Abstract
Pharmacological blockade of the type 5 metabotropic glutamate receptor (mGluR5) attenuates cue-induced reinstatement of ethanol-seeking behavior, yet the brain regions involved in these effects are not yet known. The purpose of the present study was to determine if local blockade of mGluR5 receptors in the basolateral amygdala (BLA) and/or the nucleus accumbens (NAc), two brain regions known to be involved in stimulus-reward associations, attenuates the reinstatement of ethanol-seeking behavior induced by ethanol-paired cues. As a control for possible non-specific effects, the effects of mGluR5 blockade in these regions on cue-induced reinstatement of sucrose-seeking were also assessed. Male Wistar rats were implanted with bilateral microinjection cannulae aimed at the BLA or NAc. Following recovery, animals were trained to self-administer ethanol (10% w/v) or 45 mg sucrose pellets on an FR1 schedule of reinforcement in 30 min daily sessions using a sucrose fading procedure. Following stabilization of responding, animals underwent extinction training. Next, animals received infusions of vehicle or the selective mGluR5 antagonist MTEP (3 μg/μl) into the BLA or NAc prior to cue-induced reinstatement testing sessions. mGluR5 blockade eliminated cue-induced reinstatement of alcohol-but not sucrose-seeking behavior. Results from this study indicate that mGluR5 receptors in the BLA and NAc mediate cue-induced reinstatement of ethanol-seeking behavior, and provide two potential neuroanatomical sites of action where systemically administered mGluR5 antagonists attenuate cue-induced reinstatement. These data are consistent with previous findings that cue-induced reinstatement of ethanol-seeking increases neuronal activity and glutamatergic transmission in these two regions.
Keywords: ethanol, self-administration, reinstatement glutamate, mGluR5, antagonist, cue
1. Introduction
One of the biggest obstacles to the successful treatment of alcoholism is the problem of relapse. The probability that an abstinent alcoholic will relapse to drinking at least once their lifetime exceeds 80% (Boothby and Doering, 2005; Carr, 2011; Spanagel, 2009). These high relapse rates are often a result of noncompliance with treatment, poor coping skills, and neuroadaptive changes in the brain that occur as a result of chronic ethanol consumption (Breese et al., 2011).
Another consequence of long-term ethanol consumption is the increase in incentive salience of ethanol-associated stimuli. Environmental stimuli that have been repeatedly paired with ethanol consumption can elicit conditioned responses such as craving for ethanol, which can result in ethanol-seeking and ultimately relapse (Adinoff, 2004; Sinha and Li, 2007). Both human and animal studies have begun to elucidate the neural circuitry underlying cue-induced relapse to ethanol-seeking (Heinz et al., 2009). For example, animal experiments have revealed that ethanol- and drug-associated cues increase neuronal activity and dopamine release in ventral striatal regions such as the nucleus accumbens (NAc) (Dayas et al., 2007; Di Chiara, 2002; Jupp et al., 2011; Schroeder et al., 2008; Shalev et al., 2000; Weiss et al., 2000) which is an integral part of the mesolimbic reward circuitry that has been hypothesized to act as a sensorimotor gateway to integrate the influence of salient environmental stimuli on adaptive behavioral responses (Mogenson et al., 1980; Salamone et al., 2005; Tobler et al., 2005; Wrase et al., 2007). The nucleus accumbens consists of two primary functional subdivisions, the core and shell subregions. Inactivation of the NAc core has been shown to attenuate the reinstatement of reward-seeking behavior induced by exposure to drug or natural reward (i.e., food) associated environmental cues (Di Ciano et al., 2008; Floresco et al., 2008; Fuchs et al., 2004; Lalumiere and Kalivas, 2008; Rocha and Kalivas, 2010). Human imaging studies have also revealed that the NAc is activated by ethanol-associated cues in human alcoholic subjects (Myrick et al., 2008; Vollstadt-Klein et al., 2011).
