC1 can rescue only the proliferation defect elicited by DISC1 knockdown, whereas a phosphor-mimic mutant of DISC1 can exclusively recover impaired migration. The question arises whether the phosphorylation of DISC1 at Serine 710 may be involved in the pathophysiologies of major mental disorders, such as schizophrenia. It is obviously impractical to investigate the phosphorylation status of DISC1 in the developing human brain from subjects at risk of developing schizophrenia. Nonetheless, recent progress in induced pluripotent stem (iPS) cell technology will open new avenues to characterize such findings from preclinical studies using patient-derived neuronal cells, which might in turn identify biological markers for major mental disorders.
DISC1 and Glutamate Signaling for Synaptic Function
Disrupted in schizophrenia 1 impacts upon brain development may be a challenge for treatment intervention. However, synaptic deficits revealed by the DISC1 pathway offer some potential for development of targeted pharmacologic intervention. Early reports suggested a role for DISC1 in neurite outgrowth (Miyoshi et al., 2003; Ozeki et al., 2003). Subsequent findings underline roles for DISC1 in regulating dendritic spines of the glutamate synapse (Hayashi-Takagi et al., 2010). Rac1 is activated by Karilin-7, leading to increased spine size following NMDA glutamate receptor activation. However, DISC1 appears to interact with Karilin-7, preventing access to and activation of Rac1 until NMDA receptor activation promotes release of Kal-7 and spine enlargement. Pharmacologic tools to modulate the Karilin-7/DISC1 interaction might be a means to regulate spine maintenance.
TRAF2- and NCK-interacting kinase (TNIK) represents another potential pharmacological target in the DISC1 protein interaction network. TNIK is found in postsynaptic densities and regulates c-Jun kinase, the actin cytoskeleton and a number of Wnt pathway effectors (Fu et al., 1999; Taira et al., 2004; Mahmoudi et al., 2009). Genetic association studies have found single-nucleotide polymorphisms of TNIK associated with schizophrenia (Potkin et al., 2009; Shi et al., 2009). TNIK mRNA expression was increased in the dorsolateral prefrontal cortex of schizophrenia subjects (Glatt et al., 2005) and in lymphoblasts of monozygotic twins discordant for bipolar disorder (Matigian et al., 2007). A yeast-two-hybrid screen using DISC1 as “bait” identified TNIK as an interactor (Camargo et al., 2007). Subsequently, TNIK and DISC1 were shown to interact in mouse brain (Wang et al., 2011). DISC1 was found to inhibit the kinase activity of TNIK, an action that could be reproduced by a small peptide derived from the DISC1 interaction site. This DISC1 peptide led to increased actin polymerization and decreased expression of a number of postsynaptic density proteins, including PSD95, stargazin, AMPA receptor subunit GluR1 and TNIK, itself (Wang et al., 2011).
DISC1 and Neuroimmune/Inflammatory Processes
Microbial infections have been recognized as environmental factors responsible for the increased incidence of schizophrenia and associated disorders (Brown and Derkits, 2010; Sham et al., 1992; Torrey and Yolken, 2003). These reports have been supported by the epidemiological findings of an association between elevated cytokines in maternal serum and schizophrenia in the offspring (DeLisi and Wyatt, 1982; Patterson, 2007; Miller et al., 2009). Subsequently, it has been demonstrated that it is the maternal immune response to a microbe that may contribute to the increased risk of schizophrenia. The role of cytokines in innate immune response makes them promising candidates for studying their functions in disruption of fetal brain development in vulnerable individuals (Dantzer et al., 2008). Most studies with prenatal immune activation have thus far used wild-type mice and rats. However, recently, there have been several reports on developing and characterizing animal models based on combining prenatal immune activation with genetic mutations relevant to schizophrenia (Ibi et al., 2010; Ehninger et al., 2012).
We have been studying possible roles for DISC1 in modulation of poly I:C-induced immune activation in pregnant mice to mimic prenatal in utero exposure to viruses as a model of gene–environment interactions relevant to schizophrenia (Abazyan et al., 2010). Our findings have suggested that DISC1 may be involved in mediating neuroimmune interplay in this mouse model. Given the extended interactome of DISC1, it is not surprising that this protein is at the crossroads of the signaling transduction pathways activated by immune factors.
One can envision multiple interactions between the pathways impacted by mutant DISC1 and activated by cytokines and/or bacterial lipopolysaccharide (LPS) and poly I:C itself via cytokine receptors or toll-like receptors (TLR) expressed by neurons or glia cells, respectively. One of the major common pathways is the phosphoinositide-3 kinase/AKT-signaling network (PI3K/AKT) that is activated by cytokines and poly IC and has been demonstrated to interact with DISC1 partners (Camargo et al., 2007). Another example is interactions with GSK3β, a key regulator of the host inflammatory response and the production of pro- and anti-inflammatory cytokines (Hayden et al., 2006). As described above, DISC1 inhibits GSK3β activity through a direct interaction (Mao et al., 2009). We also found altered poly I:C-induced phosphorylation of GSK3β in mutant DISC1 newborn mice that might at least in part explain altered basal and poly I:C-induced production of cytokines in fetal brains and resultant affective behaviors in adult offspring (Abazyan et al., 2010). These observations are consistent with an emerging role for GSK3β in inflammation-associated depression and anxiety (Jope, 2011).
