Abstract
Acute emotional arousal moderates the effects of cortisol on memory. However, it is currently unknown how stable inter-individual differences (i.e., traits) moderate cortisol’s effects on memory. In two studies using within-subjects designs -- 31 healthy males in Study 1 and 42 healthy subjects (22 female) in Study 2 -- we measured trait negative affect (NA) and presented emotional and neutral pictures. In Study 1, we manipulated endogenous cortisol levels using a speech stressor following encoding. In Study 2, using a randomized placebo-controlled design, we pharmacologically manipulated cortisol levels prior to encoding (0.1 mg/kg hydrocortisone vs. saline infused over 30 min). Free recall for pictures was subsequently assessed. Trait NA repeatedly moderated the relationship between cortisol and memory formation. Findings suggested the speculative conclusion that the direction of effects may vary by sex. In males, cortisol was related to memory facilitation in subjects with lower Trait NA. Conversely, females with higher Trait NA showed greater cortisol-related increases in memory. Trait NA may be a stable inter-individual difference predicting neurocognitive effects of cortisol during stressors.
Keywords: affect, cognition, cortisol, emotion, endocrine, hormones, learning, memory, stress, trait
Introduction
Stable (i.e., trait-like) negative cognitive biases increase vulnerability for affective psychopathology (Alloy et al., 2006). In addition, stress triggers psychopathological symptomatology in some but not all individuals (Monroe and Harkness, 2005). However, the mechanisms responsible for inter-individual differences in vulnerability to stressors are not completely understood. One aspect of vulnerability to stress may involve inter-individual differences in the effects of stress on neurocognitive processes (e.g., emotional memory biases in depression; over-consolidation of threat-related material in Posttraumatic Stress Disorder; PTSD).
Elevation in the stress hormone cortisol is a primary mechanism through which stress alters neurocognitive processes (de Kloet et al., 1999). The direction of effects of glucocorticoids (GCs; i.e., cortisol in primates and corticosterone in rodents) on learning vary depending on the magnitude of GC elevation -- mild-to-moderate elevations in GCs enhance many neurobiological processes associated with memory formation, but extremely elevated cortisol levels often dampen memory formation (Lupien and McEwen, 1997; Pittenger and Duman, 2008). In addition, the facilitatory effects of GCs on memory formation depend on emotional arousal at the time of GC elevation (Okuda et al., 2004; Abercrombie et al., 2006). Arousal-related noradrenergic activation in the basolateral amygdala is required in order for GCs to affect memory (Roozendaal et al., 2006b). In summary, extensive work has examined how factors at the time of GC elevations (e.g., current neural milieu and emotional state) moderate GC’s effects on memory. However, it is not known how long-lasting inter-individual differences (or “traits”) moderate effects of stress hormones on emotional memory.
Dispositional “affective style” refers to consistent inter-individual differences in mood, emotional reactivity, and emotion regulation (Davidson, 2000). Studies examining a constellation of behavioral and physiological measures (Davidson, 2000) show that affective style can be indexed by biological measures as well as measures of self-reported trait affect. In the current project, we use the Positive Affect and Negative Affect Schedule (PANAS) – Trait Version (Watson et al., 1988) as an index of inter-individual differences in trait affective arousal.
We used two different paradigms to examine in healthy individuals how trait affective arousal moderates the relation between acute cortisol elevations and memory formation. In Study 1 we included only males, and manipulated endogenous cortisol levels using a laboratory-based stressor immediately after encoding emotional and neutral stimuli. Data from Study 1 examining the moderating effects of acute increases in negative affect (i.e., “State NA”) on cortisol’s relation with memory facilitation have been previously published (Abercrombie et al., 2006). Study 2 included males and females, and we manipulated cortisol levels exogenously using hydrocortisone or placebo administration during memory encoding.
Because research shows that cortisol facilitates memory formation preferentially in individuals in an emotionally aroused state (mentioned above), we hypothesized that these findings would extend to trait measures of emotional arousal. In other words, we hypothesized that cortisol would facilitate emotional memory formation preferentially in individuals reporting higher levels of trait emotional arousal (in particular, negative emotional arousal). Furthermore, a rapidly growing literature has established sex differences in the relation between memory and stress (Shors, 2006; Andreano and Cahill, 2009; Wolf, 2009). We therefore hypothesized that the role of trait emotional arousal as a moderator may vary by sex.
In order to make firm inferences about the role of cortisol in cognition, it is essential to jointly examine studies that pharmacolocially manipulate cortisol and studies that manipulate cortisol levels naturalistically (e.g., with a stressor). Studies that manipulate cortisol levels using a lab-based stressor limit inferences about the role of cortisol per se because other elements of a stress response (e.g., autonomic response; activation of neural circuitry) could be responsible for observed effects (which may simply co-vary with cortisol elevations). Studies that pharmacologically manipulate cortisol (vs. placebo) permit firm conclusions regarding the causal role of cortisol elevation, but do not readily allow for generalization of conclusions because of the artificial drug-induced physiological state (i.e., a cortisol elevation absent of other aspects of a stress response). Thus, we used two different studies (one with manipulation of endogenous cortisol, and the other manipulating cortisol exogenously) to examine whether findings replicate across both types of studies.
Study 1
Method
Participants
Thirty-four healthy college-aged males met eligibility criteria. Exclusion criteria were:<18 years old, medical illness, history of head injury, self-reported mental or substance use disorder, daily tobacco use, night shift work, or treatment with medication affecting endocrine or nervous systems. Written informed consent was obtained in accordance with the University of Wisconsin Health Sciences Institutional Review Board.
Three participants were excluded from analyses: One participant revealed marijuana use that was suspected to have altered his data. Two participants were excluded due to experimenter error during stimulus presentation. The final sample contained 31 participants.
Procedure
Participants took part in two laboratory visits: an initial session (beginning at 1630h) including memory encoding followed by a speech stressor (Session 1), followed two evenings later by recall testing in Session 2, which began at either 1700h or 1800h. Additional information regarding procedures is included in the original report (Abercrombie et al., 2006).
Session 1
Participants were instructed to refrain from eating, exercising, and drinking anything but water for the hour prior to the session. Participants encoded 21 pleasant, 21 neutral, and 21 unpleasant photographs (each presented for 6 seconds) from the International Affective Picture System (IAPS; Lang et al., 2001). Endogenous cortisol levels were manipulated using a speech stressor immediately following encoding, which involved 5 minutes of anticipation and 15 minutes of videotaped public speaking in front of a two-person evaluative audience.