An additional brain region that is believed to influence behavioral responses to drug-associated cues is the basolateral amygdala (BLA), which mediates the emotional salience of various types of stimuli and is involved in the formation of stimulus-reward associations and emotional learning (Balleine and Killcross, 2006; Cador et al., 1989; Cardinal et al., 2002). In rodents, experimental inactivation of the BLA attenuates the reinstatement of drug-seeking behavior induced by exposure to environmental cues (Fuchs and See, 2002; Kruzich and See, 2001; Meil and See, 1997), and cue-induced reinstatement of ethanol-seeking is associated with increased activation of this region (Dayas et al., 2007; Jupp et al., 2011; Schroeder et al., 2008). There is also evidence that the BLA and other amygdaloid subregions are activated by ethanol-associated cues (Schneider et al., 2001).
The excitatory amino acid glutamate, via actions on both ionotropic (iGluR) and metabotropic (mGluR) receptors, is involved in relapse-like behavior (Gass and Olive, 2008; Kalivas et al., 2009). One glutamate receptor subtype that has received a considerable amount of attention is the type 5 metabotropic glutamate receptor (mGluR5). In rodents, systemic administration of mGluR5 antagonists attenuate cue-evoked reinstatement of ethanol-seeking behavior (Adams et al., 2008; Backstrom et al., 2004; Schroeder et al., 2008), as well as cue-evoked activation of the BLA (Schroeder et al., 2008). In addition, reinstatement of cocaine-seeking behavior evoked by drug-associated cues is attenuated by blockade of mGluR5 receptors in the NAc core (Backstrom and Hyytia, 2007); however, the effects of mGluR5 receptor blockade in specific brain regions on cue-evoked reinstatement of ethanol-seeking have not yet been evaluated. This is of particular interest since we have previously shown that ethanol-associated cues increase extracellular levels of glutamate in the NAc core and BLA as measured by glutamate oxidase-coated biosensors (Gass et al., 2011).
Therefore, the primary purpose of this study was to examine the effects of site-specific infusions of an mGluR5 antagonist into specific brain regions on cue-induced reinstatement of ethanol-seeking behavior. The BLA and NAc core were chosen based on the aforementioned evidence for mediation of cue-evoked ethanol-seeking by these regions, as well as our recent findings that cue-induced ethanol-seeking is associated with increased levels of extracellular glutamate in these regions (Gass et al., 2011). We also sought to determine if any observed attenuation of alcohol-seeking behavior could be attributed to non-specific effects of MTEP by assessing the impact of local administration of MTEP into these regions on cue-induced sucrose-seeking behavior.
2. Methods
2.1. Animals
Male Wistar rats (250–275 g upon arrival, Harlan, Indianapolis, IN) were housed individually in standard polycarbonate cages. Access to food and water in the home cage was continuous throughout the experiment except during behavioral testing. The animal colony room was maintained on a 12:12 light-dark cycle with lights on at 07:00, and experimental testing was performed during the light portion of the cycle. All experimental procedures were conducted with the approval of the Institutional Animal Care and Use Committee at the Medical University of South Carolina, and within guidelines set forth by the National Research Council’s Guideline for the Care and Use of Mammals in Neuroscience and Behavioral Research (2003).
2.2. Microinjection Cannula Implantation
Rats were anesthetized with isoflurane vaporized in medical grade breathing air at a flow rate of 0.4 L/min and placed in a stereotaxic instrument (Kopf Instruments, Tujunga, CA). Bilateral microinjection guide cannulae (26 ga O.D., Plastics One, Roanoke, VA) were aimed to terminate 1 mm dorsal to the BLA and NAc. The stereotaxic coordinates used for the BLA were (in mm from bregma and skull surface) anterior-posterior −2.5, medial lateral 5.0, and dorsal-ventral −7.4 mm, and the coordinates used for the NAc were anterior-posterior +1.18, medial lateral 1.2, and dorsal-ventral −5.0 (Paxinos and Watson, 2007). Microinjection cannulae were secured to the skull with stainless steel screws and dental cement. Removable obturators (33 ga O.D.) were inserted in the full length of the guide cannulae to limit obstruction by tissue and contamination by external debris. The wound was treated with topical 2% xylocaine and 2% triple antibiotic ointments and sutured closed using 3-0 Vicryl sutures. Following surgery, all rats were given carprofen (0.1 mg/kg, s.c. twice daily for 3 days) for post-operative pain management.