Many immune effects of GSK3β are related to its regulation of critical transcription factors, including nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB; Hayden et al., 2006). A family of TLRs acts as primary sensors that detect a wide variety of microbial components and elicit innate immune responses. All TLR signaling pathways culminate in activation of NF-κB, which controls the expression of an array of inflammatory cytokine genes. Stimulation with TLR ligands triggers the rapid phosphorylation of specific serine residues of inhibitor of κB (IκB) proteins by the IκB kinase (IKK) complex. Phosphorylated IκB proteins are subsequently polyubiquitinated and degraded, allowing NF-κB to move into the nucleus. This so-called “canonical pathway” is involved in TLR-mediated induction of inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6; Hayden et al., 2006). Prior studies with DISC1 have demonstrated that DISC1, particularly a nuclear isoform of the protein, can play an important role in regulation of transcription activity in the nucleus (Sawamura et al., 2008). Our pilot in vitro experiments demonstrated that DISC1 may impact NF-κB signaling. We found that expression of mutant DISC1 in N2 a neuronal cells led to delaying a recovery of IκBα after TNF-α-induced phosphorylation and ubiquitination of IκBα. This prolonged degradation due to expression of mutant DISC1 seems to suggest that perturbation in functions of DISC1 could also affect (e.g., stimulate) pro-inflammatory signaling transduction cascades in neurons.
In addition to immune signaling pathways, DISC1 and perhaps other candidate genes can play a significant role in the cellular processes utilized by microbes during their life cycles (Carter, 2009). It has been proposed that the involvement of DISC1 in the control of the microtubule network might be important both in viral traffic and in the rerouting of microtubules to the vacuoles formed by T. gondii (Carter, 2009).
Recent clinical trials of anti-inflammatory add-on therapy in schizophrenia have demonstrated superior beneficial treatment effects when antipsychotics were co-administered with anti-inflammatory compounds, as compared with treatment outcomes using antipsychotics alone (Meyer et al., 2011). However, a broad non-specific anti-inflammatory or immunosuppressive treatments that may have several unwanted effects such as increased sensitivity to infections (Meyer et al., 2011). Ultimately, future therapeutic approaches will result from deciphering intracellular pathways that underlie convergence of environmental influences and genetic predisposition and their influence on neurodevelopmental processes.
Conclusion Remarks
Disrupted in schizophrenia 1-mediated pathways play multiple roles for critical cellular processes through many protein binding partners in a context dependent manner. Nonetheless, it is still unknown which functional aspect of DISC1 directly affects molecular mechanisms underlying disease susceptibility. Are all DISC1 functions in such cellular events implicated in disease processes or are only some specific functional aspects critical? This is a tremendously difficult question, because the molecular disposition of DISC1 is complex as reflected by multiple isoforms at both mRNA and protein levels (Ishizuka et al., 2006; Nakata et al., 2009). Nonetheless, biological functions of DISC1 are currently being explored without waiting for the complete identification of DISC1 isoforms, resulting in the identification of multiple roles of DISC1 in various functional contexts. In fact, in addition to the roles in cerebral cortex we reviewed here, DISC1 also contributes to brain development and function in other brain regions, such as hippocampal regions (Enomoto et al., 2009; Kim et al., 2009; Meyer and Morris, 2009). Further investigations with advanced genetic engineering techniques, which allow us to dissect region and cell type-specific DISC1 functions in a temporal manner, might contribute to more clearly elucidate DISC1 functions relevant to psychiatric disorders.
As complete functional recovery is unlikely for neurodevelopmental disorders, such as schizophrenia, developing preventive strategies is particularly important. Indeed, if the findings on microbial etiologies and resultant immune dysfunction are replicated, simple public health measures may prove beneficial in diminishing the incidence of infections during pregnancy to prevent an appreciable proportion of schizophrenia cases. For example, influenza vaccination, improved hygiene to prevent T. gondii infection, and antibiotics to treat genital/reproductive infections are feasible strategies already employed (Brown and Derkits, 2010).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Dr. Hanna Jaaro-Peled and Ms. Sandra P. Zoubovsky for critical reading of the manuscript. We also thank Ms. Yukiko Lema for preparation of the figure. This work was supported by grants from MH091230 (Atsushi Kamiya), MH083728 (Mikhail V. Pletnikov), MH094268 (Atsushi Kamiya; Mikhail V. Pletnikov), and foundation grants from NARSAD (Atsushi Kamiya; Mikhail V. Pletnikov), S-R (Atsushi Kamiya), BSF (Atsushi Kamiya), and SMRI (Mikhail V. Pletnikov).