Session 2
Free recall was assessed 48h after Session 1. Participants were given 10 minutes to list short descriptions of all pictures they could remember from Session 1. During Session 2 after all other study procedures were completed, inter-individual differences in trait affective arousal1 were measured using the PANAS – Trait Version (Watson et al., 1988).
Salivary Cortisol
Salivary cortisol samples were obtained using Salivettes (Sarstedt Inc., Newton, NC), at multiple timepoints throughout the session. In order to capture cortisol output associated with the speech stressor we used samples taken after the 5-min anticipation, after the 15-min speech, and 10 min after the speech, when cortisol levels typically peaked (see Abercrombie et al. 2006). Salivary cortisol was assayed using Salimetrics (State College, PA) cortisol enzyme immunoassay kits. Mean inter-assay CV% was 7.4%, and mean intra-assay CV% was 3.8%. Cortisol output associated with the speech stressor was computed using area under the curve with respect to ground (AUC; Pruessner et al., 2003) for the 3 cortisol samples taken at the timepoints mentioned above (after anticipation, after speech, and 10 minutes after speech). AUC was computed using log-transformed cortisol values (which are negative, as all our samples fell below 1 µg/dL).
Data Analysis
We conducted a hierarchical regression to test the hypothesis that inter-individual differences in Trait NA and endogenous cortisol output during stress interactively predict subsequent recall performance. In the regression analysis, free recall performance (number of pictures recalled) was the dependent variable, and independent variables were entered as follows: 1st, cortisol AUC; 2nd, Trait NA; and 3rd, the interaction between AUC and Trait NA. Regression analysis was followed by post hoc analyses. We used a median split on Trait NA to create Low and High Trait NA groups. Correlations between cortisol AUC and memory performance were compared for Low vs. High Trait NA groups. Additionally, we examined whether findings varied depending on valence. In order to confirm that these findings were not a recapitulation of findings previously reported for State NA (Abercrombie et al., 2006), we also examined how State NA and Trait NA together predicted memory performance.
Results and Discussion
Interactive effects of Trait NA and inter-individual differences in stress-induced cortisol-output
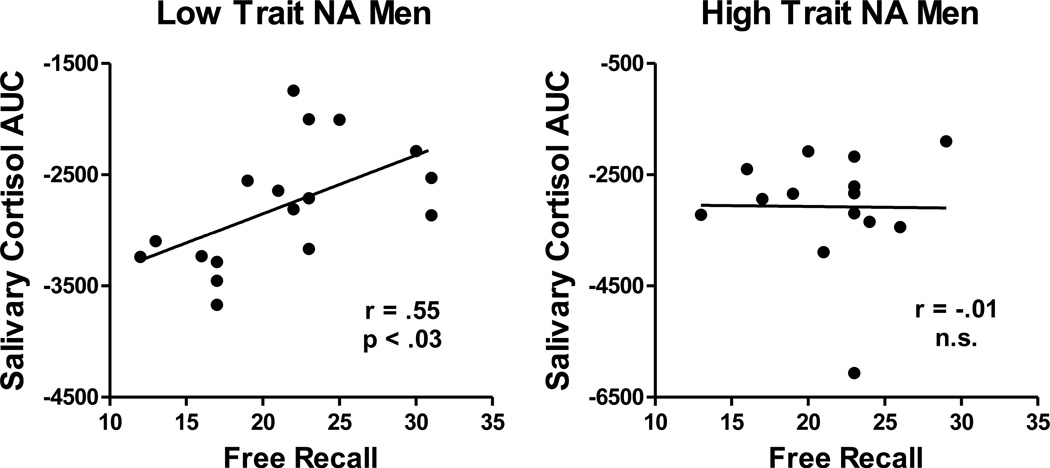
Table 1 displays results from a hierarchical regression predicting free recall performance. The interaction between cortisol AUC and Trait NA significantly predicted free recall performance, accounting for 21% of variance in free recall scores, over and above variance accounted for by cortisol AUC and Trait NA. In order to disentangle the significant interaction, a post hoc median split on Trait NA was used to create Low and High Trait NA groups, with means for Trait NA of 11.9 (1.03) and 17.5 (3.5), respectively.2 Higher cortisol AUC was related to better total recall scores in the Low Trait NA group, r(16) = .55, p < .03, but not in the High Trait NA group, r(13) = -.01, n.s. (Figure 1). Thus, in individuals with lower Trait NA, greater stress-related cortisol output was related to memory facilitation. In individuals with higher Trait NA, stress-related cortisol output was unrelated to memory formation.
Table 1.
Study 1. Log cortisol AUC and Trait NA interactively predict free recall performance (DV = number of words recalled).
| R2 | Increment in R2 | F | p | |
|---|---|---|---|---|
| Log Cortisol AUC | .05 | -- | 1.52 | n.s. |
| Trait NA | .06 | .01 | 0.26 | n.s. |
| Log Cortisol AUC X Trait NA | .27 | .21 | 7.62 | <.02 |
Figure 1. Study 1.
Scatter plots of the relation between log cortisol AUC and free recall performance (total number of pictures recalled), presented separately for Low and High Trait NA groups for men in Study 1. Higher salivary cortisol was related to memory facilitation only in men with Low Trait NA.
Removal of the outlier with very low cortisol levels from the High Trait NA group does not alter the moderating effects of Trait NA. After removal of the outlier, the zero-order correlation reported here for the High Trait NA group is r = .14, n.s. After removal of the outlier, the effects reported in Table 1 are R2 = .30 for the total model with a partial R2 (i.e., increment in R2) of .15, p < .03 for the interaction of cortisol AUC and Trait NA.
Findings presented separately by stimuli valence category
In the Low Trait NA group higher cortisol AUC was related to better recall for both pleasant, r(16) = .56, p < .02, and neutral r(16) = .58, p < .02, but not unpleasant, r(16) = .29, n.s., pictures. In the High Trait NA group, cortisol AUC was unrelated to recall across all emotion categories, r’s ranged .11 to −.26.
Additional analyses to ensure that the findings for Trait NA were not simply a recapitulation of the findings for change in State NA previously reported for this sample (Abercrombie et al., 2006)
In this sample, lower Trait NA was positively related to lower State NA at baseline, r(30) = .43, p < .02, and lower Trait NA was marginally related to greater increase in State NA, r(30) = −.35, p = .06, potentially suggesting that individuals with lower Trait NA tended to show greater increase in State NA. Therefore, for the current analyses, we added the variables tested in the previous report (i.e., change in State NA, the interaction between Trait NA and change in State NA, and the interaction between cortisol AUC and change in State NA) to the model predicting subsequent recall performance. Even after inclusion of these State NA variables in the model, the interaction between Trait NA and cortisol AUC remained, F(6,24) = 4.10, p = .05. Furthermore, the median split on Trait NA used here produced different subsets of subjects than did the median split on change in State NA (used in the previous report); only 32% of the Low Trait NA group also belonged to the High State NA group, and only 39% of subjects fell in the same group (i.e., either Low or High) for both State and Trait NA median splits. Thus, the finding presented herein is novel with respect to our previous finding in this sample (Abercrombie et al., 2006).