2.3. Self-Administration Apparatus
Ethanol and sucrose self-administration, extinction, and reinstatement test sessions were conducted in Plexiglas chambers (32 cm W × 25 cm D × 11 cm H, Med Associates, model ENV-008, St. Albans, VT) located in melamine sound attenuating cubicles. Each cubicle was equipped with an exhaust fan to provide air circulation and mask external noise. Mounted on one wall of the self-administration chamber were two response levers that flanked a liquid receptacle connected to a single speed syringe infusion pump with polyethylene tubing. For sucrose experiments, a pellet receptacle connected to a pellet dispenser was located between the levers. Responses on one lever, designated the active lever, resulted in delivery of the liquid or sucrose pellet reinforcer (see below), while responses on the other (designated inactive) lever had no programmed consequences. Located above the active lever was a 2.5 cm diameter stimulus light, which was illuminated for 3 sec during each reinforcer delivery. Located atop the chambers were a house light to provide general illumination, and a Sonalert speaker that emitted a tone (2900 Hz, ~65 dB) for 3 sec during each reinforcer delivery. Chambers were interfaced to a PC computer that controlled experimental sessions and recorded data using commercially available software (MED Associates, MED-PC IV).
2.4. Self-Administration Procedures
After at least 5 days of post-surgical recovery, rats were trained to lever press on the active lever to receive a sucrose solution on an FR1 schedule of reinforcement. Each active lever press activated the syringe pump to deliver 90 μl of a liquid solution over a 3 second period. During reinforcer delivery, the stimulus light above the active lever was illuminated and the tone was presented. Following each reinforcer delivery, a 4-sec timeout period was initiated during which additional active lever presses were recorded but had no programmed consequences.
Daily 30 min sessions were then conducted with sucrose (10% w/v) as the reinforcer five days per week. When response patterns stabilized (the number of active lever presses per 30 min session varied less than 15% across two consecutive sessions), the rats were trained to self-administer ethanol (10% v/v) using a sucrose fading procedure (Samson, 1986). Briefly, ethanol was gradually added to the sucrose solution (2%, 5%, and then 10%, with one week at each concentration) while sucrose was concurrently faded out of the solution (10%, 5%, 2%, then 0%) until rats were self-administering 10% ethanol in 30 min daily sessions conducted Mon–Fri.
Following stabilization of responding for 10% ethanol (approximately 1 week), blood samples (20 μl) were taken from the tail vein using heparin-coated borosilicate capillary tubes immediately following a 30 min self-administration session for subsequent analysis of blood ethanol levels (see below). After an additional week of daily self-administration sessions, extinction training procedures commenced.
A separate group of control rats was trained to self-administer sucrose pellets. To initiate a preference for the sucrose pellets, 2–3 pellets were placed in the home cage for 4 days prior to the initiation of operant self-administration, Rats were then placed in the self-administration chambers for daily 30 min self-administration sessions whereby each press on the designated active lever delivered a 45-mg sucrose pellet (TestDiets, Richmond, IN, USA) into the pellet receptacle on a FR1 schedule of reinforcement. As with the alcohol self-administration group, reinforcer delivery was paired with illumination of the stimulus light above the active lever and the tone was presented for 3 seconds. Sucrose pellet delivery was followed by a 20-s timeout period, during which additional active lever presses were recorded but produced no programmed consequences.
2.5. Extinction, Microinjection, and Cue-Induced Reinstatement Procedures
During 30 min extinction training sessions, presses on the active lever no longer produced any programmed consequences (i.e., no tone/light presentation and no reinforcer delivery). Ten minutes prior to extinction training sessions, an investigator lightly restrained animals and the obturators were removed and replaced to habituate the animals to microinjection procedures. Extinction training sessions were conducted until extinction criteria were met by all animals in each group (i.e., the number of active lever presses was <20% than that observed during the final two days prior to commencement of extinction training).
