Abstract
Objective
To determine whether cerebrospinal fluid (CSF) biomarkers for Alzheimer disease fluctuate significantly over time in a cohort of older, mildly symptomatic individuals.
Design
Biomarker validation in a clinical cohort.
Setting
University hospital inpatient unit.
Participants
Ten patients admitted for CSF drainage for diagnostic purposes.
Main Outcome Measures
The CSF levels of Aβ1–40, Aβ1–42, tau, and phosphorylated tau on threonine 181 (p-tau181) were measured every 6 hours for 24 or 36 hours.
Results
The mean coefficient of variation values for each biomarker assessed in our 10 patients were 5.5% (95% CI, 3.8%–10.0%) for Aβ1–42, 12.2% (9.0%–24.2%) for Aβ1–40, 8.2% (5.7%–15.1%) for total tau, and 11.9% (8.5%–23.0%) for p-tau181. These values are only slightly higher than the variability in the assay. In addition, no significant circadian fluctuation in any Alzheimer disease biomarker was observed given the limitations of our sampling frequency.
Conclusion
In a cohort of elderly patients, little fluctuation in the levels of important Alzheimer disease biomarkers in lumbar CSF is seen as a function of time.
Despite intensive research during the past 2 decades that has led to a better understanding of the pathogenesis of Alzheimer disease (AD), a therapy that alters its progression remains elusive. Several medications are available for symptomatic treatment of AD; however, none modifies the underlying evolution of the disease. Multiple trials1–3 of disease-modifying drugs in AD have failed thus far. One potential reason for this failure is the late stage in which these drugs are administered, a time at which neuronal injury may be irreversible. This has encouraged the identification of biomarkers for AD, which would facilitate early identification of the disease and in turn would enable trials of drug therapy when the course of the disease is still modifiable. Of all the biologically plausible biomarkers under study, only a few have been repeatedly identified by independent multicenter studies to be candidates that closely reflect the hallmark findings of the disease process. These include the serum apolipo-protein E allele status,4 positron emission tomographic imaging of brain amyloid,5,6 and the cerebrospinal fluid (CSF) levels of Aβ1–42, total tau, and phosphorylated tau onthreonine 181 (p-tau181).7–9 The last 3 CSF biomarkers are particularly important, since they also have significant implications for disease-modifying therapy.10–12
One obstacle to widespread use of these CSF biomarkers has been their diurnal variability seen in younger individuals.13 The fluctuations in CSF Aβ1–42 values, in particular, are as much as 3-fold during a 24-hour cycle. This variability poses a significant threat to the usefulness of CSF Aβ1–42 levels as a disease predictor, given that reductions of 30% to 40% can predict conversion from mild cognitive impairment to dementia.9,14 To examine this important issue further, we determined whether the levels of Aβ1–40, Aβ1–42, tau, and p-tau181 in lumbar CSF fluctuate in elderly patients.
METHODS
PARTICIPANTS
Ten patients suspected of having idiopathic normal pressure hydrocephalus (n=9) or pseudotumor cerebri (n=1) who were admitted to the hospital for intracranial pressure monitoring and extended CSF drainage as part of their routine clinical care were recruited. All patients provided consent to participate. Most had relatively modest cognitive problems associated with their suspected diagnosis (mean [SD] Mini-Mental State Examination score, 26.3 [2.9]; range, 20–30). All patients were monitored for at least 1 year after their initial evaluation. Clinical diagnoses of dementia and mild cognitive impairment were made by two of us (A.M. and R.J.O.) on the basis of informant history as well as cognitive testing,15 without knowledge of AD biomarker levels. A diagnosis of mild cognitive impairment required cognitive abnormalities documented by history and testing but no functional loss due to the abnormalities.16 This study was approved by the Johns Hopkins Institutional Review Board.