References
- Abazyan B., Nomura J., Kannan G., Ishizuka K., Tamashiro K. L., Nucifora F., Pogorelov V., Ladenheim B., Yang C., Krasnova I. N., Cadet J. L., Pardo C., Mori S., Kamiya A., Vogel M. W., Sawa A., Ross C. A., Pletnikov M. V. (2010). Prenatal interaction of mutant DISC1 and immune activation produces adult psychopathology. Biol. Psychiatry 68, 1172–1181 10.1016/j.biopsych.2010.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann R. F., Schloesser R. J., Gould T. D., Manji H. K. (2005). Mood stabilizers target cellular plasticity and resilience cascades: implications for the development of novel therapeutics. Mol. Neurobiol. 32, 173–202 10.1385/MN:32:2:173 [DOI] [PubMed] [Google Scholar]
- Boules M., Shaw A., Fredrickson P., Richelson E. (2007). Neurotensin agonists: potential in the treatment of schizophrenia. CNS Drugs 21, 13–23 10.2165/00023210-200721010-00002 [DOI] [PubMed] [Google Scholar]
- Bradshaw N. J., Christie S., Soares D. C., Carlyle B. C., Porteous D. J., Millar J. K. (2009). NDE1 and NDEL1: multimerisation, alternate splicing and DISC1 interaction. Neurosci. Lett. 449, 228–233 10.1016/j.neulet.2008.10.095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon N. J., Sawa A. (2011). Linking neurodevelopmental and synaptic theories of mental illness through DISC1. Nat. Rev. Neurosci. 12, 707–722 10.1038/nrn3120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. S., Derkits E. J. (2010). Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am. J. Psychiatry 167, 261–280 10.1176/appi.ajp.2010.10040535r [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdick K. E., Kamiya A., Hodgkinson C. A., Lencz T., DeRosse P., Ishizuka K., Elashvili S., Arai H., Goldman D., Sawa A., Malhotra A. K. (2008). Elucidating the relationship between DISC1, NDEL1 and NDE1 and the risk for schizophrenia: evidence of epistasis and competitive binding. Hum. Mol. Genet. 17, 2462–2473 10.1093/hmg/ddn146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callicott J. H., Straub R. E., Pezawas L., Egan M. F., Mattay V. S., Hariri A. R., Verchinski B. A., Meyer-Lindenberg A., Balkissoon R., Kolachana B., Goldberg T. E., Weinberger D. R. (2005). Variation in DISC1 affects hippocampal structure and function and increases risk for schizophrenia. Proc. Natl. Acad. Sci. U.S.A. 102, 8627–8632 10.1073/pnas.0500515102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo A. C., Caldo H., Emson P. C. (1983). Degradation of neurotensin by rabbit brain endo-oligopeptidase A and endo-oligopeptidase B (proline-endopeptidase). Biochem. Biophys. Res. Commun. 116, 1151–1159 10.1016/S0006-291X(83)80263-0 [DOI] [PubMed] [Google Scholar]
- Camargo L. M., Collura V., Rain J. C., Mizuguchi K., Hermjakob H., Kerrien S., Bonnert T. P., Whiting P. J., Brandon N. J. (2007). Disrupted in schizophrenia 1 Interactome: evidence for the close connectivity of risk genes and a potential synaptic basis for schizophrenia. Mol. Psychiatry 12, 74–86 10.1038/sj.mp.4001880 [DOI] [PubMed] [Google Scholar]
- Carter C. J. (2009). Schizophrenia susceptibility genes directly implicated in the life cycles of pathogens: cytomegalovirus, influenza, herpes simplex, rubella, and Toxoplasma gondii. Schizophr. Bull. 35, 1163–1182 10.1093/schbul/sbn157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chubb J. E., Bradshaw N. J., Soares D. C., Porteous D. J., Millar J. K. (2008). The DISC locus in psychiatric illness. Mol. Psychiatry 13, 36–64 10.1038/sj.mp.4002106 [DOI] [PubMed] [Google Scholar]
- Dantzer R., O’Connor J. C., Freund G. G., Johnson R. W., Kelley K. W. (2008). From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 9, 46–56 10.1038/nrn2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisi L. E., Wyatt R. J. (1982). Abnormal immune regulation in schizophrenic patients. Psychopharmacol. Bull. 18, 158–163 [PubMed] [Google Scholar]