Next, microinjections were performed 10 min prior to cue-induced reinstatement testing. Rats were lightly restrained by an investigator and obturators were removed. Sterile 33-gauge microinjection needles (Plastics One) were connected via microbore tubing to two 100 μl syringes (Hamilton, Reno, NV). Syringes were mounted on a micro-infusion pump (Harvard Apparatus, Holliston, MA) set to deliver fluids at a flow rate of 0.5 μl/min. Microinjection needles were inserted bilaterally to a depth 1 mm beyond the ventral tip of the guide cannula. Drug solutions were infused in a volume of 0.5 μl/side over a 1-min period. Microinjection needles were left in place for an additional 60-sec period to allow drug diffusion. Next, injectors were removed, obturators were replaced, and 10 minutes later cue-induced reinstatement procedures were conducted.
For cue-induced reinstatement, each press on the active lever resulted in the presentation of the light-tone stimulus complex that was presented during active ethanol self-administration, as well as activation of the computer-controlled syringe; however, no reinforcer was presented. Each animal underwent a total of two cue-induced reinstatement test sessions, with one session being preceded by vehicle treatment and the other preceded by drug treatment. All animals received treatment with vehicle and MTEP (3 μg/μl) in a randomized, counterbalanced within-subjects design to prevent order effects associated with the microinjections. This dose of MTEP was chosen as we have previously found that it alters ethanol self-administration when administered into the NAc shell without producing motor impairment (Gass and Olive, 2009). Microinjections were performed only on Tuesday through Friday due to increased variability in lever responding on Monday. Additionally, after each microinjection session rats were given at least two extinction sessions (without microinjections) to ensure that responding had returned to post-extinction levels. MTEP and vehicle microinjections were randomly counterbalanced across the two reinstatement test sessions. After completion of the second cue-induced reinstatement test session, animals were euthanized and their brains were removed for histological verification of microinjector needle location (see below).
2.6. Histology
After completion of the second cue-induced reinstatement test session, rats were administered sodium pentobarbital (150 mg/kg, ip.) and perfused transcardially with phosphate buffered saline followed by 10% v/v formalin. Brains were removed and stored in 10% formalin at 4°C for 24 hr prior to be transferred to a solution of 30% w/v sucrose for a minimum of 48 hr. Brains were then sectioned at 40 μm thickness on a cryostat (Leica Microsystems, Bannockburn, IL), mounted onto gelatin-coated microscope slides, and stained with cresyl violet. Brain sections were then examined under a light microscope to verify the location of the tip of the microinjection needle using the atlas of Paxinos and Watson (2007).
2.7. Blood Ethanol Level Determination
Immediately following blood sample collection, samples were centrifuged at 800 × g for 10 min to obtain a plasma supernatant, which was then stored at 4°C for a maximum of 24 hr. Next, approximately a 10 μl sample of plasma was used for determination of blood ethanol levels using an Analox AM-1 analyzer (Analox Instruments, Lunenberg, MA).
2.8. Drugs
3-((2-Methyl-4-thiazolyl)ethynyl)pyridine (MTEP) hydrochloride was purchased from Ascent Scientific (Princeton, NJ), and dissolved in sterile artificial cerebrospinal fluid (aCSF) at a concentration of 3 μg/μl. Ethanol (95%, MUSC Pharmacy Distribution Services) and sucrose (Sigma-Aldrich, St. Louis, MO) were diluted in tap water for oral self-administration.
2.9. Statistical Analyses
For correlational analysis of the number of ethanol deliveries and resulting blood ethanol levels, a Pearson’s product-moment correlation was conducted. For operant responding, total number of lever presses during ethanol self-administration (SA, average of the last 2 days), extinction training (Ext, average of the last two days), and during each cue-induced reinstatement tests were analyzed using repeated measures analysis of variance (ANOVA). Separate ANOVAs were conducted for active and inactive lever presses. To rule out potential between-groups differences in ethanol or sucrose self-administration patterns between rats implanted with guide cannula aimed at the NAc or BLA, a one-way ANOVA of the number of active lever presses during self-administration (average of the last 2 days) was conducted. Post-hoc comparisons were conducted using Holm-Sidak procedures. All statistics were conducted with SPSS version 18 (SPSS, inc., Chicago, IL). All p-values less than 0.05 were considered statistically significant.