CSF COLLECTION
All patients underwent insertion of a catheter into the lumbar subarachnoid space on the first day of hospitalization. After monitoring of intracranial pressure for 18 hours, drainage of CSF was initiated at noon the following day. Collection of CSF for analysis was commenced at 6 pm on the first day of drainage (the second hospital day). Forty milliliters of CSF was withdrawn from the lumbar catheter every 6 hours for 24 or 36 consecutive hours. The difference in collection duration was the result of investigator availability. The first 10 mL of CSF collected at each time point was discarded to eliminate CSF that may have pooled in the lumbar catheter. The next 30 mL of fresh CSF was drip-collected into 2-mL aliquots in polypropylene tubes and stored at −80°C until further analysis. Mean time from collection of CSF to storage at −80°C was approximately 15 minutes. The levels of measured AD biomarkers did not differ significantly between the first and last aliquot of the 30-mL CSF collection (data available from the authors on request). We did not record sleeping times; however, all patients were asleep (and remained asleep) when the midnight sample was withdrawn (the investigator used a flashlight and was careful to not awaken the patient) and awake at the noon and 6 pm withdrawals. The unit to which the patients were admitted has a policy to promote sleep at night. Lights on the unit are dimmed between 8 pm and 6 am; noise and noncritical interruptions of the patients’ sleep are minimized. Vital signs are measured at 8 pm and 8 am to allow uninterrupted nighttime sleep. Patients were ambulatory at least once each day and were served meals at 7 am, 11 am, and 6 pm.
CSF ANALYSIS
Different systems were used to analyze CSF for full-length Aβ1–42, total tau, and p-tau181 (xMAP-based AlzBio3 kit [Innogenetics] run on the Bioplex 200 system) vs Aβ1–40 (sandwich enzyme-linked immunoassay kit [Wako] using BAN50 and BA27 antibodies). We used the first 2-mL aliquot of CSF for all the analyses in this study. Each patient had all samples (run in triplicate) analyzed on the same plate. Intra-assay coefficients of variation (CVs) for the plates used in this study were 3.4% (Aβ1–42), 5.8% (tau), 6.8% (p-tau181), and 9.1% (Aβ1–40). Interassay (plate-to-plate) CVs for a single CSF standard run on all plates used in this study were 8.3% (Aβ1–42), 6.9% (tau), 9.3% (p-tau181), and 13.8% (Aβ1–40). Compared with studies9,17 using the same kits and platforms, our absolute results are at the upper (high) end of the center-to-center variability for Aβ1–42 and are at the median levels for tau, p-tau181, and Aβ1–40. Our CVs, plate-to-plate variability, and the dynamic range of our assay are well within published norms.9,17
STATISTICAL ANALYSIS
To detect the presence of significant fluctuations in biomarker levels across participants, a repeated-measures analysis of variance was performed using time (up to 6 time points for each of the 10 patients) as the independent variable, with Greenhouse-Geisser adjustment for nonsphericity. To evaluate overall variation of the biomarkers, within-subject CVs during the sampling period were computed.18 The 95% CIs for these CVs provide information on the range of expected variation in the population (95% of the time) given the data from this sample, and these were determined by assuming a noncentral T distribution.19 Similar results were obtained assuming a normal distribution. To compare different groups (eg, young vs elderly patients and low vs high CSF Aβ1–42), we used an independent-samples t test.