- Di Giorgio A., Blasi G., Sambataro F., Rampino A., Papazacharias A., Gambi F., Romano R., Caforio G., Rizzo M., Latorre V., Popolizio T., Kolachana B., Callicott J. H., Nardini M., Weinberger D. R., Bertolino A. (2008). Association of the SerCys DISC1 polymorphism with human hippocampal formation gray matter and function during memory encoding. Eur. J. Neurosci. 28, 2129–2136 10.1111/j.1460-9568.2008.06482.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehninger D., Sano Y., de Vries P. J., Dies K., Franz D., Geschwind D. H., Kaur M., Lee Y. S., Li W., Lowe J. K., Nakagawa J. A., Sahin M., Smith K., Whittemore V., Silva A. J. (2012). Gestational immune activation and Tsc2 haploinsufficiency cooperate to disrupt fetal survival and may perturb social behavior in adult mice. Mol. Psychiatry 17, 62–70 10.1038/mp.2010.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto A., Asai N., Namba T., Wang Y., Kato T., Tanaka M., Tatsumi H., Taya S., Tsuboi D., Kuroda K., Kaneko N., Sawamoto K., Miyamoto R., Jijiwa M., Murakumo Y., Sokabe M., Seki T., Kaibuchi K., Takahashi M. (2009). Roles of disrupted-in-schizophrenia 1-interacting protein girdin in postnatal development of the dentate gyrus. Neuron 63, 774–787 10.1016/j.neuron.2009.08.015 [DOI] [PubMed] [Google Scholar]
- Fu C. A., Shen M., Huang B. C., Lasaga J., Payan D. G., Luo Y. (1999). TNIK, a novel member of the germinal center kinase family that activates the c-Jun N-terminal kinase pathway and regulates the cytoskeleton. J. Biol. Chem. 274, 30729–30737 10.1074/jbc.274.49.34527 [DOI] [PubMed] [Google Scholar]
- Glatt S. J., Everall I. P., Kremen W. S., Corbeil J., Sasik R., Khanlou N., Han M., Liew C. C., Tsuang M. T. (2005). Comparative gene expression analysis of blood and brain provides concurrent validation of SELENBP1 up-regulation in schizophrenia. Proc. Natl. Acad. Sci. U.S.A. 102, 15533–15538 10.1073/pnas.0507666102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori T., Baba K., Matsuzaki S., Honda A., Miyoshi K., Inoue K., Taniguchi M., Hashimoto H., Shintani N., Baba A., Shimizu S., Yukioka F., Kumamoto N., Yamaguchi A., Tohyama M., Katayama T. (2007). A novel DISC1-interacting partner DISC1-binding zinc-finger protein: implication in the modulation of DISC1-dependent neurite outgrowth. Mol. Psychiatry 12, 398–407 10.1038/sj.mp.4001945 [DOI] [PubMed] [Google Scholar]
- Hayashi M. A., Portaro F. C., Bastos M. F., Guerreiro J. R., Oliveira V., Gorrao S. S., Tambourgi D. V., Sant’Anna O. A., Whiting P. J., Camargo L. M., Konno K., Brandon N. J., Camargo A. C. (2005). Inhibition of NUDEL (nuclear distribution element-like)-oligopeptidase activity by disrupted-in-schizophrenia 1. Proc. Natl. Acad. Sci. U.S.A. 102, 3828–3833 10.1073/pnas.0506595102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi-Takagi A., Takaki M., Graziane N., Seshadri S., Murdoch H., Dunlop A. J., Makino Y., Seshadri A. J., Ishizuka K., Srivastava D. P., Xie Z., Baraban J. M., Houslay M. D., Tomoda T., Brandon N. J., Kamiya A., Yan Z., Penzes P., Sawa A. (2010). Disrupted-in-schizophrenia 1 (DISC1) regulates spines of the glutamate synapse via Rac1. Nat. Neurosci. 13, 327–332 10.1038/nn.2487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden M. S., West A. P., Ghosh S. (2006). NF-kappaB and the immune response. Oncogene 25, 6758–6780 10.1038/sj.onc.1209943 [DOI] [PubMed] [Google Scholar]
- Hennah W., Varilo T., Kestila M., Paunio T., Arajarvi R., Haukka J., Parker A., Martin R., Levitzky S., Partonen T., Meyer J., Lonnqvist J., Peltonen L., Ekelund J. (2003). Haplotype transmission analysis provides evidence of association for DISC1 to schizophrenia and suggests sex-dependent effects. Hum. Mol. Genet. 12, 3151–3159 10.1093/hmg/ddg341 [DOI] [PubMed] [Google Scholar]
- Hirotsune S., Fleck M. W., Gambello M. J., Bix G. J., Chen A., Clark G. D., Ledbetter D. H., McBain C. J., Wynshaw-Boris A. (1998). Graded reduction of Pafah1b1 (Lis1) activity results in neuronal migration defects and early embryonic lethality. Nat. Genet. 19, 333–339 10.1038/1221 [DOI] [PubMed] [Google Scholar]
- Hodgkinson C. A., Goldman D., Jaeger J., Persaud S., Kane J. M., Lipsky R. H., Malhotra A. K. (2004). Disrupted in schizophrenia 1 (DISC1): association with schizophrenia, schizoaffective disorder, and bipolar disorder. Am. J. Hum. Genet. 75, 862–872 10.1086/425586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur E. M., Zhou F. Q. (2010). GSK3 signalling in neural development. Nat. Rev. Neurosci. 11, 539–551 10.1038/nrm2939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibi D., Nagai T., Koike H., Kitahara Y., Mizoguchi H., Niwa M., Jaaro-Peled H., Nitta A., Yoneda Y., Nabeshima T., Sawa A., Yamada K. (2010). Combined effect of neonatal immune activation and mutant DISC1 on phenotypic changes in adulthood. Behav. Brain Res. 206, 32–37 10.1016/j.bbr.2009.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka K., Kamiya A., Oh E. C., Kanki H., Seshadri S., Robinson J. F., Murdoch H., Dunlop A. J., Kubo K., Furukori K., Huang B., Zeledon M., Hayashi-Takagi A., Okano H., Nakajima K., Houslay M. D., Katsanis N., Sawa A. (2011). DISC1-dependent switch from progenitor proliferation to migration in the developing cortex. Nature 473, 92–96 10.1038/nature09859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka K., Paek M., Kamiya A., Sawa A. (2006). A review of disrupted-in-schizophrenia-1 (DISC1): neurodevelopment, cognition, and mental conditions. Biol. Psychiatry 59, 1189–1197 10.1016/j.biopsych.2006.03.065 [DOI] [PubMed] [Google Scholar]
