Abstract
Methamphetamine (MA) use increases the likelihood of engaging in risky sexual behavior and most MA-using women are of child-bearing age. Therefore, cognitive effects following MA exposure to the developing brain are concerning. Exposure of mice to MA during hippocampal development causes cognitive impairments in adulthood. These effects are more severe in female than male mice and mimicked by the H3 receptor antagonist thioperamide (THIO). In this study, we assessed whether neonatal exposure to MA or THIO also affects cognition in adolescence. As these effects might be associated with alterations in circadian activity, we also assessed circadian activity in a subgroup of neonatally exposed mice. Sex-dependent treatment effects were seen in the water maze. While THIO-, but not MA-, treated female mice showed hippocampus-dependent spatial memory retention in the first probe trial, MA-, but not THIO-treated female mice showed spatial memory retention in the probe trial following reversal training. In contrast, MA- and THIO-treated male mice showed spatial memory retention in both probe trials. When sensorimotor gating was assessed, MA-treated male mice showed greater pre-pulse inhibition than MA-treated female mice. Regardless of sex, THIO-treated mice gained on average more weight each day and showed an enhanced startle response. In addition, MA increased the length of the circadian period, with an intermediate effect following THIO treatment were observed. No treatment effects in exploratory behavior, measures of anxiety, or contextual or cued fear conditioning. Thus, the water maze is particularly sensitive to detect sex-dependent effects of neonatal MA and THIO exposure on spatial memory retention in adolescence.
Keywords: Methamphetamine, thioperamide, cognition, circadian, adolescence, hippocampus, postnatal
1. Introduction
Compared to other drugs of abuse, methamphetamine (MA) use by women is high. [1] and MA use increases the likelihood of engaging in risky sexual behavior [2]. Fetuses are at high risk of MA exposure since most MA-using women are of child-bearing age and MA use is the most common reason for pregnant women to seek drug counseling [3, 4]. There are significant concerns about the short- and long-term effects following MA exposure to the developing brain. Maternal MA use is associated with increased risk of preterm birth, complications associated with hypertension, placental abruption, and physical abnormalities at birth [5, 6], as well as cognitive impairments [7], including hippocampus-dependent cognitive impairments [8], in childhood.
Consistent with the human studies, exposure of rodents to MA during hippocampal development (the first three postnatal weeks in rodents, modeling human hippocampal development during the third trimester [9]) causes hippocampus-dependent and hippocampus-independent cognitive impairments [10–12] in adulthood. Adult female mice are more severely affected than adult male mice [11, 12], perhaps due to enhanced release of histamine (HA) [11]. HA levels in the brains of MA-exposed neonatal mice are elevated [13]. In addition, increasing HA release with the H3 receptor antagonist thioperamide (THIO) mimicked the detrimental effects of MA on cognition at 3 months of age [11]. Following MA administration, neonatal exposure to MA and THIO administration might also have similar effects on cognition during adolescence.
HA plays a role in the generation and entrainment of circadian rhythms. HA, involving H1 and H3 receptor-mediated signaling, is a key regulator of wakefulness and proposed as the final neurotransmitter in the entrainment of circadian rhythms [14, 15]. The effects of HA likely involve projections from the tuberomamillary nucleus to the suprachiasmatic nucleus (SCN). Potential cognitive effects of neonatal exposure to MA and THIO might be associated with alterations in circadian activity. For example, in aged female Rhesus macaques, the level of circadian activity is associated with improved spatial learning and memory [16]. Animals with better hippocampus-dependent spatial learning and memory show more overall activity, higher daytime activity and a larger ratio of day activity to night activity. In the current study, we assessed behavioral performance in adolescence following neonatal exposure to MA or THIO and assessed whether these exposures affect circadian activity by analyzing the behavioral free running period determined by the endogenous circadian clock.
2. Materials and methods
2.1. Animals
Sixteen total litters of C57BL/6J mouse pups were bred in our colony using breeding cages containing one 76–103 day-old male and two 135–158 day-old female mice. Litter sizes of 2–10 pups were injected using a within-litter design to balance the number of distinct treatment injections within a litter and across sexes. Every litter had up to 4 MA-injected pups per litter. Female mice were singly housed from the first sign of pregnancy. Lab chow (PicoLab Rodent Diet 20, #5053; PMI Nutrition International, St. Louis, MO) and water were given ad libitum. On PND21, pups were weaned and group housed with five mice per cage according to sex. They were provided soft foods from day 1 to day 45 to maintain stable weight gain. The mice were kept on a 12 hr light/dark schedule (lights on at 06:00). Behavioral testing took place during the light cycle. All procedures conformed to the standards of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee of the Oregon Health and Science University.
2.2. Injections
(d)-MA hydrocholoride (5 mg/kg), obtained from the Research Triangle Institute (Research Triangle Park, NC) through the National Institute on Drug Abuse drug supply program or the histamine H3 receptor antagonist thioperamide (THIO) (5 mg/kg) synthesized by Dr. de Esch (Free University, Amterdam, The Netherlands), was diluted with 0.9% sodium chloride (saline (SA)) to the appropriate concentration. The MA and THIO dose produces activation of the hypothalamic-pituitary-adrenal axis in 11–20 day-old mice [13] and cognitive impairments and reduced levels of the dendritic marker microtubule-associated protein 2 (MAP-2) immunoreactivity in the hippocampus and cortex of 90-day-old mice [11–13]. Male and female pups were weighed and injected with MA, THIO, or SA from PND 11–20 (injection volume of 0.1mL), once daily between 6:30 and 9:00 AM on postnatal days 11–20.