RESULTS
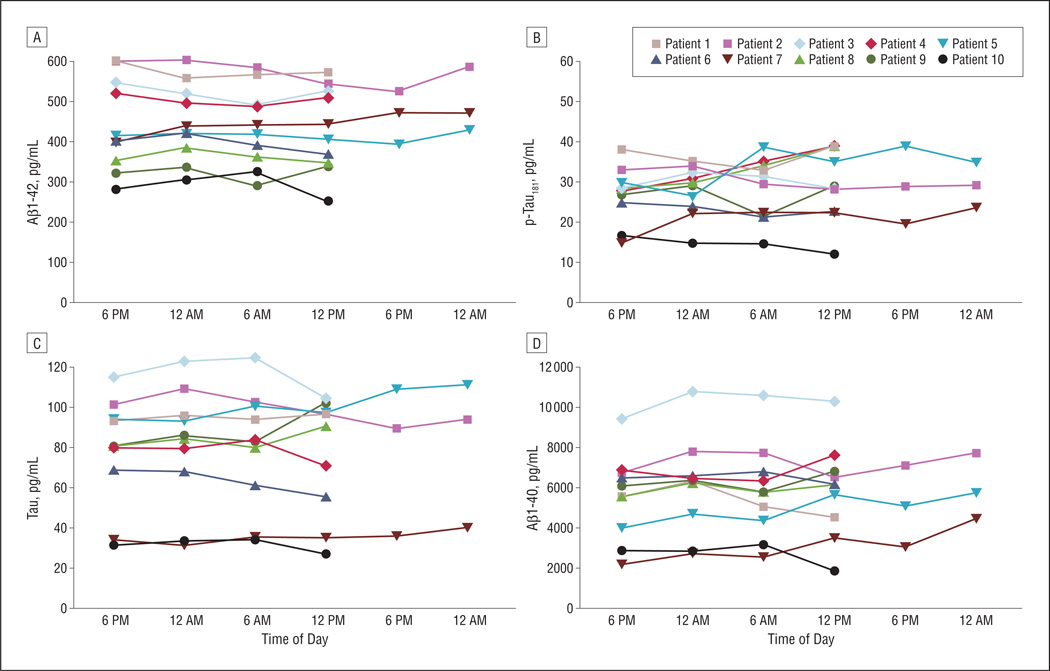
There were 6 men and 4 women in the study, with a mean age of 72 years (Table 1). The age of the patients spanned 5 decades, from 38 years to 87 years. Six patients demonstrated improvement in gait following drainage, indicating that they had a high likelihood for idiopathic normal pressure hydrocephalus, although the condition of 1 patient (patient 5) deteriorated after placement of a permanent ventriculo-peritoneal shunt; she is presumed to have a neurodegenerative disorder. Patient 4, who had elevated CSF pressure and headaches, had pseudotumor cerebri diagnosed (she was not receiving acetazolamide at the time of the drainage). The other 3 patients had an undiagnosed neurodegenerative process associated with cognitive impairment and gait disorder. The CSF was sampled during 24 hours in 7 patients and during 36 hours in 3 patients. The levels of Aβ1–42, Aβ1–40, total tau, and p-tau181, although significantly different between the patients, did not fluctuate appreciably over time within any of them (Figure). The mean CVs were 5.5% (95% CI, 3.8%–10.0%) for Aβ1–42, 12.2% (9.0%–24.2%) for Aβ1–40, 8.2% (5.7%–15.1%) for total tau, and 11.9% (8.5%–23.0%) for p-tau181. Thus, the CV in the population is expected to fall within these CIs 95% of the time given these sample data. Each of these is slightly above the variability of the assay. Significant fluctuations in Aβ1–42 did not occur in the patients with the highest CSF Aβ1–42 levels (ie, those at least risk for coexistent AD pathologic characteristics) as well as in those with the lowest CSF Aβ1–42 levels (ie, those at highest risk for coexistent AD pathologic characteristics).8,9 The mean (SD) CV for Aβ1–42 in patients with the 4 highest mean values of Aβ1–42 (536 [33] pg/mL) was 4.7% (1.2%). This value was not significantly different from that observed in the 4 patients with the lowest mean Aβ1–42 values (346 [43] pg/mL; CV 6.5% [3.3%]; P<.001 for means and P=.32 for CV). Moreover, the mean CV for Aβ1–42 in the 3 youngest subjects in our cohort (mean age, 54.6 [14.0] years; CV 5.6% [3.0%]) is similar to that in the 3 oldest patients (mean age, 84.6 [2.0] years; CV 3.8% [2.3%]; P=.01 for age and P=.41 for CV). When concentrations of individual CSF analytes at different times were pooled across all 10 participants, there was no evidence of a circadian fluctuation (Table 2). In addition, the 6 am value (when all patients had been fasting for at least 10 hours) did not differ significantly from any of the other values determined when the patients were able to eat (Table 2).
Table 1.
Patient Characteristicsa
| Patient No./Sex/Age, y | Initial MMSE Score | Short-term Response to Drainage | Gait/Cognition Response to Permanent CSF Shunt | Cognitive Assessment 1 y After Initial Evaluation |
|---|---|---|---|---|
| 1/M/83 | 27 | Improved gait | Awaiting shunt | Normal |
| 2/F/70 | 27 | No improvement | NA | Dementia |
| 3/M/84 | 26 | Improved gait | Improvement | Normal |
| 4/F/38 | 30 | Improved headache | NA | Normal |
| 5/F/87 | 26 | Improved gait | Deterioration | Dementia |
| 6/M/78 | 29 | No improvement | NA | MCI |
| 7/F/62 | 27 | Improved gait | Improvement | Normal |
| 8/M/78 | 28 | No improvement | NA | Dementia |
| 9/M/75 | 23 | Improved gait | Improvement | MCI |
| 10/M/64 | 20 | Improved gait | Improvement | Dementia |
Abbreviations: CSF, cerebrospinal fluid; MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination; NA, not applicable.