Summary
Altogether, these findings in males show that Trait NA moderates the relation between stress-related endogenous cortisol elevations and subsequent recall performance. However, the direction of these findings is opposite to the prediction based on findings regarding acute emotional arousal (i.e., that cortisol and memory would be related only in individuals with high Trait NA). Our current findings show that cortisol output is related to memory facilitation only in males with low levels of Trait NA.
The opposite findings for State vs. Trait NA are consistent with research showing opposite effects of acute vs. chronic stress on emotional learning in males. Acute stress and/or GC elevation has been found to facilitate learning on a number of tasks in males, while chronic stress often impairs learning (Andreano and Cahill, 2009). One could speculate that high Trait NA and a negative affective style (possibly mirroring certain aspects of chronic stress) might weaken or block facilitatory effects of acute GC elevation on explicit memory formation in males.
Study 2
Method
Participants
Fifty-two healthy males and females met eligibility criteria (age between 18 and 35; self-reported good health; English fluency). Exclusion criteria included pregnancy, lactation, daily tobacco use, fear of needles, history of adverse responses to IV or blood draw, medical or psychiatric symptomatology, medication affecting central nervous system function, steroidal medications, adverse responses to steroid medications, and night shift work. Only women using hormonal contraceptives were included to reduce risk of pregnancy and somewhat reduce variability in endogenous HPA activity and reproductive hormones due to menstrual phase (Kirschbaum et al., 1999). Women on different formulations of hormonal contraceptives were included (e.g., monophasic and triphasic formulations). Study sessions were scheduled such that neither drug administration session fell within the “placebo” week of oral contraceptives.
Five participants did not complete memory testing. Data from three additional participants were dropped due to failure to follow instructions or experimenter error. An additional subject fell asleep during a memory encoding session, and was therefore excluded. Another subject showed recall performance that was 3 SDs lower than mean recall performance, and was therefore dropped. The final sample included 22 women and 20 men.
Procedure
Study sessions took place at the Clinical and Translational Research Core (CTRC) at the University of Wisconsin Hospital. Participants completed three sessions, each beginning at 1600h. Participants refrained from food and caffeine intake and exercise for 2h prior to each session. Additional information regarding study procedures can be found in a report not addressing recall performance (Wirth et al., 2011). Written informed consent was obtained in accordance with the University of Wisconsin Health Sciences Institutional Review Board.
In the first two sessions (48 hours apart), participants received received 0.1 mg/kg body weight intravenous hydrocortisone (CORT; synthetic cortisol) or physiological (0.9%) saline (placebo) in a double-blind randomized order, administered over 30 minutes using a programmed pump. Blood samples for measurement of plasma cortisol were taken as follows: 3 samples prior to drug infusion, 1 sample during infusion, 4 samples in the 1st hour following infusion while cortisol levels were elevated, and 3 additional samples were taken during the 2nd and 3rd hours after infusion as cortisol levels began to drop (see Figures 1 and 2 from Wirth et al., 2011). This CORT dose resulted in plasma cortisol levels higher than those caused by moderate stressors like public speaking (Kirschbaum et al., 1993), but were still within the physiological range, comparable to levels resulting from strenuous exercise or asthma-related distress (Fry et al., 1991; Cydulka and Emerman, 1998). It should be noted that despite adjustment of CORT dose by weight, sex differences emerged in peak plasma cortisol, with average (SD) peak of 28.75 (6.88) µg/dL for men and 52.94 (8.15) µg/dL for women.
During the second half of drug infusion, when cortisol levels were significantly elevated on the CORT day (see Wirth et al., 2011), a picture viewing task was administered for memory encoding. During picture viewing in each session, participants encoded 23 unpleasant and 23 neutral IAPS pictures which were displayed for 5 sec3 (Lang et al., 2001). Two sets of psychometrically matched pictures were created to allow for presentation of different pictures in CORT and placebo sessions. To keep participants engaged during picture viewing, they rated pictures for emotional qualities.
The third session (4 days following Session 2) included free recall testing for pictures encoded during CORT and placebo sessions. During the third session after all other study procedures were completed, a packet of questionnaires was administered that included the PANAS-Trait Version (Watson et al., 1988), for measurement of inter-individual differences in Trait NA.
Cortisol Processing
Blood samples were centrifuged for extraction of plasma, which was aliquoted and stored at −80° C until analysis. Cortisol assays were performed with Coat-A-Count radioimmunoassay (RIA) kits (Siemens Healthcare Diagnostics), which have a lower limit of detection of 0.2 µg/dL Mean inter-assay CV% was 5.9%, and mean intra-assay CV% was 4.0%. Post-drug plasma cortisol levels were computed using area under the curve with respect to ground (AUC; Pruessner et al., 2003) for the 6 samples starting immediately after drug infusion and spanning until 2 hours after infusion. The final sample occurring approximately 3 hours after drug infusion was not included in AUC because 5 subjects were missing this sample because of time constraints. AUC was computed using log-transformed cortisol values.
Data Analysis
We analyzed the data using hierarchical regression. The dependent variable was the difference between recall for pictures encoded during CORT vs. during placebo, which reflects magnitude and direction of effects of CORT on subsequent recall for each individual (relative to his/her own recall performance for pictures encoded during placebo). Independent variables were entered as follows: 1st Drug Order, 2nd Cortisol AUC, 3rd Sex, 4th Trait NA, and 5th the interaction between Sex and Trait NA. Drug Order and Cortisol AUC were entered first to test for any effects of order of drug administration (i.e., CORT first vs. placebo first) or variation in magnitude of cortisol elevation, and to adjust for any variance related to these potentially confounding variables. Sex and Trait NA and their interaction were entered to test hypotheses about whether Trait NA moderated effects of CORT on memory formation, and whether these effects varied by Sex.
Next, we conducted a set of analyses analogous to Study 1 analyses in which we examined variation in post-drug cortisol levels after CORT administration in relation to memory performance. Last, we examined whether results varied based on stimuli valence.