2.3. Behavioral testing
Beginning on PND 28, mice were individually housed. Behavioral testing began on PND 30. All mice were tested consecutively using the following sequence of tests: open field and elevated zero maze (week 1), water maze test (week 2), and pre-pulse inhibition and contextual and cued fear conditioning (week 3). This order of testing was used to begin with the least stressful assessment and end with those thought to be most stressful. Following behavioral testing circadian activity was assessed in a subgroup of MA, THIO, and saline-treated female and male mice.
2.3.1. Open Field
The open field test (Kinder Scientific, San Diego, CA) was used to assess exploratory and anxiety-like behaviors, as previously described [17]. Mice were placed in a brightly lit (800 lux) open chamber (40.64 cm × 40.64 cm) for 10 minutes. Mice that are more anxious spend less time exploring the center of the chamber compared to less anxious mice. Measures included total distance moved as measure of exploratory behavior and percent time spent in the center of the chamber as measure of anxiety. After each trial, the equipment was cleaned with 5% acetic acid to remove residual orders.
2.3.2. Elevated Zero Maze
The elevated zero maze (Kinder Scientific, San Diego, CA) was used to assess anxiety-like behaviors during a 10 min trial. In the elevated zero maze. The percent time spent in the open areas was analyzed as measures of anxiety, with more anxious mice spending less time in the open areas. Total distance moved in the elevated zero maze was analyzed as a measure of exploratory activity and to assure that potential alterations in measures of anxiety were not due to differences in activity level. After each trial, the equipment was cleaned with 5% acetic acid to remove residual orders.
2.3.3. Water Maze
The water maze was used to assess spatial learning and memory requiring navigation. The mice were first trained to locate four visible platform locations (Sessions 1–4) and subsequently, trained to locate two hidden platform locations (Sessions 5–8 and 9–12, respectively). Probe trial tests (no platform) were used to assess whether mice show a bias towards the learned hidden platform location.
A circular pool (140cm diameter) was filled with water made opaque by adding chalk (22°C± 2°C) and contained a platform (20 cm wide) approximately 1 cm below water level. On the first two days of water maze testing, mice were first trained to locate a visible platform (platform flagged with a visible beacon) in two trials per session with five-minute inter-trial intervals and two sessions per day (two hours apart). Trials ended when mice reached the platform and remained on it for 10 seconds or when 60 seconds elapsed, at which point the mice were guided to the platform and allowed to remain on it for 10 seconds. Swim speeds during visible platform training were used to assess potential differences in vision, locomotion, motivation, and differences in task learning. After visible platform training, the mice were trained to locate a hidden platform in two trials per session with five-minute inter-trial intervals and two sessions per day (two hours apart). On days 3 and 4, the location of the hidden platform remained constant although the drop location varied for each trial. On days 5 and 6, the platform was moved to a new location (reversal training) and the mice were trained to locate the novel platform location using the same number of trials and sessions used for the first platform location. A 60 sec probe trial test (platform removed) was conducted 1 hour after the last trial of fourth and sixth day of hidden platform training. Performance measures for visible and hidden platform location training included swim speeds, time to reach the platform (latency), and total distance swam. For the probe trials, spatial memory retention (target preference or bias) was assessed by analyzing how much time the mice spent in the quadrant where the platform was located during hidden training (target quadrant) versus the three non-target quadrants. In addition, the % of mice in each group that showed a spatial bias and spent more time in the target quadrant than any other quadrant was also analyzed. The swimming patterns of the mice were analyzed using the Ethovision video tracking system set at 6 samples/sec.
2.3.4. Pre-pulse inhibition
Mice were placed in an enclosure within a startle monitor sound-attenuated chamber and startle response amplitudes were measured with a force transducer (Kinder Scientific, Poway, CA). Following a 5 min acclimation period, mice were exposed to three acoustic stimuli (40 ms, 110 db). The testing phase consisted of 20 ms pre-pulses (70–80 db) followed by 50 ms delays and 40 ms acoustic stimuli (110 db). The same pattern of acoustic stimuli and testing with pre-pulses then occurred with a 120 db stimulus. Random inter-trial intervals were used between trials (15–30 sec). Pre-Pulse inhibition (PPI) was calculated using the following formula: % response = 100 × ((SPS)/S), where S was the mean startle amplitude without a pre-pulse and PS was the mean startle response following a pre-pulse. Thus, a 100% response in the PPI test indicates complete inhibition of the startle response during pre-pulse trials.
2.3.5. Contextual and Cued Fear Conditioning
Contextual and cued fear conditioning tests were performed as previously described [18]. In contextual fear conditioning, the mice learn to associate the environment (fear conditioning chamber, conditioned stimulus (CS)) with a mild foot shock (unconditioned stimulus (US)), while in cued fear conditioning, the mice learn to associate a cue (tone (CS)) with a mild foot shock (US). During training on day 1, the mice were placed in a fear conditioning chamber and allowed to explore for 2 min before the delivery of a 30 second tone which was immediately followed by a 2 second foot shock. Two minutes later a second CS-US pair was delivered. After 24 hours, the mice were first tested for contextual testing by placing them in the same context in the absence of the US, and cued testing where the mouse was placed in a different context in the absence of the US for 5 min. Freezing behavior was analyzed for the first 3 min. One hour later, the mice were tested for cued fear conditioning by placing them in a new context (containing a different odor, floor texture, walls, and shape). After 3 min, the mice were re-exposed to the tone for 3 min. Freezing was analyzed for the entire 6 min of the cued fear conditioning test. Freezing behavior was analyzed using Ethovision XT video tracking and 2% tresholding (Noldus, Leesburg, VA).