- Izzo J. L., Jr., Weir M. R. (2011). Angiotensin-converting enzyme inhibitors. J. Clin. Hypertens. (Greenwich) 13, 667–675 10.1111/j.1751-7176.2011.00508.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jope R. S. (2011). Glycogen synthase kinase-3 in the etiology and treatment of mood disorders. Front. Mol. Neurosci. 4:16. 10.3389/fnmol.2011.00016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya A., Kubo K., Tomoda T., Takaki M., Youn R., Ozeki Y., Sawamura N., Park U., Kudo C., Okawa M., Ross C. A., Hatten M. E., Nakajima K., Sawa A. (2005). A schizophrenia-associated mutation of DISC1 perturbs cerebral cortex development. Nat. Cell Biol. 7, 1167–1178 10.1038/ncb1328 [DOI] [PubMed] [Google Scholar]
- Kamiya A., Tan P. L., Kubo K., Engelhard C., Ishizuka K., Kubo A., Tsukita S., Pulver A. E., Nakajima K., Cascella N. G., Katsanis N., Sawa A. (2008). Recruitment of PCM1 to the centrosome by the cooperative action of DISC1 and BBS4: a candidate for psychiatric illnesses. Arch. Gen. Psychiatry 65, 996–1006 10.1001/archpsyc.65.9.996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang U. G., Seo M. S., Roh M. S., Kim Y., Yoon S. C., Kim Y. S. (2004). The effects of clozapine on the GSK-3-mediated signaling pathway. FEBS Lett. 560, 115–119 10.1016/S0014-5793(04)00082-1 [DOI] [PubMed] [Google Scholar]
- Kilpinen H., Ylisaukko-Oja T., Hennah W., Palo O. M., Varilo T., Vanhala R., Nieminen-von Wendt T., von Wendt L., Paunio T., Peltonen L. (2008). Association of DISC1 with autism and Asperger syndrome. Mol. Psychiatry 13, 187–196 10.1038/sj.mp.4002031 [DOI] [PubMed] [Google Scholar]
- Kim J. Y., Duan X., Liu C. Y., Jang M. H., Guo J. U., Pow-anpongkul N., Kang E., Song H., Ming G. L. (2009). DISC1 regulates new neuron development in the adult brain via modulation of AKT-mTOR signaling through KIAA1212. Neuron 63, 761–773 10.1016/j.neuron.2009.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo K., Tomita K., Uto A., Kuroda K., Seshadri S., Cohen J., Kaibuchi K., Kamiya A., Nakajima K. (2010). Migration defects by DISC1 knockdown in C57BL/6, 129X1/SvJ, and ICR strains via in utero gene transfer and virus-mediated RNAi. Biochem. Biophys. Res. Commun. 400, 631–637 10.1016/j.bbrc.2010.08.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Rosborough K. M., Friedman A. B., Zhu W., Roth K. A. (2007). Regulation of mouse brain glycogen synthase kinase-3 by atypical antipsychotics. Int. J. Neuropsychopharmacol. 10, 7–19 10.1017/S1461145705006425 [DOI] [PubMed] [Google Scholar]
- Mahmoudi T., Li V. S., Ng S. S., Taouatas N., Vries R. G., Mohammed S., Heck A. J., Clevers H. (2009). The kinase TNIK is an essential activator of Wnt target genes. EMBO J. 28, 3329–3340 10.1038/emboj.2009.285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y., Ge X., Frank C. L., Madison J. M., Koehler A. N., Doud M. K., Tassa C., Berry E. M., Soda T., Singh K. K., Biechele T., Petryshen T. L., Moon R. T., Haggarty S. J., Tsai L. H. (2009). Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3beta/beta-catenin signaling. Cell 136, 1017–1031 10.1016/j.cell.2008.12.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieson I., Munafo M. R., Flint J. (2011). Meta-analysis indicates that common variants at the DISC1 locus are not associated with schizophrenia. Mol. Psychiatry. [Epub ahead of print]. 10.1038/mp.2011.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matigian N., Windus L., Smith H., Filippich C., Pantelis C., McGrath J., Mowry B., Hayward N. (2007). Expression profiling in monozygotic twins discordant for bipolar disorder reveals dysregulation of the WNT signalling pathway. Mol. Psychiatry 12, 815–825 10.1038/sj.mp.4001998 [DOI] [PubMed] [Google Scholar]