3. Results
3.1. Histological verification of microinjector placement
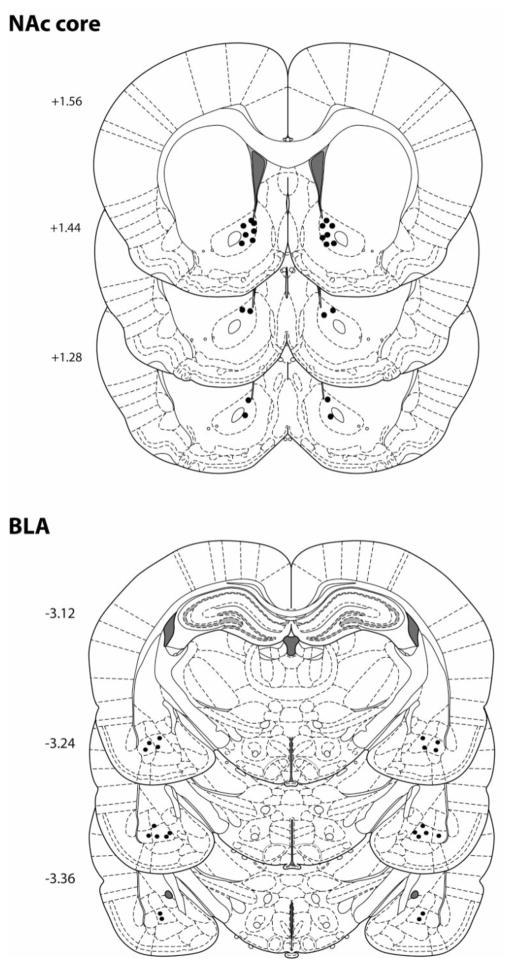
Figure 1 shows the approximate location of the ventral tip of bilateral microinjectors in the NAc and BLA, as assessed from histological staining. As can be observed, most of the placements within the NAc were located within the core subregion, but some were found to be localized to the core-shell border. As a result of this and possible diffusion of test substances between the two NAc subregions, we designated all of these animals as only being confined to the NAc and not to one specific subregion. One animal in which the BLA was targeted was found to have erroneous cannula placement, and data from this animal were discarded.
Figure 1.
Diagrams of coronal rat brain section showing the location of microinjector tips in the NAc and BLA. Illustrations were adapted from the atlas of Paxinos and Watson (2007). The numbers along the left side of each section represent the distance (in mm) of that section from bregma.
3.2. Correlation of number of ethanol deliveries and blood ethanol levels
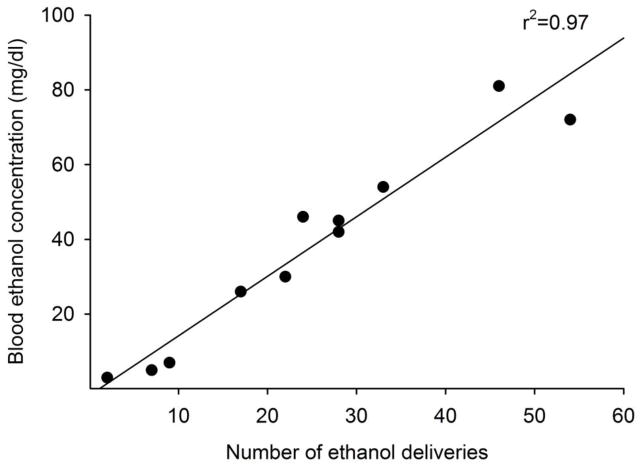
In order to establish that rats obtained physiologically relevant blood ethanol levels during the self-administration sessions, we conducted a correlational analysis between the number of ethanol deliveries obtained during a single self-administration session and blood ethanol levels as assessed immediately following the session (Figure 2). Data from both groups with guide implanted into either the BLA or NAc were combined (n=12 total) for this analysis. We failed to obtain enough blood for analysis from one animal. A Pearson’s product-moment correlation analysis revealed a statistically significant correlation between the number of ethanol deliveries and resulting blood ethanol levels [r (11) = 0.97, p < .0001].
Figure 2.
Correlation between the number of ethanol reinforcer deliveries obtained in a single operant ethanol administration session and resulting blood ethanol levels determined immediately following the session. Animals (n=12) from both experimental groups (i.e., guide cannula implanted in the BLA and NAc) are shown.