Results and Discussion
No main effects of CORT on memory
Drug (i.e., CORT vs. placebo administration) did not significantly affect subsequent recall performance, nor did Sex interact with Drug, p’s > .60. Thus, across all individuals, CORT did not significantly affect memory.
Trait NA and Sex moderated the effects of CORT on memory
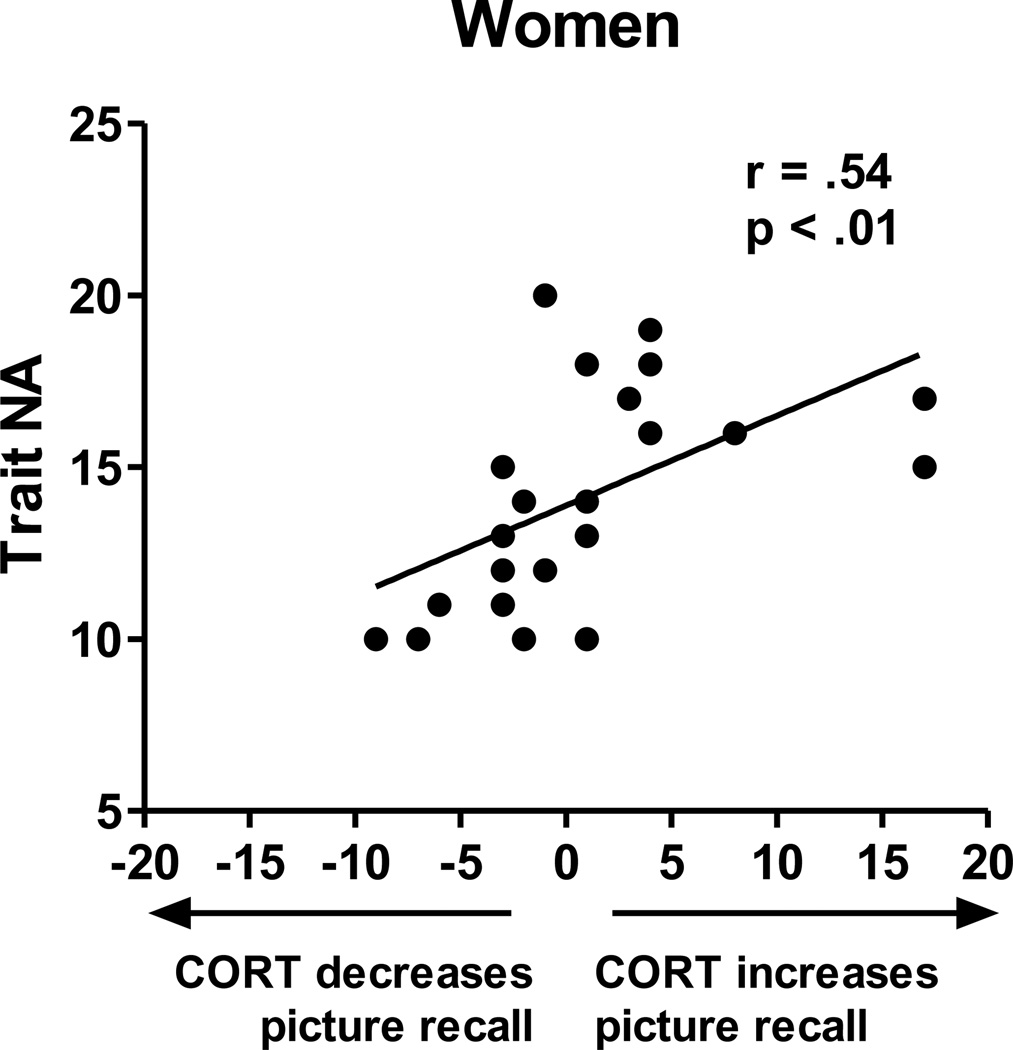
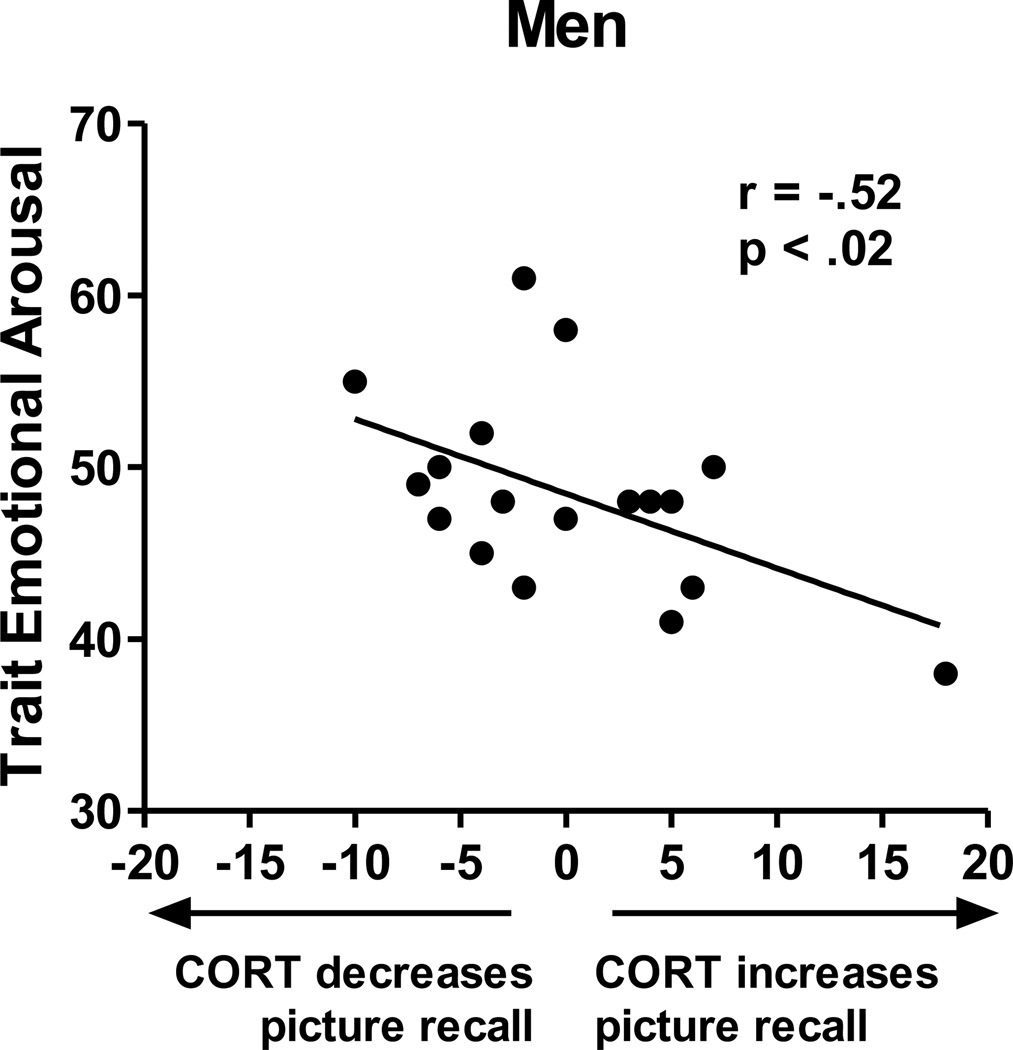
Table 2 displays results from a hierarchical regression predicting the effects of CORT vs. placebo on subsequent recall (DV = subsequent recall for pictures encoded during CORT minus placebo administration). The table shows that 1) Neither Drug Order nor Cortisol AUC significantly affected subsequent recall, 2) neither Sex nor Trait NA alone predicted the effects of CORT on recall, and 3) the interaction between Sex and Trait NA significantly predicted CORT’s effects on subsequent recall performance, accounting for 16% of the variance over and above the already-entered variables. Posthoc tests showed that in women, Trait NA predicted CORT’s effects on total recall, r(21) = .54, p < .01, such that CORT facilitated memory formation predominantly in women with higher Trait NA (Figure 2). In men, Trait NA and recall performance were not significantly related. However, when Trait NA and Trait PA were summed, which reflects overall emotional arousal, trait emotional arousal predicted CORT’s effects on total recall, r(19) = −.52, p < .02, such that CORT facilitated memory formation predominantly in men with lower trait emotional arousal (Figure 3). Thus, findings for overall trait emotional arousal in men in Study 2 were similar to findings in Study 1 in which endogenous cortisol elevations were related to memory facilitation only in men with lower Trait NA.