Patient 1 had not had a shunt placed because of recurrent meningitis. Patient 4 received a diagnosis of pseudotumor cerebri after the results of pressure monitoring, response to drainage, and subsequent improvement while receiving acetazolamide. No definitive diagnosis was made for patients 2, 6, and 8.
Figure.
Temporal fluctuations of cerebrospinal fluid (CSF) Aβ1–42, Aβ1–40, tau, and phosphorylated tau on threonine 181 (p-tau181). The CSF levels of each of the 4 biomarkers were determined in specimens collected 6 hours apart, starting at 6 pm. Each point represents the mean of each sample run in triplicate. Data on individual patients are color coded.
Table 2.
Circadian Fluctuations in CSF Alzheimer Disease Biomarkers in 10 Patients
| Concentration by Time Point, Mean (SD), pg/mL |
|||||
|---|---|---|---|---|---|
| Biomarker Level | 6 pm | 12 am | 6 ama | 12 pm | P Valueb |
| Aβ1–40 | 5515 (2058) | 6023 (2280) | 5752 (2254) | 5871 (2244) | .21 |
| Aβ1–42 | 443 (110) | 447 (91) | 437 (92) | 429 (105) | .50 |
| Total tau | 78.6 (25.4) | 81.3 (29.1) | 80.9 (29.3) | 78.5 (29.7) | .72 |
| p-tau181 | 27.0 (7.8) | 28.1 (6.5) | 26.4 (7.1) | 28.1 (9.2) | .66 |
Abbreviations: CSF, cerebrospinal fluid; p-tau181, phosphorylated tau on threonine 181.
All patients were fasting at 6 am.
Determined by repeated-measures analysis of variance.
COMMENT
Multiple studies in the past decade,17 including the most recent from the Alzheimer’s Disease Neuroimaging Initiative,9 have suggested that CSF levels of Aβ1–42, total tau, or p-tau181 could serve as biomarkers for AD risk. The International Working Group for New Research Criteria for the Diagnosis of Alzheimer’s Disease20 has recommended incorporating levels of these biomarkers into routine clinical practice. Indeed, new criteria for AD and mild cognitive impairment have been proposed that rely in part on measurements of levels of these biomarkers in the CSF.
There are many uncertainties regarding the validity of these biomarkers to assess the risk of AD. First, several studies21–23 have shown significant laboratory-to-laboratory variability in CSF levels of Aβ1–42, total tau, or p-tau181, even when using the same reagents and samples. Second, CSF levels of Aβ1–40 and Aβ1–42 have been shown to fluctuate significantly in young volunteers (2- to 3-fold for Aβ1–42) during 24 hours in a partially noncircadian rhythm.13 If this also occurs in older impaired individuals (ie, the ones most likely to receive this test), this variability could prevent recognition of the underlying disease (30%–40% lower levels of CSF Aβ1–42 in individuals at risk for AD).9 Given that the reported variability in CSF Aβ values was not completely explained by circadian factors, standardizing the time of CSF collection would not necessarily correct this problem.
The lack of significant fluctuations in any of the biomarkers we assayed in our study of older, cognitively impaired individuals shows promise for the real-world application of CSF AD biomarkers, as these people are most likely to undergo testing for these biomarkers. Moreover, it is reassuring that changes in the levels of these biomarkers in a clinically relevant cohort imply a disease- or age-relevant variation rather than fluctuations resulting from preanalytical variables such as time of collection.