3.3. mGluR5 antagonism in the BLA and NAc core suppresses cue-induced reinstatement of ethanol-seeking behavior
Prior to examining effects of MTEP microinjections on cue-induced reinstatement, we first sought to rule out potential differences in the level of ethanol self-administration between rats with guide cannula aimed at the NAc and BLA. We analyzed the number of active and inactive lever presses over the last two days of self-administration prior to extinction training in both groups of animals. One-way ANOVA revealed no statistically significant difference between the two groups (F1,10=1.77, p>0.05).
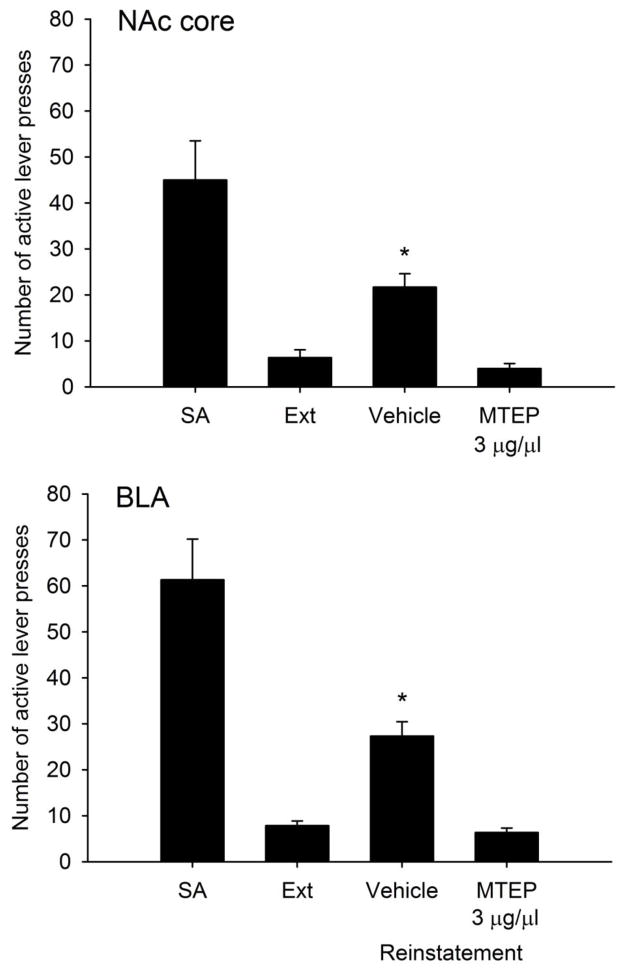
In animals with guide cannula aimed at the NAc, one-way repeated measures ANOVA demonstrated a significant effect of experimental phase (i.e., SA, Ext, or reinstatement) on the number of active lever presses (F3,15=22.01, p< 0.001), and post-hoc analyses revealed the number of active lever presses was significantly reduced during the last 2 days of extinction training as compared with the last 2 days of self-administration. During reinstatement testing following vehicle infusions into the NAc, animals demonstrated a significant increase in the number of active lever presses as compared with Ext values, but not following infusions of MTEP, suggesting a blockade of reinstatement (Figure 3A). With regards to inactive lever presses, there was no overall effect of experimental phase (F3,15=2.42, p>0.05), indicating a lack of non-specific effects of intra-NAc infusions of MTEP on general lever pressing behavior.
Figure 3.
Number of active lever presses during the final two days of ethanol self-administration (SA), extinction training (Ext), and during reinstatement test sessions in animals with guide cannula aimed at the NAc (A) or BLA (B). The selective mGluR5 antagonist MTEP (3 μg/μl) or vehicle (aCSF) was infused 10 min prior to reinstatement testing in a counterbalanced randomized order. Data represent mean (±SEM) responses of n=6 rats per group. * indicates p<0.05 vs. values for Ext and MTEP.