2.4. Determination of the Circadian Period
Finally, a subgroup of the mice was individual housed in Nalgene cages equipped with running wheels and magnetic switches (Minimitter, Bend, OR, USA). The cages were placed into Intellus Control System chambers (Percival Scientific, Perry, IA, USA) maintained at 21–22°C on a 12:12 hr light:dark cycle. The wheel revolutions were counted and stored on a computer using Vitalview software (Minimitter, Bend, OR, USA). The data were analyzed and actograms generated using Clocklab (Actimetrics Software, Wilmette, IL, USA) and MATLAB software (Mathworks, Natick, MA, USA). Wheel running behavior was analyzed following 14 days of entrainment to a 12:12 light dark cycle followed by up to 28 days of constant darkness. During the constant dark period, circadian periods (τ) were calculated from at least 9 days of free running behavior with a F periodogram (Clocklab) to determine the circadian period. In addition, the onset of running wheel activity was calculated each day as the difference in the onset of activity and the time of lights-off on the last day of the light-dark cycle [19].
2.5. Statistical analysis
The data are presented as Means ± S.E.M. All statistical analyses were performed using SPSS (Chicago, IL) or Statistica (Tulsa, OK) software and were considered significant at P < 0.05. The pups were individually analyzed during the injection period, and repeated measures ANOVA was used to assess the amount of weight gained each day during the injection period. Performance on all of the behavioral and cognitive tests was assessed using ANOVA with sex and/or treatment as between factors. Data were assessed for normality and homogeneity of variance to determine whether to use parametric or non-parametric statistical analyses. For the open field test, elevated zero maze, and conditioned fear tests, treatment and/or sex were used as between-subject factors to assess potential group differences using 2-way ANOVAs. Post-hoc tests were assessed where appropriate and the type of post-hoc indicated for each test in the results section. For the water maze platform training trials, body weight gains, and PPI, data were analyzed using repeated measures ANOVAs with sex and/or treatment as between subject-factors and sessions as within subject factors. For the water maze test, visible and hidden platform training sessions were analyzed separately. The outcome of Mauchly's Test of Sphericity was assessed for analysis involving repeated measures ANOVA. Violations of Maulchy's Test of Sphericity were addressed using the Green-Geisser correction. For the water maze probe trial analysis of target preference, one-way ANOVAs were used to assess the effect of quadrant within each group. When the overall ANOVA was significant, Tukey's post-hoc test was used to compare time in the target quadrant versus time in the three non-target quadrants.
3. Results
3.1. Weight gain
The pups were weighed each day during the injection period (PND 11–20) to monitor weight gain. Repeated measures ANOVA showed an effect of postnatal day on the amount of weight gained from the previous day (F (4.46,241.09) = 5.175, p < 0.001, Table 1). Post hoc tests showed that all of the mice tended to gain more weight at the beginning of the injection period and the amount of weight gained from the previous day declined toward the end of the injection period. The repeated measures ANOVA also showed an effect of treatment on weight gained from the previous day (F (2, 54) = 3.253, p = 0.046, Table 1). Post hoc tests showed that THIO-treated mice gained on average more weight each day than SA-treated mice, regardless of sex (p=0.034; Table 1). There was no effect of sex on weight gained from the previous day during the injection period (F (1, 54) = 0.128, p > 0.70).
Table 1.
Body weights, Open Field, and Elevated Zero Maze Performance
| Body | ||||||
|---|---|---|---|---|---|---|
| Weightsa Treatment | Sex | Weight Gain (g)(P20–P11) | Weight (g) PD21 | Sex | Weight Gain (g)(P20–P11) | Weight (g) PD21 |
| SA | F | 2.644±0.308 | 7.844±0.450 | M | 2.633±0.233 | 7.941±0.465 |
| THIO | F | 3.270±0.396 | 8.080±0.198 | M | 3.250±0.102 | 8.60±0.305 |
| MA | F | 2.756±0.154 | 8.033±0.267 | M | 2.958±0.164 | 8.667±0.210 |
|
| ||||||
| Open Field b | ||||||
| Treatment | Sex | Distance Moved (cm) | % Time in Center | Sex | Distance Moved (cm) | % Time in Center |
| SA | F | 4406.9±466.86 | 9.463±1.37 | M | 3903.98±133.26 | 11.525±1.48 |
| THIO | F | 4894.58±313.09 | 9.606±1.37 | M | 3909.695±260.58 | 8.521±1.623 |
| MA | F | 4064.95±399.123 | 7.62±0.929 | M | 3693.5±181.211 | 9.550±1.532 |
|
| ||||||
| Elevated | ||||||
| ZeroMazec Treatment | Sex | Distance Moved (cm) | % Time in Open Areas | Sex | Distance Moved (cm) | % Time in Open Areas |
|
| ||||||
| SA | F | 1868.457±84.606 | 22.032±2.544 | M | 1647.277±62.709 | 20.993±3.937 |
| THIO | F | 1895.627±97.535 | 24.027±2.777 | M | 1607.831±34.927 | 19.002±2.305 |
| MA | F | 1795.512±144.673 | 17.869±2.043 | M | 1605±109.92 | 19.829±2.290 |
All measures shown are averages ± SEM. SA=saline; MA=methamphetamine; THIO=thioperamide. N=8–12 mice/sex/treatment.
RM ANOVA indicated a main effect of postnatal day (P < 0.001) on weight gain and of treatment (p<0.05) with THIO-treated mice gaining significantly more weight than SA-treated mice.
ANOVA indicated that in the open field
elevated zero maze female mice moved more than male mice, regardless of treatment (P < 0.05) and P < 0.001; respectively).