There are several differences between our study and that of Bateman and colleagues,13 which showed significant variability in CSF Aβ values. First, our patient population was significantly older. However, Bateman and colleagues examined a group of 5 older individuals and found the same variation in AD biomarkers as in the younger controls, and we found no significant difference in the fluctuation of Aβ1–42 between the youngest and oldest patients in our cohort. Thus, the role of age in the discrepant results is uncertain. A second difference between the 2 studies is the sampling frequency. Bateman and colleagues sampled CSF every hour compared with every 6 hours in our study. However, since the peak-to-peak variability for Aβ followed a 12-hour cycle in the prior study, a significant level of variability would have been apparent in our study, which sampled at twice that frequency, consistent with the Nyquist rate.24 Third, all patients in the prior study were in good general health and without neurologic disease in contrast to our participants, each of whom had some ongoing neurologic abnormality. Our reliance on a clinical cohort without healthy controls is a shortcoming of this study and limits comparison with that of Bateman and colleagues. Finally, some technical aspects of CSF collection were different. Because we did not collect samples as frequently, we could discard the first 10 mL of CSF, ensuring that the collected fluid was fresh. In addition, our CSF was dripped directly into polypropylene tubes and frozen almost immediately to minimize any adherence of Aβ to tubing.
Fluctuating levels of CSF Aβ concentrations have been taken as evidence of circadian- or activity-dependent processing of the amyloid precursor protein that produces Aβ.25 Because of our sampling frequency (6 hours) and duration of sampling (36 hours maximum), we cannot exclude fast or slow oscillations in CSF Aβ. Sampling in the lumbar spine is also a limitation to detecting rapid physiologic fluctuations in Aβ, since the lumbar subarachnoid space is far removed from the generation of most Aβ in the cerebral cortex. However, the absence of variability of these AD biomarkers in the lumbar CSF of a clinically relevant cohort of older individuals with neurologic diseases should provide some reassurance as to the validity of these biomarkers in clinical practice.
Acknowledgments
Funding/Support: This study was supported by grants P50 AG05146 and U01AG033655 from the National Institute on Aging, by the Burroughs Wellcome Fund for Translational Research, and by the Intramural Research Program, National Institute on Aging, National Institutes of Health.
Footnotes
Author Contributions: Study concept and design: O’Brien. Acquisition of data: Moghekar and Li. Analysis and interpretation of data: Moghekar, Goh, and Albert. Drafting of the manuscript: Moghekar, Li, and O’Brien. Critical revision of the manuscript for important intellectual content: Goh, Albert, and O’Brien. Statistical analysis: Goh. Obtained funding: Albert and O’Brien. Administrative, technical, and material support: Moghekar and Li.
Financial Disclosure: None reported.
REFERENCES
- 1.Green RC, Schneider LS, Amato DA, et al. Tarenflurbil Phase 3 Study Group. Effect of tarenflurbil on cognitive decline and activities of daily living in patients with mild Alzheimer disease: a randomized controlled trial. JAMA. 2009;302(23):2557–2564. doi: 10.1001/jama.2009.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holmes C, Boche D, Wilkinson D, et al. Long-term effects of Aβ42 immunisation in Alzheimer’s disease: follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008;372(9634):216–223. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- 3.McGuinness B, O’Hare J, Craig D, Bullock R, Malouf R, Passmore P. Statins for the treatment of dementia. Cochrane Database Syst Rev. 2010;(8):CD007514. doi: 10.1002/14651858.CD007514.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Saunders AM, Strittmatter WJ, Schmechel D, et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology. 1993;43(8):1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- 5.Clark CM, Schneider JA, Bedell BJ, et al. AV45-A07 Study Group. Use of florbetapir-PET for imaging beta-amyloid pathology [published correction appears in JAMA. 2011;305(11):1096] JAMA. 2011;305(3):275–283. doi: 10.1001/jama.2010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55(3):306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 7.Sunderland T, Hampel H, Takeda M, Putnam KT, Cohen RM. Biomarkers in the diagnosis of Alzheimer’s disease: are we ready? J Geriatr Psychiatry Neurol. 2006;19(3):172–179. doi: 10.1177/0891988706291088. [DOI] [PubMed] [Google Scholar]