- Meyer K. D., Morris J. A. (2009). Disc1 regulates granule cell migration in the developing hippocampus. Hum. Mol. Genet. 18, 3286–3297 10.1093/hmg/ddp266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U., Schwarz M. J., Muller N. (2011). Inflammatory processes in schizophrenia: a promising neuroimmunological target for the treatment of negative/cognitive symptoms and beyond. Pharmacol. Ther. 132, 96–110 10.1016/j.pharmthera.2011.06.003 [DOI] [PubMed] [Google Scholar]
- Millar J. K., Pickard B. S., Mackie S., James R., Christie S., Buchanan S. R., Malloy M. P., Chubb J. E., Huston E., Baillie G. S., Thomson P. A., Hill E. V., Brandon N. J., Rain J. C., Camargo L. M., Whiting P. J., Houslay M. D., Blackwood D. H., Muir W. J., Porteous D. J. (2005). DISC1 and PDE4B are interacting genetic factors in schizophrenia that regulate cAMP signaling. Science 310, 1187–1191 10.1126/science.1112915 [DOI] [PubMed] [Google Scholar]
- Millar J. K., Wilson-Annan J. C., Anderson S., Christie S., Taylor M. S., Semple C. A., Devon R. S., St Clair D. M., Muir W. J., Blackwood D. H., Porteous D. J. (2000). Disruption of two novel genes by a translocation co-segregating with schizophrenia. Hum. Mol. Genet. 9, 1415–1423 10.1093/hmg/9.9.1415 [DOI] [PubMed] [Google Scholar]
- Miller A. H., Maletic V., Raison C. L. (2009). Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol. Psychiatry 65, 732–741 10.1016/j.biopsych.2008.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi K., Asanuma M., Miyazaki I., Diaz-Corrales F. J., Katayama T., Tohyama M., Ogawa N. (2004). DISC1 localizes to the centrosome by binding to kendrin. Biochem. Biophys. Res. Commun. 317, 1195–1199 10.1016/j.bbrc.2004.03.163 [DOI] [PubMed] [Google Scholar]
- Miyoshi K., Honda A., Baba K., Taniguchi M., Oono K., Fujita T., Kuroda S., Katayama T., Tohyama M. (2003). Disrupted-in-schizophrenia 1, a candidate gene for schizophrenia, participates in neurite outgrowth. Mol. Psychiatry 8, 685–694 10.1038/sj.mp.4001352 [DOI] [PubMed] [Google Scholar]
- Morris J. A., Kandpal G., Ma L., Austin C. P. (2003). DISC1 (disrupted-in-schizophrenia 1) is a centrosome-associated protein that interacts with MAP1A, MIPT3, ATF4/5 and NUDEL: regulation and loss of interaction with mutation. Hum. Mol. Genet. 12, 1591–1608 10.1093/hmg/ddg162 [DOI] [PubMed] [Google Scholar]
- Nakata K., Lipska B. K., Hyde T. M., Ye T., Newburn E. N., Morita Y., Vakkalanka R., Barenboim M., Sei Y., Weinberger D. R., Kleinman J. E. (2009). DISC1 splice variants are upregulated in schizophrenia and associated with risk polymorphisms. Proc. Natl. Acad. Sci. U.S.A. 106, 15873–15878 10.1073/pnas.0903413106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata K., Ujike H., Sakai A., Takaki M., Imamura T., Tanaka Y., Kuroda S. (2003). The human dihydropyrimidinase-related protein 2 gene on chromosome 8p21 is associated with paranoid-type schizophrenia. Biol. Psychiatry 53, 571–576 10.1016/S0006-3223(02)01729-8 [DOI] [PubMed] [Google Scholar]
- Osbun N., Li J., O’Driscoll M. C., Strominger Z., Wakahiro M., Rider E., Bukshpun P., Boland E., Spurrell C. H., Schackwitz W., Pennacchio L. A., Dobyns W. B., Black G. C., Sherr E. H. (2011). Genetic and functional analyses identify DISC1 as a novel callosal agenesis candidate gene. Am. J. Med. Genet. A 155A, 1865–1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozeki Y., Tomoda T., Kleiderlein J., Kamiya A., Bord L., Fujii K., Okawa M., Yamada N., Hatten M. E., Snyder S. H., Ross C. A., Sawa A. (2003). Disrupted-in-schizophrenia-1 (DISC-1): mutant truncation prevents binding to NudE-like (NUDEL) and inhibits neurite outgrowth. Proc. Natl. Acad. Sci. U.S.A. 100, 289–294 10.1073/pnas.0136913100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y. U., Jeong J., Lee H., Mun J. Y., Kim J. H., Lee J. S., Nguyen M. D., Han S. S., Suh P. G., Park S. K. (2010). Disrupted-in-schizophrenia 1 (DISC1) plays essential roles in mitochondria in collaboration with Mitofilin. Proc. Natl. Acad. Sci. U.S.A. 107, 17785–17790 10.1073/pnas.0909565107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson P. H. (2007). Neuroscience. maternal effects on schizophrenia risk. Science 318, 576–577 10.1126/science.1150196 [DOI] [PubMed] [Google Scholar]
- Pilz D. T., Matsumoto N., Minnerath S., Mills P., Gleeson J. G., Allen K. M., Walsh C. A., Barkovich A. J., Dobyns W. B., Ledbetter D. H., Ross M. E. (1998). LIS1 and XLIS (DCX) mutations cause most classical lissencephaly, but different patterns of malformation. Hum. Mol. Genet. 7, 2029–2037 10.1093/hmg/7.13.2029 [DOI] [PubMed] [Google Scholar]