3.2. Exploration and measures of anxiety in the open field and elevated zero maze
Regardless of treatment, female mice moved more than male mice in the open field (F (1,42) = 4.254, p < 0.05) and elevated zero maze (F (1, 42) = 8.907, p < 0.005) tests (Table 1). There was no effect of either treatment or sex on measures of anxiety in the open field or elevated plus maze (Table 1).
3.3 Water maze
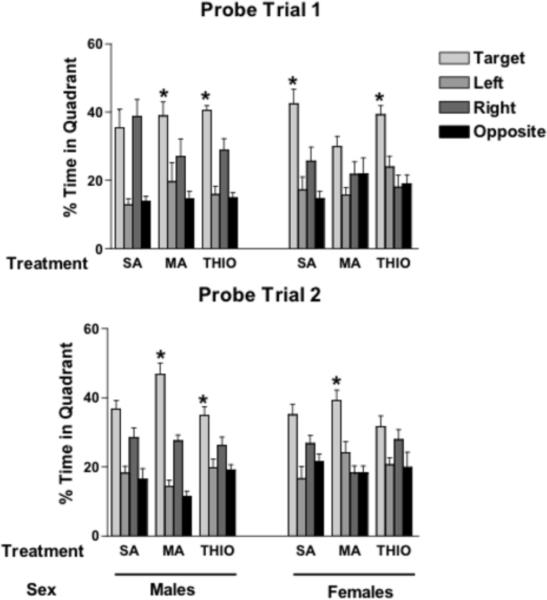
The latency to the platform was used as a measure of performance since there were no group differences in swim speeds during the visible platform training sessions in the water maze (Table 2). All groups of mice learned the task and improved their performance during the visible platform training (effect of session: F(1.96,82.47) = 56.46; p < 0.001). There were no significant effects of the treatments, sex, or interactions between them (data not shown). During the hidden platform training sessions, all groups of mice learned the first (F(2.51,105.34) = 10.89; p < 0.001) and second (F(3,126) = 29.45; p < 0.001) platform location. There were no significant effects of the treatments, sex, or interactions between them (data not shown). Similar data were obtained when total distance swam was analyzed as performance measure (Table 2). However, group differences were seen when spatial memory retention was assessed in the probe trials (Fig. 1). Following hidden platform training for the first platform location, MA- and THIO-treated male mice showed spatial memory retention and searched longer in the target quadrant than any other quadrant but SA-treated male mice did not. In contrast, SA- and THIO-treated female mice showed spatial memory retention in the first probe trial but MA-treated female did not. These data show that neonatal MA exposure causes sex-dependent impairments in spatial memory retention in the first probe trial.
Table 2.
Mean swim speeds and distance swam in the water maze
| Treatment | Sex | Mean velocity visible sessions (cm/sec) | Mean distance swam visible platform (cm) | Mean distance swam first hidden platform (cm) | Mean distance swam second hidden platform (cm) |
|---|---|---|---|---|---|
| SA | Males | 15.0 ± 0.5 | 310 ± 40 | 562 ± 54 | 539 ± 51 |
| MA | Males | 16.1 ± 0.5 | 369 ± 49 | 531 ± 59 | 405 ± 52 |
| THIO | Males | 16.8 ± 0.5 | 366 ± 42 | 525 ± 61 | 356 ± 50 |
| SA | Females | 15.4 ± 0.7 | 343 ± 48 | 709 ± 59 | 576 ± 62 |
| MA | Females | 16.7 ± 0.6 | 391 ± 48 | 567 ± 62 | 551 ± 61 |
| THIO | Females | 17.0 ± 0.6 | 398 ± 51 | 604 ± 57 | 596 ± 58 |
1 Data are expressed as means ± standard error of the mean. SA=saline; MA=methamphetamine; THIO=thioperamide; n = 8 mice/sex/treatment.
Figure 1.
Spatial memory retention in the water maze probe trials. Data are expressed as mean ± S.E.M. SA=saline; MA=methamphetamine; THIO=thioperamide. n = 8 mice/sex/treatment. *p < 0.05 versus any other quadrant.
In male mice, the same pattern was seen in the second probe trial, following reversal training, as was seen in the first probe trial. MA- and THIO-treated male mice showed spatial memory retention and searched longer in the target quadrant than any other quadrant but SA-treated male mice did not. In contrast, in female mice, the SA- and MA-treated mice that showed spatial memory retention in the first probe trial did not show spatial memory retention in the second probe trial, while MA-treated female mice showed spatial memory retention in the second, but not first, probe trial. It is conceivable the spatial memory retention for the fist platform location interfered with the ability of the SA- and THIO-treated female mice to remember the second platform location.
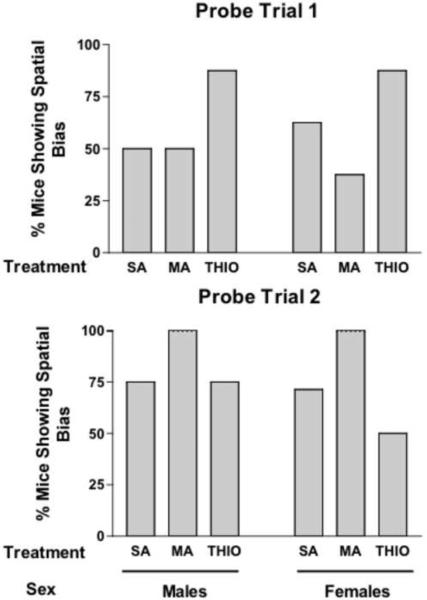
The percentage of mice in each group spending more time in the target quadrant than any other quadrant was also analyzed (Fig. 2). In male mice, in the first probe trial, 50% of the SA- and MA-treated mice demonstrated spatial bias, while the 87% of THIO-treated mice showed spatial bias. In contrast, in female mice, in the first probe trial, less than 50% of the MA-treated mice showed spatial bias (37%) while the majority of SA- and THIO-treated mice did. In male mice, the majority of SA-, MA-, and THIO-treated mice showed spatial memory retention in the second probe trial (SA: 75%; MA: 100%; and THIO: 75%). In contrast, in female mice, while the majority of SA- and MA-treated mice showed spatial bias (SA: 71%; and MA: 100%), the majority of THIO-treated mice did not (THIO: 50%,). These data show that compared to neonatal SA exposure, neonatal THIO exposure causes in subtle impairment in spatial memory retention in the second probe trial not seen in male mice. Together with the first probe trial data, these data indicate that female mice are more susceptible to developmental MA or THIO exposure.