- 8.Sunderland T, Mirza N, Putnam KT, et al. Cerebrospinal fluid beta-amyloid1–42 and tau in control subjects at risk for Alzheimer’s disease: the effect of APOE epsilon4 allele. Biol Psychiatry. 2004;56(9):670–676. doi: 10.1016/j.biopsych.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 9.Shaw LM, Vanderstichele H, Knapik-Czajka M, et al. Alzheimer’s Disease Neuroimaging Initiative. Cerebrospinal fluid biomarker signature in Alzheimer’s Disease Neuroimaging Initiative subjects. Ann Neurol. 2009;65(4):403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blennow K. Biomarkers in Alzheimer’s disease drug development. Nat Med. 2010;16(11):1218–1222. doi: 10.1038/nm.2221. [DOI] [PubMed] [Google Scholar]
- 11.Shaw LM, Korecka M, Clark CM, Lee VM-Y, Trojanowski JQ. Biomarkers of neurodegeneration for diagnosis and monitoring therapeutics. Nat Rev Drug Discov. 2007;6(4):295–303. doi: 10.1038/nrd2176. [DOI] [PubMed] [Google Scholar]
- 12.De Meyer G, Shapiro F, Vanderstichele H, et al. Alzheimer’s Disease Neuroimaging Initiative. Diagnosis-independent Alzheimer disease biomarker signature in cognitively normal elderly people. Arch Neurol. 2010;67(8):949–956. doi: 10.1001/archneurol.2010.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bateman RJ, Wen G, Morris JC, Holtzman DM. Fluctuations of CSF amyloid-beta levels: implications for a diagnostic and therapeutic biomarker. Neurology. 2007;68(9):666–669. doi: 10.1212/01.wnl.0000256043.50901.e3. [DOI] [PubMed] [Google Scholar]
- 14.Okonkwo OC, Mielke MM, Griffith HR, et al. Alzheimer’s Disease Neuroimaging Initiative. Cerebrospinal fluid profiles and prospective course and outcome in patients with amnestic mild cognitive impairment. Arch Neurol. 2011;68(1):113–119. doi: 10.1001/archneurol.2010.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Driscoll I, Resnick SM, Troncoso JC, An Y, O’Brien R, Zonderman AB. Impact of Alzheimer’s pathology on cognitive trajectories in nondemented elderly. Ann Neurol. 2006;60(6):688–695. doi: 10.1002/ana.21031. [DOI] [PubMed] [Google Scholar]
- 16.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 17.Mattsson N, Zetterberg H, Hansson O, et al. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA. 2009;302(4):385–393. doi: 10.1001/jama.2009.1064. [DOI] [PubMed] [Google Scholar]
- 18.Bland JM, Altman DG. Measurement error proportional to the mean. BMJ. 1996;313(7049):106. doi: 10.1136/bmj.313.7049.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelley K. Sample size planning for the coefficient of variation from the accuracy in parameter estimation approach. Behav Res Methods. 2007;39(4):755–766. doi: 10.3758/bf03192966. [DOI] [PubMed] [Google Scholar]
- 20.Dubois B, Feldman HH, Jacova C, et al. Revising the definition of Alzheimer’s disease: a new lexicon. Lancet Neurol. 2010;9(11):1118–1127. doi: 10.1016/S1474-4422(10)70223-4. [DOI] [PubMed] [Google Scholar]
- 21.Lewczuk P, Beck G, Esselmann H, et al. Effect of sample collection tubes on cerebrospinal fluid concentrations of tau proteins and amyloid beta peptides. Clin Chem. 2006;52(2):332–334. doi: 10.1373/clinchem.2005.058776. [DOI] [PubMed] [Google Scholar]
- 22.Schoonenboom NSM, Mulder C, Vanderstichele H, et al. Effects of processing and storage conditions on amyloid beta (1–42) and tau concentrations in cerebrospinal fluid: implications for use in clinical practice. Clin Chem. 2005;51(1):189–195. doi: 10.1373/clinchem.2004.039735. [DOI] [PubMed] [Google Scholar]
- 23.Mattsson N, Blennow K, Zetterberg H. Inter-laboratory variation in cerebrospinal fluid biomarkers for Alzheimer’s disease: united we stand, divided we fall. Clin Chem Lab Med. 2010;48(5):603–607. doi: 10.1515/CCLM.2010.131. [DOI] [PubMed] [Google Scholar]
- 24.Nyquist H. Certain topics in telegraph transmission theory. Am Inst Electrical Eng Trans. 1928;47(2):617–644. [Google Scholar]
- 25.Kang J-E, Lim MM, Bateman RJ, et al. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326(5955):1005–1007. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]