- Porteous D. J., Millar J. K., Brandon N. J., Sawa A. (2011). DISC1 at 10: connecting psychiatric genetics and neuroscience. Trends. Mol. Med. 17, 699–706 10.1016/j.molmed.2011.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potkin S. G., Turner J. A., Guffanti G., Lakatos A., Fallon J. H., Nguyen D. D., Mathalon D., Ford J., Lauriello J., Macciardi F. (2009). A genome-wide association study of schizophrenia using brain activation as a quantitative phenotype. Schizophr. Bull. 35, 96–108 10.1093/schbul/sbn155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prata D. P., Mechelli A., Fu C. H., Picchioni M., Kane F., Kalidindi S., McDonald C., Kravariti E., Toulopoulou T., Miorelli A., Murray R., Collier D. A., McGuire P. K. (2008). Effect of disrupted-in-schizophrenia-1 on pre-frontal cortical function. Mol. Psychiatry 13, 909, 915–917. 10.1038/mp.2008.76 [DOI] [PubMed] [Google Scholar]
- Purcell S. M., Wray N. R., Stone J. L., Visscher P. M., O’Donovan M. C., Sullivan P. F., Sklar P. (2009). Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature 460, 748–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe M. K., Wiest C., Chuang D. M. (2007). GSK-3 is a viable potential target for therapeutic intervention in bipolar disorder. Neurosci. Biobehav. Rev. 31, 920–931 10.1016/j.neubiorev.2007.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawamura N., Ando T., Maruyama Y., Fujimuro M., Mochizuki H., Honjo K., Shimoda M., Toda H., Sawamura-Yamamoto T., Makuch L. A., Hayashi A., Ishizuka K., Cascella N. G., Kamiya A., Ishida N., Tomoda T., Hai T., Furukubo-Tokunaga K., Sawa A. (2008). Nuclear DISC1 regulates CRE-mediated gene transcription and sleep homeostasis in the fruit fly. Mol. Psychiatry 13, 1069, 1138–1148. 10.1038/mp.2008.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sham P. C., O’Callaghan E., Takei N., Murray G. K., Hare E. H., Murray R. M. (1992). Schizophrenia following pre-natal exposure to influenza epidemics between 1939 and 1960. Br. J. Psychiatry 160, 461–466 10.1192/bjp.160.4.461 [DOI] [PubMed] [Google Scholar]
- Shi J., Levinson D. F., Duan J., Sanders A. R., Zheng Y., Pe’er I., Dudbridge F., Holmans P. A., Whittemore A. S., Mowry B. J., Olincy A., Amin F., Cloninger C. R., Silverman J. M., Buccola N. G., Byerley W. F., Black D. W., Crowe R. R., Oksenberg J. R., Mirel D. B., Kendler K. S., Freedman R., Gejman P. V. (2009). Common variants on chromosome 6p22.1 are associated with schizophrenia. Nature 460, 753–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda T., Taya S., Tsuboi D., Hikita T., Matsuzawa R., Kuroda S., Iwamatsu A., Kaibuchi K. (2007). DISC1 regulates neurotrophin-induced axon elongation via interaction with Grb2. J. Neurosci. 27, 4–14 10.1523/JNEUROSCI.3825-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K. K., Ge X., Mao Y., Drane L., Meletis K., Samuels B. A., Tsai L. H. (2010). Dixdc1 is a critical regulator of DISC1 and embryonic cortical development. Neuron 67, 33–48 10.1016/j.neuron.2010.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares D. C., Carlyle B. C., Bradshaw N. J., Porteous D. J. (2011). DISC1: structure, function, and therapeutic potential for major mental illness. ACS Chem. Neurosci. 2, 609–632 10.1021/cn200062k [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W., Li W., Feng J., Heston L. L., Scaringe W. A., Sommer S. S. (2008). Identification of high risk DISC1 structural variants with a 2% attributable risk for schizophrenia. Biochem. Biophys. Res. Commun. 367, 700–706 10.1016/j.bbrc.2007.12.117 [DOI] [PubMed] [Google Scholar]
- Song W., Li W., Noltner K., Yan J., Green E., Grozeva D., Jones I. R., Craddock N., Longmate J., Feng J., Sommer S. S. (2010). Identification of high risk DISC1 protein structural variants in patients with bipolar spectrum disorder. Neurosci. Lett. 486, 136–140 10.1016/j.neulet.2010.09.027 [DOI] [PubMed] [Google Scholar]
- Stambolic V., Ruel L., Woodgett J. R. (1996). Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr. Biol. 6, 1664–1668 10.1016/S0960-9822(02)70790-2 [DOI] [PubMed] [Google Scholar]