Figure 2.
Percent of mice spending more time in the target quadrant than any other quadrant. SA=saline; MA=methamphetamine; THIO=thioperamide. n = 8 mice/sex/treatment.
3.4. Sensorimotor gating in the pre-pulse inhibition test
THIO-treated mice showed more baseline startle than MA- or SA-treated mice (F(2,42)=3.456, p = 0.04, Table 3). At 110 db, there was a sex by treatment interaction for the baseline-adjusted percent PPI. MA-treated male mice showed greater PPI than MA-treated female mice (p < 0.0068). In contrast, at 120 db there were no treatment effects on baseline-adjusted PPI (Table 3). These data show that neonatal MA or THIO exposure does not impair PPI in adolescence.
Table 3.
Baseline Acoustic Startle and Pre-Pulse Inhibitiona
| Treatment | Sex | bBaseline startle (N) | c110 db Pre-Pulse Inhibition | d120 db Pre-Pulse Inhibition |
|---|---|---|---|---|
| SA | M | 0.214±0.030 | 76.367±4.073 | 48.690±6.124 |
| THIO | M | 0.253±0.039 | 71.893±4.792 | 52.889±4.425 |
| MA | M | 0.125±0.019 | 83.2460±9.940 | 57.748±8.646 |
| SA | F | 0.154±0.010 | 66.193±3.922 | 43.483±3.697 |
| THIO | F | 0.243±0.041 | 81.471±5.810 | 53.295±9.892 |
| MA | F | 0.216±0.035 | 59.959±8.124 | 33.415±9.456 |
Data are expressed as mean ± standard error of the mean.
Main effect of treatment on baseline startle (F(2,42)=3.456, p = 0.04) with THIO-treated mice having higher PPI.
Baseline-adjusted % PPI at 110 db. A significant sex × treatment interaction (F(2,42) = 3.81, p = 0.029 and a trend for a main effect of sex (p = 0.09) with males having higher PPI than females. Simple effects showed that male MA-treated mice had greater PPI than female MA-treated mice 110 db (p < 0.0068).
No effect of tx on 120 db baseline adjusted % PPI.
1SA=saline; MA=methamphetamine; THIO=thioperamide. n = 8 mice/sex/treatment.
3.5. Contextual and cued fear conditioning
The first two minutes of the training (prior to tone-US presentation) were analyzed to exclude potential locomotor or anxiety-like effects contributing to freezing behavior (baseline freezing behavior). There was no difference between the treatment groups or sexes in baseline percent freezing during the training phase (Table 4).
Table 4.
Percent contextual and cued fear conditioning1
| Treatment | Sex | Baselinea | Contextual test | Pre-tone cued testa | During tone cued test |
|---|---|---|---|---|---|
| SA | M | 23.866±2.165 | 46.521±4.630 | 31.067±4.619 | 62.677±4.728 |
| THIO | M | 24.786±2.232 | 49.440±4.114 | 30.617±2.311 | 67.949±4.238 |
| MA | M | 21.410±1.378 | 48.076±3.371 | 30.970±2.837 | 70.075±1.882 |
| SA | F | 17.593±1.784 | 44.800±6.186 | 25.796±3.125 | 63.728±3.869 |
| THIO | F | 21.121±1.816 | 42.809±3.039 | 25.104±2.609 | 68.147±3.477 |
| MA | F | 25.421±2.848 | 48.864±4.670 | 26.179±2.420 | 67.526±4.108 |
Data are expressed as mean percentages ± standard error of the mean. SA=saline; THIO=thioperamide; MA=methamphetamine. n = 8 mice/sex/treatment.
Trend for a sex*treatement interaction (P = 0.055). MA-treated females showed slightly more freezing during baseline than SA- and THIO-treated females. This was not seen in male mice.
Trend for females to freeze less than males during pre-tone cued test (P = 0.099).
There were no differences between the treatment groups or sexes in the percent time freezing during the first three minutes of the contextual fear conditioning test (Table 4). For the cued fear conditioning test, the first three minutes (prior to tone onset) and last three minutes (during tone presentation) were analyzed separately. Similar to the contextual fear conditioning results, there was no difference between the treatment groups or sexes in the percent time freezing during either phase of the cued fear conditioning test (Table 4). When the pre- and post-tone freezing behavior was analyzed as a repeated measure ANOVA, there were also no effects of treatment or sex (data not shown). These data show that neonatal MA or THIO exposure does not impair contextual or cued fear conditioning in adolescence.