Results from animals with guide cannula aimed at the BLA, one-way repeated measures ANOVA demonstrated a significant effect of experimental phase on the number of active lever presses (F3,15=37.13, p< 0.001), and post-hoc analyses revealed the number of active lever presses was significantly reduced during the last 2 days of extinction training as compared with the last 2 days of self-administration. During reinstatement testing following vehicle infusions into the BLA, animals demonstrated a significant increase in the number of active lever presses as compared with Ext values, but not following infusions of MTEP, suggesting a blockade of reinstatement (Figure 3B). With regards to inactive lever presses, there was no overall effect of experimental phase (F3,15=1.67, p>0.05), indicating a lack of non-specific effects of infusions of MTEP into the BLA on general lever pressing behavior.
3.4. mGluR5 antagonism in the BLA and NAc core does not suppress cue-induced reinstatement of sucrose-seeking behavior
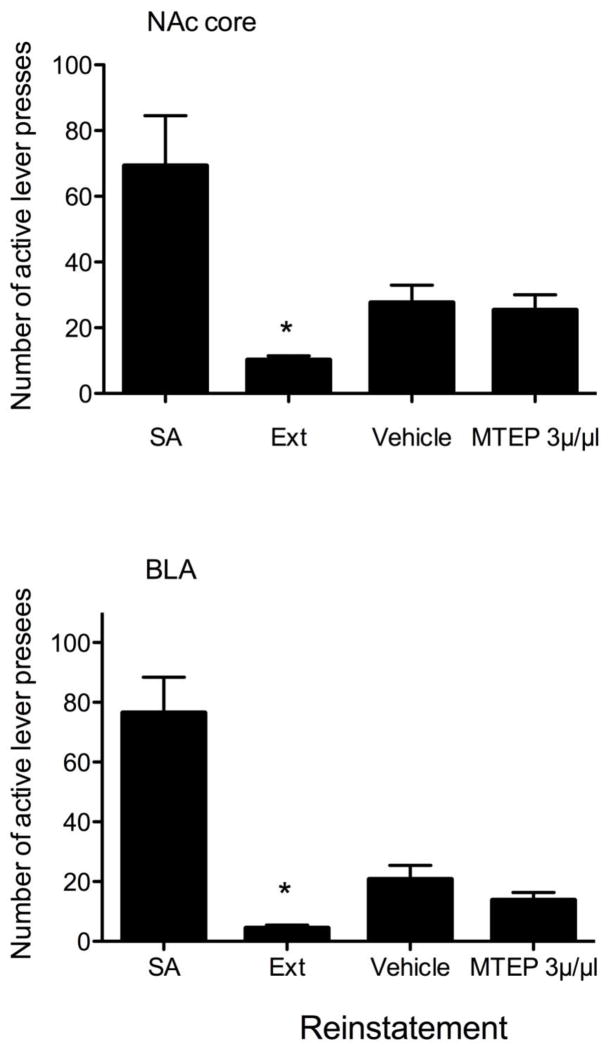
There were no significant differences in active or inactive lever responding during self-administration training between the groups (NAc versus BLA) (p’s >0.05). In animals trained to self-administer sucrose with guide cannula aimed at the NAc (n=5), one-way repeated measures ANOVA demonstrated a significant effect of experimental phase (i.e., SA, Ext, or reinstatement) on the number of active lever presses (F3,16=9.042, p = 0.001). Post-hoc analyses revealed the number of active lever presses was significantly reduced during the last 2 days of extinction training as compared with the last 2 days of self-administration. However, there were no significant differences between responding on the active lever during vehicle and MTEP microinjections, suggesting that MTEP administration into the NAc core had no effect on cue-induced sucrose responding (Figure 4A).
Figure 4.
Number of active lever presses during the final two days of sucrose self-administration (SA), extinction training (Ext), and during reinstatement test sessions in animals with guide cannula aimed at the NAc (A) or BLA (B). The selective mGluR5 antagonist MTEP (3 μg/μl) or vehicle (aCSF) was infused 10 min prior to reinstatement testing in a counterbalanced randomized order. Data represent mean (± SEM) responses of n=5 rats per group. * indicates p<0.05 vs. values for Ext and MTEP.
Similarly, in animals with guide cannula aimed at the BLA (n=5), statistical analysis revealed a significant effect of experimental phase (F3,16=24.786, p < 0.001). Post-hoc analyses revealed that active lever responding was significantly decreased during extinction training (compared to self-administration training). However, microinjection of MTEP into the BLA did not significantly reduce cue-induced sucrose-seeking behavior (when compared to vehicle microinjection) (Figure 4B). These data indicate that blockade of the mGluR5 receptor in the BLA and NAc does not attenuate sucrose-seeking behavior induced by environmental cues.