Table 2.
Study 2. Sex and Trait NA interactively predict CORT’s effects on free recall performance (DV = recall performance for number of words encoded during CORT minus placebo administration). Drug Order and cortisol levels (i.e., Log Cortisol AUC) are potential confounds, and have been placed in the model to adjust for variance related to these factors. It should be noted that the Sex X Trait NA interaction remains significant even when Drug Order and/or Log Cortisol AUC are removed from the model.
| R2 | Increment in R2 | F | p | |
|---|---|---|---|---|
| Drug Order | .03 | -- | 1.52 | n.s. |
| Log Cortisol AUC | .04 | .01 | 0.36 | n.s. |
| Sex | .05 | .01 | 0.14 | n.s. |
| Trait NA | .08 | .03 | 1.07 | n.s. |
| Sex X Trait NA | .24 | .16 | 7.90 | < .01 |
Figure 2. Study 2.
Scatter plot showing the relation between Trait NA and CORT’s effects on total subsequent recall in women. In women, greater Trait NA predicted greater facilitatory effects of CORT administration (vs. placebo) on subsequent recall.
Figure 3. Study 2.
Scatter plot showing the relation between trait emotional arousal (i.e., the sum of PANAS NA and PA) and CORT’s effects on total subsequent recall in men. In men, lower trait emotional arousal predicted greater facilitatory effects of CORT administration (vs. placebo) on subsequent recall.
Tying together Studies 1 and 2
Because we used a placebo-controlled drug manipulation in Study 2, we were able to use each participant as his/her own control (examining the difference in memory performance for words encoded during CORT minus placebo). However, in Study 1, which did not include a control condition, we were limited to examining how Trait NA moderated the relations between memory and inter-individual variation in stress-induced cortisol elevations. In order to directly compare results across both studies, for Study 2 we conducted a set of analyses analogous to Study 1 -- we used a median split on Trait NA to examine whether Trait NA moderated the relation between memory and inter-individual variation in post-drug cortisol elevations on the CORT day.4
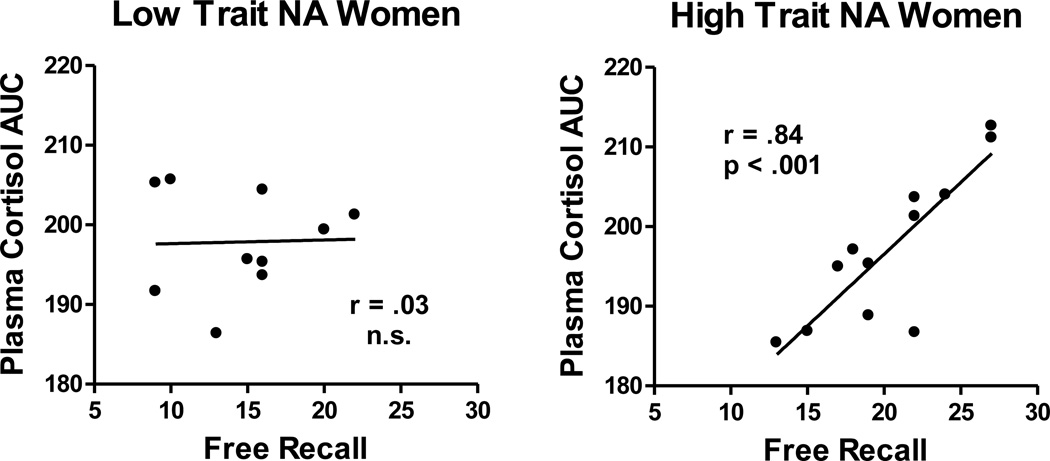
In women in Study 2, variation in post-drug cortisol AUC was related to total recall performance only in women with higher Trait NA. In the group of women with higher Trait NA, the correlation between post-drug cortisol AUC and total recall was r(9) = 0.84, p < 0.001. In the group of women with low Trait NA, the correlation was r(11) = 0.03, n.s. (Figure 4). Thus, both sets of analyses for Study 2 suggest that CORT is related to greater facilitation of memory in women with higher Trait NA.
Figure 4. Study 2.
Scatter plots of the relation between log cortisol AUC and free recall performance (total number of pictures recalled), presented separately for Low and High Trait NA groups for women in Study 2. These plots present result from analyses analogous to those conducted in Study 1. In Study 2, higher salivary cortisol was related to memory facilitation only in the group of women with high Trait NA (based on median split).
However, in men, results were not significant using these analysis methods. After median split based on Trait NA, correlations between post-drug Cortisol AUC and recall performance were not significant in men in Study 2, r’s < 0.30, n.s. Thus, for men in Study 2, only the first analysis strategy presented above suggested that trait emotional arousal moderated the relation between cortisol and memory formation.