3.6. Circadian Rhythms
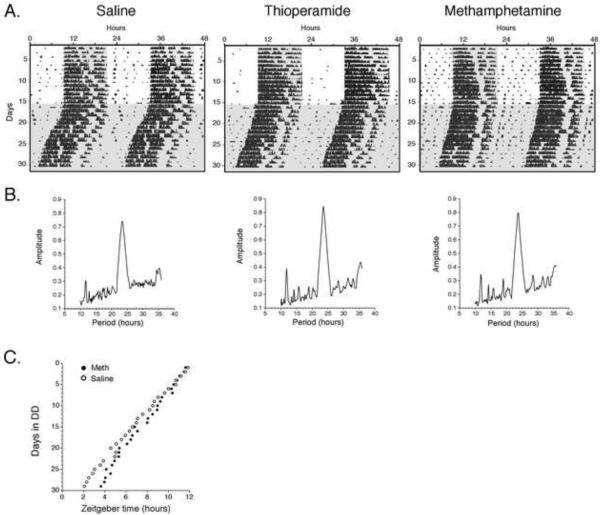
Mice were released into constant darkness (DD) after being entrained on a 12:12 hour light:dark cycle. The free running period was determined after at least 10 days in DD. The SA-treated mice had a free running period (τ) of 23.59 hrs (n = 13). The MA treated mice had a τ of 23.71 hrs (n = 19) while the THIO-treated mice had a τ value of 23.69 hrs (n = 13) (Fig. 3A, B). Compared to SA, MA caused a significant increase in τ duration (F = 5.149, p = 0.031), while the τ in THIO-treated mice was the intermediate between that in SA- and MA-treated mice. The period of the circadian clock is dependent on the previous duration and intensity of the light-dark cycle – the “after-effects” [20]. Our results demonstrate that in the first ten days of the constant darkness there is a small but significantly longer circadian of period in the methamphetamine mice compared to the saline treated mice. The longer the mice are in DD the onset of activity of the two groups diverges consistent with the methamphetamine treated mice having a longer circadian period (Figure 3C). There was no effect of sex or sex x treatment interaction; therefore, they were combined in the analysis.
Figure 3.
Effects of THIO and MA on the circadian free running period. A. Examples of wheel running actograms from SA-, THIO-, and METH-treated mice. B. Below each actogram is the F periodogram calculated from data obtained during constant darkness. C. Mean daily onsets of wheel running activity for saline and methamphetamine treated animals maintained in DD. The onset of activity is normalized to lights-off on the last day of the light-dark cycle. The error bars are not shown for clarity.
4. Discussion
In this study, sex-dependent treatment effects were seen in the water maze and pre-pulse inhibition. THIO-, but not MA-, treated female mice showed hippocampus-dependent spatial memory retention in the first probe trial. In contrast, MA-, but not THIO-, treated female mice showed spatial memory retention in the probe trial following reversal training. MA- and THIO-treated male mice showed spatial memory retention in both probe trials. A similar pattern was seen when the percent mice showing spatial memory retention was analyzed. In female mice, compared to SA treatment, MA treatment mice reduced the percent of mice showing spatial memory retention in the first probe trial while THIO treatment reduced the percent of mice showing spatial memory retention following reversal training. Such changes were not seen in male mice. In addition, MA-treated male mice showed greater pre-pulse inhibition than MA-treated female mice.
Regardless of sex, THIO-treated mice gained on average more weight each day. A similar pattern was seen in our previous study [11]. Consistent with these data in neonatal mice, THIO increased appetite and body weight in adult wild-type rats [21] and mice [22]. However, as described in [22] and reviewed in [23], no or opposite effects of THIO on body weight in adult rodents have also been reported. These divergent effects in adults might be related to differences in administration route, species, and strain. The fact that THIO increased body weights in neonates in the current study indicates that the use of H3 receptor antagonists as anti-obesity treatment in women of child-bearing age should be especially carefully considered.
Neonatal MA or THIO exposure caused sex-dependent spatial memory retention in the first water maze probe trial in adolescent mice. Similar results were seen in adult mice following neonatal MA exposure [11]. While MA-treated female mice did not show spatial memory retention, MA-treated male mice did. In contrast to MA, THIO-treated adolescent and adult female mice did not show impairments in spatial memory retention in the first probe trial. However, when in this study adolescent mice were trained to locate a second platform location during reversal training, THIO-treated female mice did show impairment in spatial memory retention in the second probe trial. It is possible that a higher dose of THIO might cause also impairments in spatial memory retention in the first probe trial. As both the first water maze probe trial and contextual fear conditioning are hippocampus-dependent, these data show that the water maze is particularly sensitive to detect sex-dependent effects of neonatal MA exposure on spatial memory retention.
Following neonatal exposure to MA or THIO, no treatment effects in exploratory behavior or measures of anxiety were seen in the open field or elevated zero maze in adolescent female or male mice. Similarly, no treatment effects following neonatal exposure to MA or THIO were seen in adult female or male mice [11]. These data indicate that potential treatments effect on exploratory behavior or measures of anxiety did not contribute to the treatment effects seen in the water maze.
This study shows long-term effects of exposure to MA and THIO during hippocampal development on lengthening of the free-running period. While chronic MA exposure in adult rodents has acute effects on the length of the free-running period and duration of activity in rats [24–26] and mice [27, 29], potential long-term effects of MA on circadian rhythms post-treatment seem inconsistent in adult male rats [26] and complex in adult male mice [29]. There are some long-lasting effects of subcutaneous MA injections on wheel running in adult male ddY mice under a 12-hr light dark schedule, but the MA dose, time of day, and interval between MA administrations, critically determine the direction of the effect. Thus, exposure to the developing brain might be required for long-term effects of MA on circadian rhythms to occur. As the effects on circadian rhythm were not sex-dependent while those on spatial memory retention in the water maze were, these data show that there is no simple relationship between the observed changes in circadian rhythm and spatial memory retention.