- Stefansson H., Ophoff R. A., Steinberg S., Andreassen O. A., Cichon S., Rujescu D., Werge T., Pietilainen O. P., Mors O., Mortensen P. B., Sigurdsson E., Gustafsson O., Nyegaard M., Tuulio-Henriksson A., Ingason A., Hansen T., Suvisaari J., Lonnqvist J., Paunio T., Borglum A. D., Hartmann A., Fink-Jensen A., Nordentoft M., Hougaard D., Norgaard-Pedersen B., Bottcher Y., Olesen J., Breuer R., Moller H. J., Giegling I., Rasmussen H. B., Timm S., Mattheisen M., Bitter I., Rethelyi J. M., Magnusdottir B. B., Sigmundsson T., Olason P., Masson G., Gulcher J. R., Haraldsson M., Fossdal R., Thorgeirsson T. E., Thorsteinsdottir U., Ruggeri M., Tosato S., Franke B., Strengman E., Kiemeney L. A., Melle I., Djurovic S., Abramova L., Kaleda V., Sanjuan J., de Frutos R., Bramon E., Vassos E., Fraser G., Ettinger U., Picchioni M., Walker N., Toulopoulou T., Need A. C., Ge D., Yoon J. L., Shianna K. V., Freimer N. B., Cantor R. M., Murray R., Kong A., Golimbet V., Carracedo A., Arango C., Costas J., Jonsson E. G., Terenius L., Agartz I., Petursson H., Nothen M. M., Rietschel M., Matthews P. M., Muglia P., Peltonen L., St Clair D., Goldstein D. B., Stefansson K., Collier D. A. (2009). Common variants conferring risk of schizophrenia. Nature 460, 744–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taira K., Umikawa M., Takei K., Myagmar B. E., Shinzato M., Machida N., Uezato H., Nonaka S., Kariya K. (2004). The Traf2- and Nck-interacting kinase as a putative effector of Rap2 to regulate actin cytoskeleton. J. Biol. Chem. 279, 49488–49496 10.1074/jbc.M406370200 [DOI] [PubMed] [Google Scholar]
- Taya S., Shinoda T., Tsuboi D., Asaki J., Nagai K., Hikita T., Kuroda S., Kuroda K., Shimizu M., Hirotsune S., Iwamatsu A., Kaibuchi K. (2007). DISC1 regulates the transport of the NUDEL/LIS1/14-3-3epsilon complex through kinesin-1. J. Neurosci. 27, 15–26 10.1523/JNEUROSCI.3826-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson P. A., Harris S. E., Starr J. M., Whalley L. J., Porteous D. J., Deary I. J. (2005). Association between genotype at an exonic SNP in DISC1 and normal cognitive aging. Neurosci. Lett. 389, 41–45 10.1016/j.neulet.2005.07.004 [DOI] [PubMed] [Google Scholar]
- Tomppo L., Hennah W., Lahermo P., Loukola A., Tuulio-Henriksson A., Suvisaari J., Partonen T., Ekelund J., Lonnqvist J., Peltonen L. (2009a). Association between genes of disrupted in schizophrenia 1 (DISC1) interactors and schizophrenia supports the role of the DISC1 pathway in the etiology of major mental illnesses. Biol. Psychiatry 65, 1055–1062 10.1016/j.biopsych.2009.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomppo L., Hennah W., Miettunen J., Jarvelin M. R., Veijola J., Ripatti S., Lahermo P., Lichtermann D., Peltonen L., Ekelund J. (2009b). Association of variants in DISC1 with psychosis-related traits in a large population cohort. Arch. Gen. Psychiatry 66, 134–141 10.1001/archgenpsychiatry.2008.524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrey E. F., Yolken R. H. (2003). Toxoplasma gondii and schizophrenia. Emerging Infect. Dis. 9, 1375–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Charych E. I., Pulito V. L., Lee J. B., Graziane N. M., Crozier R. A., Revilla-Sanchez R., Kelly M. P., Dunlop A. J., Murdoch H., Taylor N., Xie Y., Pausch M., Hayashi-Takagi A., Ishizuka K., Seshadri S., Bates B., Kariya K., Sawa A., Weinberg R. J., Moss S. J., Houslay M. D., Yan Z., Brandon N. J. (2011). The psychiatric disease risk factors DISC1 and TNIK interact to regulate synapse composition and function. Mol. Psychiatry 16, 1006–1023 10.1038/mp.2010.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M., Yoshida Y., Mori D., Takitoh T., Kengaku M., Umeshima H., Takao K., Miyakawa T., Sato M., Sorimachi H., Wynshaw-Boris A., Hirotsune S. (2009). Inhibition of calpain increases LIS1 expression and partially rescues in vivo phenotypes in a mouse model of lissencephaly. Nat. Med. 15, 1202–1207 10.1038/nm.2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura T., Kawano Y., Arimura N., Kawabata S., Kikuchi A., Kaibuchi K. (2005). GSK-3beta regulates phosphorylation of CRMP-2 and neuronal polarity. Cell 120, 137–149 10.1016/j.cell.2004.11.012 [DOI] [PubMed] [Google Scholar]
- Young-Pearse T. L., Suth S., Luth E. S., Sawa A., Selkoe D. J. (2010). Biochemical and functional interaction of disrupted-in-schizophrenia 1 and amyloid precursor protein regulates neuronal migration during mammalian cortical development. J. Neurosci. 30, 10431–10440 10.1523/JNEUROSCI.1445-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]