Findings presented separately by stimuli valence category
Women. Higher Trait NA predicted greater memory facilitation by CORT for both negative and neutral pictures in women, findings which were significant for negative pictures, r(21) = .51, p < .02, and trend-level for neutral pictures, r(21) = .39, p = .07. When using the analysis strategy similar to Study 1 (examination of the relation between subsequent recall and variation in post-drug cortisol levels on the CORT day), higher cortisol levels were related to memory facilitation in the high Trait NA group but not in the low Trait NA group for neutral pictures: high Trait NA group, r(11) = .82, p < .002, and low Trait NA group r(9) = .04, n.s. A similar pattern was apparent for negative pictures at a trend-level: high Trait NA group, r(11) = .49, p = .10, and low Trait NA group r(9) = .02, n.s. Thus, for both analysis strategies in women, higher Trait NA predicted greater cortisol-related memory facilitation for both negative and neutral stimuli, findings which were statistically significant or at trend-levels when broken down by stimulus type.
Men. As mentioned above, Study 2 did not reveal significant moderation of CORT’s effects by Trait NA in men. However, lower trait emotional arousal (including both positive and negative affective arousal) predicted CORT-related memory facilitation for total recall in men, a finding most apparent for recall of negative pictures, r(19) = −.56, p < .01, but also apparent at a trend-level for neutral stimuli, r(19)< −.37, p = .11. Thus, findings for Study 2 do not significantly differ for negative vs. neutral stimuli.
General Discussion
In two studies with very different designs, Trait NA moderated the relation between cortisol elevations and memory formation. In Study 1, in men with lower Trait NA, greater endogenous cortisol elevations during stress were related to memory facilitation. These data suggest that in men with low Trait NA, cortisol elevations during stress may enhance learning. In males with higher Trait NA, no relation between cortisol elevations and recall performance was observed. These data possibly suggest that high Trait NA weakens or blocks the facilitatory effects of acute cortisol elevations on memory formation in males. These findings are somewhat counter-intuitive, given data showing that higher state emotional arousal appears to play a permissive effect in GCs’ facilitatory effects on memory (Okuda et al., 2004; Abercrombie et al., 2006; Roozendaal et al., 2006a). Some data even suggest that higher state emotional arousal is a necessary prerequisite for GCs’ effects on memory (Roozendaal et al., 2006b). Our findings, however, suggest that even after adjusting for state emotional arousal, lower (but not higher) Trait NA is related to memory facilitation.
In Study 2, men and women received both CORT and placebo, such that each participant served as his/her own pharmacological control. Study 2 showed that Trait NA moderated the effects of CORT on subsequent recall, which was apparent when the interaction between Trait NA and Sex was included in the statistical model. Data in males partially replicated the counter-intuitive findings in Study 1. In Study 2, although trait NA was not related to memory performance, analyses using trait affective arousal (summed across both Trait NA and PA) replicated Study 1. These Study 2 findings in men showed that lower trait affective arousal was associated with greater facilitatory effects of CORT on memory.
Conversely, in women in Study 2, higher Trait NA was associated with greater facilitatory effects of CORT on memory. In women with higher Trait NA, cortisol elevations enhanced memory for unpleasant and neutral pictures. The data in women are more consistent with prediction based on studies manipulating state affect, which show that higher state affective arousal is related to memory facilitation (Abercrombie et al., 2006; Roozendaal et al., 2006a).
It should be noted that we did not find a main effect of CORT on memory performance in Study 2. Although cortisol and other glucocorticoids robustly affect memory, it is a variable phenomenon – with acute cortisol elevations at times impairing and other times enhancing memory (Joëls and Krugers, 2007; Champagne et al., 2008). A number of other studies using pharmacological manipulation of GCs have failed to show effects on declarative memory (Lupien et al., 1999). In addition, other studies have found effects of GCs on one type of measure of declarative memory but not other types (Buchanan and Lovallo, 2001). Many factors alter the effects of GCs on learning, such as level of cortisol elevation and time of day (Lupien and McEwen, 1997; Joëls and Krugers, 2007). The cortisol elevations in Study 2 may have been too high at the time of day we ran subjects (1600h) to observe a consistent effect on memory. Further, possibly the within-subjects design hampered our ability to detect effects. However, the goal of the analyses in humans presented here was to extend recent work in animals that shows that enduring qualities of the individual and the past history of the individual alter the effects of GCs on memory. Our goal was to show that despite null effects of cortisol for the entire group, inter-individual differences in trait affective style moderate the effects of cortisol in humans.
Cortisol and memory: Relevance of the moderating role of Trait NA
Understanding the mechanisms underlying vulnerability to stressors is of great importance. Cortisol plays a role in emotional cognition in psychopathological conditions, such as depression (Abercrombie et al., 2011) and PTSD (de Quervain, 2008). Some individuals may exhibit a heightened risk for cortisol’s facilitatory effects on memory for threatening information. Indeed, PTSD has been associated with heightened neural sensitivity to cortisol (Grossman et al., 2006; Yehuda et al., 2006). On the other hand, various data suggest that depression may be associated with cortisol insensitivity (Raison and Miller, 2003; Pariante, 2009). Some individuals may be over- or under-sensitive to cortisol’s effects on neural processing of positive or neutral information, e.g., its permissive effects on hippocampal neuronal functioning (McEwen, 1994; Abercrombie et al., 2011). Trait NA and Sex may be among the stable factors that predict cognitive and neural sensitivity to cortisol elevations. Data in rodents also suggest that variation in one’s early environment (i.e., levels of maternal care) creates lasting inter-individual differences that predict the direction of GC’s effects on learning (Champagne et al., 2008).
Behavioral and pharmacological interventions can directly target processes related to learning (Pittenger and Duman, 2008). As individuals practice behavioral techniques that alter trait emotional arousal and/or their transient emotional arousal during stress, they may alter the nature in which stress hormones affect memory and neuroplasticity. In addition, animal data show that antidepressants ameliorate the effects of chronic stress on neurobiological mechanisms associated with learning (Pittenger and Duman, 2008). Research in humans has only begun to examine the role of pharmacological agents in the enhancement of therapeutic learning (and/or prevention of maladaptive learning) (de Quervain, 2008; Norberg et al., 2008).
Why do inter-individual differences in NA alter cortisol’s effects on memory?