The mechanism underlying the effects of MA on circadian rhythms are not clear. As MA stimulates the dopaminergic system, dopamine was hypothesized to be involved in the effects of MA on circadian rhythms [27]. Our data suggest that the long-term effects of neonatal MA exposure on circadian rhythms might involve HA, as neonatal exposure to THIO also lengthened the free-running period. In addition, we previously showed that neonatal MA treatment elevates brain HA levels [13]. Interestingly, the effect of neonatal THIO treatment on lengthening of the free-running period mice was the intermediate between that of SA- and MA-treated mice. We recognize that this study was limited by the use of a single dose and that it is conceivable that a higher dose of THIO might cause a lengthening of the free-running period similar to that seen following neonatal MA treatment.
The location in the brain involved in the regulation of circadian rhythms by MA is not clear either. As MA affects circadian rhythms in animals with lesions to the site of the mammalian circadian pacemaker, the suprachiasmatic nucleus (SCN), [25, 27] or animals that contain mutations in clock genes [30], the hypothesized MA-sensitive circadian oscillator (MASCO) is expected to be distinct from the SCN. However, the longer free-running period in SCN-lesioned than intact animals indicates that MASCO can interact with the SCN [27]. Interestingly, both direct application of HA in the SCN and central injections directed towards the SCN as well as intraventricular injections of HA phase shift circadian rhythms [31]. Thus HA might mediate the long-term effects of MA on circadian rhythms in both the SCN and MASCO.
The long-term effects of neonatal exposure to MA or THIO on the free-running period were sex-independent. As the effects of neonatal exposure to MA or THIO on spatial memory retention were sex-dependent, these data show that there is no simple relationship between circadian rhythms and spatial memory retention in the water maze. Interestingly, the acute effects of the HA H1 receptor antagonist pyrilamine on wheel running were more pronounced in ovariectomized female than castrated male Swiss-Webster mice [32], indicating that there are acute HA receptor-mediated sex-dependent effects on wheel running but that sex hormones are not required for these effects. Future studies are warranted to determine the mechanisms underlying these long-term effects of neonatal exposure to MA and THIO on cognitive performance and circadian rhythms.
Research Highlights
Neonatal methamhetamine exposure impairs spatial memory in adolescent female mice.
Neonatal methamphetamine exposure prolongs the circadian period in adolescent mice.
Neonatal thioperamide exposure shows intermediate effects on these outcome measures.
The water maze is sensitive to detect effects of neonatal drug exposure in adolescence.
Acknowledgements
This work was supported by National Institute of Drug Abuse (T32DA07262), NS036607 (CNA) and the development account of Dr. Raber.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].DAWN The drug abuse warning network report: emergency department visits involving methamphetamine. 2010 [Google Scholar]
- [2].Zule W, Costenbader E, Meyer W, Wechsberg W. Methamphetamine use and risky sexual behaviors during hterosexual encounters. Sex Transm Dis. 2007;34:689–94. doi: 10.1097/01.olq.0000260949.35304.22. [DOI] [PubMed] [Google Scholar]
- [3].Terplan M, Smith E, Kosloski M, Pollack H. Methamphetamine use among pregnant women. Obstet Gynecol. 2009;113:1285–91. doi: 10.1097/AOG.0b013e3181a5ec6f. [DOI] [PubMed] [Google Scholar]
- [4].Little B, Snell M, Gilstrap III L. Methamphetamine abuse during pregnancy: outcome and fetal effects. Obstet Gynecol. 1988;75:541–4. [PubMed] [Google Scholar]
- [5].Smith L, Lagasse L, Derauf C, Grant P, Shah R, Arria A, et al. Prenatal methamphetamine use and neonatal neurobehavioral outcome. Neurotoxicol Teratol. 2008;30:20–8. doi: 10.1016/j.ntt.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Good M, Solt I, Acuna J, Rotmensch S, Kim M. Methamphetamine use during pregnancy: maternal and neonatal implications. Obstet Gynecol. 2010;116 doi: 10.1097/AOG.0b013e3181e67094. [DOI] [PubMed] [Google Scholar]
- [7].Chang L, Cloak C, Jiang C, Farnham S, Tokeshi B, Buchtal S, et al. Altered neurometabolites and motor integration in children exposed to methamphetamine in utero. NeuroImage. 2009;48:391–7. doi: 10.1016/j.neuroimage.2009.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chang L, Smith L, LoPresti C, Yonekura M, Kuo J, Walot I, et al. Smaller subcortical volumes and cognitive deficits in children with prenatal methamphetamine exposure. Psychiatr Res. 2004;132:95–106. doi: 10.1016/j.pscychresns.2004.06.004. [DOI] [PubMed] [Google Scholar]
- [9].Clancy B, Finlay BL, Darlington RB, Anand K. Extrapolating brain development from experimental species to humans. Neurotoxicology. 2007;28:931–7. doi: 10.1016/j.neuro.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Vorhees CV, Inman-Wood SL, Morford LL, Broening HW, Fukumura M, Moran MS. Adult learning deficits after neonatal exposure to D-methamphetamine: selective effects on spatial navigation and memory. J Neurosci. 2000;20:4732–9. doi: 10.1523/JNEUROSCI.20-12-04732.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Acevedo S, de Esch I, Raber J. Sex- and histamine-dependent long-term cognitive effects of methamphetamine exposure. Neuropsychoparmacology. 2007;32:665–72. doi: 10.1038/sj.npp.1301091. [DOI] [PubMed] [Google Scholar]