Animal data show that the basolateral nucleus of the amygdala is necessary for both the enhancing and impairing effects of GCs on memory (Roozendaal et al., 2006a). Individuals dispositionally prone to negative affect show enhanced negative affect-related amygdala activation (Abercrombie et al., 1998; Schwartz et al., 2003; Oler et al., 2010). Possibly, Trait NA moderates the effects of cortisol on memory by virtue of inter-individual differences in thresholds for activation of the amygdala.
Importantly, the effects of stress and/or GCs on learning are often in opposite directions for males and females, or apparent in only one but not the other sex (Andreano and Cahill, 2009). Work in rodents shows that the basolateral amygdala is necessary for induction of opposite effects of stress on learning in males vs. females on an associative learning task (Waddell et al., 2008). These rodent data are very important, because they underscore the role of the basolateral amygdala in stress effects on learning, and provide the novel finding that basolateral amygdala activation is necessary to induce opposite effects in males and females.
However, data from the current studies do not permit firm conclusions about sex differences (i.e., whether Trait NA moderates cortisol’s effects differently for men and women), because of limitations in our project: in Study 1 only males were included; in Study 2 all females were in the active phase of hormonal contraceptive use; and in Study 2, CORT produced differing cortisol levels in males and females. Although findings remain unchanged in Study 2 after adjustment for cortisol levels (log cortisol AUC), the sex differences observed here may be due to differences in the magnitude of cortisol elevations or other factors. Furthermore, variance within the female sample could be related to different formulations of hormonal contraceptives. The primary message of the current report is that Trait NA was repeatedly found to moderate the relation between cortisol and memory formation; it is unclear whether sex accounts for the differences in direction of findings. Replication of sex differences observed here is needed, and the contribution of menstrual phase and hormonal contraceptive use must be determined.
A limitation of this project is that the two studies used very different designs. Study 1 used a within subjects design with manipulation of endogenous cortisol immediately after encoding. The lack of a non-stress control condition limits conclusions that can be drawn from Study 1. Study 2 used a repeated-measures design in which each participant received IV admininistration of CORT or placebo prior to learning. Because Study 2 included a control condition, we were able to examine the effects of CORT compared to placebo, which permitted analyses that did not rely solely on correlations based on inter-individual differences in cortisol levels. Despite the differences in study designs, both studies showed that trait affective arousal moderated the relation between cortisol and memory formation.
Another limiting factor is that the current project does not show whether or not findings depend on the valence of the to-be-remembered stimuli. Although Study 1 showed moderating effects of Trait NA for positive and neutral pictures, Study 2 did not include pleasant pictures and did not show significant differences in the moderating effects of Trait NA on memory for neutral vs. negative pictures. Thus, conclusions cannot be drawn regarding differential effects based on stimuli valence. Future research must specifically examine whether the moderating effects of trait emotional arousal on cortisol’s effects on memory depend on stimuli valence.
Summary
These data are the first we know of to examine how trait affective arousal moderates the relation between acute cortisol elevations and memory formation in humans. In two studies using very different methods, we found that Trait NA moderated the relation between cortisol elevation and memory formation. Identification of trait-like factors that alter the effects of stress hormones on emotional cognition may be essential to understand why some individuals show resilience vs. pathology in the face of stress.
Acknowledgements
We are grateful to Cindy Burzinski, Camilia Cenek, Ned Kalin, Brittany Nanzig, George Nash, Patrick Roseboom, Sean Scherer, Shefaali Sharma, Kyle Swinsky, and UW CTRC nursing staff for their assistance.
Role of Funding Source
This research was funded by NIH award 1K08MH07415-012 to HCA. Data were collected at the University of Wisconsin Clinical and Translational Research Core (CTRC), which is supported by grant 1UL1RR025011 from the Clinical and Translational Science Award (CTSA) program of the NIH National Center for Research Resources. During data collection, MMW was supported by NIH institutional training grant T32MH18931. The NIH had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trait positive affect (PA) and negative affect (NA) were measured. Analyses for Trait PA and NA were conducted in parallel. Trait PA did not moderate cortisol’s relation with memory in either study and is therefore omitted.
The Low vs. High Trait NA groups did not differ on cortisol output during the speech (p > .35) nor on recall performance for total, pleasant, neutral, or unpleasant pictures (p’s > .38). It should also be noted that the “High Trait NA” group exhibits only moderate levels of negative affect, which should be considered “high” levels of NA only within the context of a healthy study sample.
For two subjects, pictures were presented for 6 seconds, which extended the task past the point of automatic shut-off tone on IV pump. Thus, picture presentation was changed to 5 seconds.
It should be noted that neither for women nor men did post-drug AUC values differ for the Low vs. High Trait NA groups, p’s > .79.
Conflict of Interest
All authors declare that they have no conflicts of interest.
Contributors
Heather Abercrombie designed and wrote the protocol for Study 1. Heather Abercrombie, Michelle Wirth, and Roxanne Hoks designed Study 2. Michelle Wirth and Roxanne Hoks wrote the protocol and collected the data for Study 2. Heather Abercrombie undertook the statistical analysis and oversaw all aspects of both studies. Heather Abercrombie wrote the first draft of the manuscript. All authors contributed to and approved the final manuscript.