- [12].Siegel J, Craytor M, Raber J. Long-term effects of methamphetamine exposure on cognitive function and muscarinic acetylcholine receptor level in mice. Behav Pharmacol. 2010;21:602–14. doi: 10.1097/FBP.0b013e32833e7e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Acevedo S, Pfankuch T, Van Meer P, Raber J. Role of histamine in short- and long-term effects of methamphetamine on the developing mouse brain. J Neurochem. 2008;107:976–86. doi: 10.1111/j.1471-4159.2008.05673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Jacobs E, Yamatodani A, Timmerman H. Is histamine the final neurotransmitter in the entrainment of circadian rhythms in mammmals? Trends in Pharmacological Sciences. 2000;8:293–8. doi: 10.1016/s0165-6147(00)01504-2. [DOI] [PubMed] [Google Scholar]
- [15].Barbier AJ, Bradbury M. Histaminergic control of sleep-wake cycles: recent therapeutic advances for sleep and wake disorders. CNS Neurol Disord Drug Targets. 2007;6:31–43. doi: 10.2174/187152707779940790. [DOI] [PubMed] [Google Scholar]
- [16].Haley G, Landauer N, Renner L, Weiss A, Hooper K, Urbanski H, et al. Circadian acitivity associated with spatial learning and memory in aging rhesus monkeys. Exp Neurol. 2009;217:55–62. doi: 10.1016/j.expneurol.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Benice T, Rizk A, Pfankuch T, Kohama S, Raber J. Sex-differences in age-related cognitive decline in C57BL/6J mice associated with increased brain microtubule-associated protein 2 and synaptophysin immunoreactivity. Neuroscience. 2006;137:413–23. doi: 10.1016/j.neuroscience.2005.08.029. [DOI] [PubMed] [Google Scholar]
- [18].Villasana L, Rosenberg J, Raber J. Sex-dependent effects of 56Fe Irradiation on contextual fear conditioning in C56BL/6J mice. Hippocampus. 2010;20:19–23. doi: 10.1002/hipo.20659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pickard GE, Ralph MR, Menaker M. The intergeniculate leaflet partially mediates effects of light on circadian rhythms. J Biol Rhythm. 1987;2:35–56. doi: 10.1177/074873048700200104. [DOI] [PubMed] [Google Scholar]
- [20].Pittendrigh CS, Daan S. A functional analysis of circadian pacemakers in nocturnal rodents. I. The stability and lability of spontaneous frequency. J Comp Physiol. 1976;106:223–52. [Google Scholar]
- [21].Itoh E, Fujimiya M, Inui A. Thioperamide, a histamine H3 receptor antagonist, powerfully suppressed peptide YY-induced food intake in rats. Biol Psychiatry. 1999;45:475–81. doi: 10.1016/s0006-3223(98)00044-4. [DOI] [PubMed] [Google Scholar]
- [22].Yoshimoto R, Miyamoto Y, Shimamura K, Ishihara A, Takahashi K, Kotani H, et al. Therapeutic potential of histamine H3 receptor agonist for the treatment of obesity and diabetes mellitus. Proc Nath Acad Sci USA. 2006;103:13866–71. doi: 10.1073/pnas.0506104103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Leurs R, Blandina P, Tedford C, Timmerman H. Therapeutic potential of histamine H3 receptor agonists and antagonists. Trends Pharmacol Sci. 1998;19:177–83. doi: 10.1016/s0165-6147(98)01201-2. [DOI] [PubMed] [Google Scholar]
- [24].Honma K, Honma S, Hiroshige T. Disorganization of the rat activity rhtythm by chronic treatment with methamphetamine. Physio Behav. 1986;38:687–95. doi: 10.1016/0031-9384(86)90265-9. [DOI] [PubMed] [Google Scholar]
- [25].Honma K, Honma S, Hiroshige T. Activity rhythms in the circadian domain appear in suprachiasmatic nuclei lesioned rats given methmamphetamine. Physio Behav. 1987;40:767–74. doi: 10.1016/0031-9384(87)90281-2. [DOI] [PubMed] [Google Scholar]
- [26].Honma S, Honma K-I, Hiroshige T. Methamphetamine effects on rat circadian clock depend on actograph. Physio Behav. 1991;49:787–95. doi: 10.1016/0031-9384(91)90319-j. [DOI] [PubMed] [Google Scholar]
- [27].Tataroglu O, Davidson A, Benvenuto L, Menaker M. The methamphetamine-sensitive circadian oscillator (MASCO) in mice. J Biol Rhythms. 2006;21:185–94. doi: 10.1177/0748730406287529. [DOI] [PubMed] [Google Scholar]
- [28].Mohawk J, Miranda-Anaya M, Tataroglu O, Menaker M. Lithium and genetic inhibition of GSK3b enhance the effect of methamphetamine on circadian rhythms in the mouse. Behav Pharmacol. 2009;20:174–83. doi: 10.1097/FBP.0b013e32832a8f43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Yoshitake U, Kuribara H, Yasuda H, Umezu T, Tadokoro S. Long-continuous observation of the effects of methamphetamine on wheel-running and drinking in mice. Prog Neuropsychopharmacol Biol Psychiatr. 1994;18:397–407. doi: 10.1016/0278-5846(94)90071-x. [DOI] [PubMed] [Google Scholar]
- [30].Masubuchi S, Honma S, Abe H, Nakamura W, Honma K. Circadian activity rhtyhm in methamphetamine-treated Clock mutant mice. Eur J Neurosci. 2001;14:1177–80. doi: 10.1046/j.0953-816x.2001.01749.x. [DOI] [PubMed] [Google Scholar]
- [31].Harrington M, Biello S, Panula P. Effects of histamine on circadian rhythms and hibernation. Biol Rhythm Res. 2000;31:374–90. [Google Scholar]
- [32].Easton A, Norton J, Goodwillie A, Paff D. Sex differences in mouse behavior following pyrilamine treatment: role of histamine 1 receptors in arousals. Pharmacol Biochem Behav. 2004;2004:563–72. doi: 10.1016/j.pbb.2004.09.014. [DOI] [PubMed] [Google Scholar]