References
- Abercrombie HC, Jahn AL, Davidson RJ, Kern S, Kirschbaum C, Halverson J. Cortisol's effects on hippocampal activation in depressed patients are related to alterations in memory formation. J Psychiatr Res. 2011;45:15–23. doi: 10.1016/j.jpsychires.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abercrombie HC, Schaefer SM, Larson CL, Oakes TR, Lindgren KA, Holden JE, Perlman SB, Turski PA, Krahn DD, Benca RM, Davidson RJ. Metabolic rate in the right amygdala predicts negative affect in depressed patients. Neuroreport. 1998;9:3301–3307. doi: 10.1097/00001756-199810050-00028. [DOI] [PubMed] [Google Scholar]
- Abercrombie HC, Speck NS, Monticelli RM. Endogenous cortisol elevations are related to memory facilitation only in individuals who are emotionally aroused. Psychoneuroendocrinology. 2006;31:187–196. doi: 10.1016/j.psyneuen.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Alloy LB, Abramson LY, Whitehouse WG, Hogan ME, Panzarella C, Rose DT. Prospective incidence of first onsets and recurrences of depression in individuals at high and low cognitive risk for depression. J Abnorm Psychol. 2006;115:145–156. doi: 10.1037/0021-843X.115.1.145. [DOI] [PubMed] [Google Scholar]
- Andreano JM, Cahill L. Sex influences on the neurobiology of learning and memory. Learn Mem. 2009;16:248–266. doi: 10.1101/lm.918309. [DOI] [PubMed] [Google Scholar]
- Buchanan TW, Lovallo WR. Enhanced memory for emotional material following stress-level cortisol treatment in humans. Psychoneuroendocrinology. 2001;26:307–317. doi: 10.1016/s0306-4530(00)00058-5. [DOI] [PubMed] [Google Scholar]
- Champagne DL, Bagot RC, van Hasselt F, Ramakers G, Meaney MJ, de Kloet ER, Joels M, Krugers H. Maternal care and hippocampal plasticity: evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. J Neurosci. 2008;28:6037–6045. doi: 10.1523/JNEUROSCI.0526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cydulka RK, Emerman CL. Adrenal function and physiologic stress during acute asthma exacerbation. Ann Emerg Med. 1998;31:558–561. doi: 10.1016/s0196-0644(98)70201-x. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Affective style, psychopathology, and resilience: Brain mechanisms and plasticity. Am Psychol. 2000;55:1196–1214. doi: 10.1037//0003-066x.55.11.1196. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Oitzl MS, Joëls M. Stress and cognition: are corticosteroids good or bad guys? Trends Neurosci. 1999;22:422–426. doi: 10.1016/s0166-2236(99)01438-1. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ. Glucocorticoid-induced reduction of traumatic memories: implications for the treatment of PTSD. Prog Brain Res. 2008;167:239–247. doi: 10.1016/S0079-6123(07)67017-4. [DOI] [PubMed] [Google Scholar]
- Fry RW, Morton AR, Garcia-Webb P, Keast D. Monitoring exercise stress by changes in metabolic and hormonal responses over a 24-h period. Eur J Appl Physiol Occup Physiol. 1991;63:228–234. doi: 10.1007/BF00233853. [DOI] [PubMed] [Google Scholar]
- Grossman R, Yehuda R, Golier J, McEwen B, Harvey P, Maria NS. Cognitive effects of intravenous hydrocortisone in subjects with PTSD and healthy control subjects. Ann N Y Acad Sci. 2006;1071:410–421. doi: 10.1196/annals.1364.032. [DOI] [PubMed] [Google Scholar]
- Joëls M, Krugers HJ. LTP after stress: up or down? Neural Plast. 2007;2007:93202. doi: 10.1155/2007/93202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom Med. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The 'Trier Social Stress Test'--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. The Center for Research in Psychophysiology. Gainsville, FL: University of Florida; 2001. International affective picture system (IAPS): Instruction manual and affective ratings. Technical Report A-5. [Google Scholar]
- Lupien SJ, Gillin CJ, Hauger RL. Working memory is more sensitive than declarative memory to the acute effects of corticosteroids: a dose-response study in humans. Behav Neurosci. 1999;113:420–430. doi: 10.1037//0735-7044.113.3.420. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS. The acute effects of corticosteroids on cognition: integration of animal and human model studies. Brain Res Rev. 1997;24:1–27. doi: 10.1016/s0165-0173(97)00004-0. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Corticosteroids and hippocampal plasticity. Ann N Y Acad Sci. 1994;746:134–142. doi: 10.1111/j.1749-6632.1994.tb39223.x. discussion 142–34, 178–139. [DOI] [PubMed] [Google Scholar]
- Monroe SM, Harkness KL. Life stress, the "kindling" hypothesis, and the recurrence of depression: considerations from a life stress perspective. Psychol Rev. 2005;112:417–445. doi: 10.1037/0033-295X.112.2.417. [DOI] [PubMed] [Google Scholar]
- Norberg MM, Krystal JH, Tolin DF. A meta-analysis of D-cycloserine and the facilitation of fear extinction and exposure therapy. Biol Psychiatry. 2008;63:1118–1126. doi: 10.1016/j.biopsych.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Okuda S, Roozendaal B, McGaugh JL. Glucocorticoid effects on object recognition memory require training-associated emotional arousal. Proc Natl Acad Sci USA. 2004;101:853–858. doi: 10.1073/pnas.0307803100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oler JA, Fox AS, Shelton SE, Rogers J, Dyer TD, Davidson RJ, Shelledy W, Oakes TR, Blangero J, Kalin NH. Amygdalar and hippocampal substrates of anxious temperament differ in their heritability. Nature. 2010;466:864–868. doi: 10.1038/nature09282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariante CM. Risk factors for development of depression and psychosis. Glucocorticoid receptors and pituitary implications for treatment with antidepressant and glucocorticoids. Ann N Y Acad Sci. 2009;1179:144–152. doi: 10.1111/j.1749-6632.2009.04978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change.[see comment] Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry. 2003;160:1554–1565. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Okuda S, de Quervain DJ, McGaugh JL. Glucocorticoids interact with emotion-induced noradrenergic activation in influencing different memory functions. Neuroscience. 2006a;138:901–910. doi: 10.1016/j.neuroscience.2005.07.049. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Okuda S, Vander Zee EA, McGaugh JL. Glucocorticoid enhancement of memory requires arousal-induced noradrenergic activation in the basolateral amygdala. Proc Natl Acad Sci U S A. 2006b;103:6741–6746. doi: 10.1073/pnas.0601874103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz CE, Wright CI, Shin LM, Kagan J, Rauch SL. Inhibited and uninhibited infants "grown up": adult amygdalar response to novelty. Science. 2003;300:1952–1953. doi: 10.1126/science.1083703. [DOI] [PubMed] [Google Scholar]
- Shors TJ. Stressful experience and learning across the lifespan. Annu Rev Psychol. 2006;57:55–85. doi: 10.1146/annurev.psych.57.102904.190205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell J, Bangasser DA, Shors TJ. The basolateral nucleus of the amygdala is necessary to induce the opposing effects of stressful experience on learning in males and females. J Neurosci. 2008;28:5290–5294. doi: 10.1523/JNEUROSCI.1129-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wirth MM, Scherer SM, Hoks RM, Abercrombie HC. The effect of cortisol on emotional responses depends on order of cortisol and placebo administration in a within-subject design. Psychoneuroendocrinology. 2011;36:945–954. doi: 10.1016/j.psyneuen.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf OT. Stress and memory in humans: twelve years of progress? Brain Res. 2009;1293:142–154. doi: 10.1016/j.brainres.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Yang RK, Buchsbaum MS, Golier JA. Alterations in cortisol negative feedback inhibition as examined using the ACTH response to cortisol administration in PTSD. Psychoneuroendocrinology. 2006;31:447–441. doi: 10.1016/j.psyneuen.2005.10.007. [DOI] [PubMed] [Google Scholar]