4. Discussion
In this study we demonstrated that local infusions of the selective mGluR5 antagonist MTEP, but not vehicle, into either the BLA or the NAc prevented the ability of ethanol-associated cues to reinstate ethanol-seeking behavior. These findings suggest that drug-associated cues increase glutamatergic transmission in these regions, as previously shown by our laboratory and others (Gass et al., 2011; Lalumiere and Kalivas, 2008), which in turn activates postsynaptic mGluR5 receptors in these regions where they are expressed in moderate to high levels (Romano et al., 1995; Shigemoto et al., 1993). The resulting activation of postsynaptic neurons in these regions, as previously documented following cue-induced reinstatement of ethanol-seeking by increased expression of activity markers such as c-fos and phosphorylated extracellular signal-regulated kinase (pERK1/2) (Dayas et al., 2007; Jupp et al., 2011; Schroeder et al., 2008), in turn, likely induce behavioral responses such as the reinstatement of operant responding. Additionally, our results indicated that the same manipulation of glutamatergic transmission in these brain regions did not alter cue-induced sucrose seeking behavior indicating that the effects of MTEP are specific to ethanol-seeking behavior elicited by ethanol-associated cues. Together these findings suggest that the BLA and NAc are regions that, at least in part, comprise the potential neuroanatomical loci at which systemically administered mGluR5 antagonists act to suppress cue-induced reinstatement of ethanol-seeking behavior (Adams et al., 2008; Backstrom et al., 2004; Schroeder et al., 2008).
The present findings are in agreement with various lines of evidence supporting a role for the BLA and NAc in cue-triggered drug-seeking behavior. For example, general inactivation of the BLA or NAc attenuates cue-induced reinstatement of cocaine-, heroin-, methamphetamine-, or food-seeking behavior (Di Ciano et al., 2008; Floresco et al., 2008; Fuchs et al., 2004; Fuchs and See, 2002; Kruzich and See, 2001; Lalumiere and Kalivas, 2008; Meil and See, 1997; Rocha and Kalivas, 2010). While many studies have shown that dopamine signaling in these two regions is an important mediator of cue-induced drug-seeking (reviewed in (Feltenstein and See, 2008; Ikemoto and Panksepp, 1999; See, 2005; Shalev et al., 2002), there is also evidence that glutamatergic signaling plays a role as well. Systemic administration of an mGluR5 antagonist was shown to inhibit the ability of ethanol-associated cues to elevated levels of pERK1/2 in the BLA (Schroeder et al., 2008), and reinstatement of cocaine-seeking behavior evoked by drug-associated cues was shown to be attenuated by blockade of mGluR5 receptors in the NAc core (Backstrom and Hyytia, 2007). Additionally, local infusion of the mGluR5 antagonist MPEP has been shown to attenuate both cocaine primed- and cue-induced reinstatement of cocaine-seeking behavior (Kumaresan et al., 2009). The current findings support this notion of an important role of mGluR5 receptors in the BLA and NAc in mediating cue-evoked drug-seeking.
It is interesting that our results showed no attenuation of cue-induced sucrose seeking behavior when MTEP was administered into the BLA and NAc. Other studies have shown that these brain structures also mediate cue-induced seeking of natural rewards such as food or sucrose (Balleine et al., 2003; Balleine and Killcross, 2006; Floresco et al., 2008; Stuber et al., 2011). Additional studies are needed to delineate the neurochemical mechanisms within these brain regions that mediate non-drug reward seeking behavior.
An analysis of the effects of local mGluR5 blockade on cue-induced reinstatement in ethanol-dependent animals is also needed, since it has recently been shown that the ability of systemically administered MTEP to suppress stress-induced reinstatement (albeit not cue-induced) of ethanol-seeking behavior is diminished in ethanol-dependent vs. non-dependent rats (Sidhpura et al., 2010). Thus, ethanol dependence may induce changes in mGluR5 expression or function that alter the ability of antagonists such as MTEP to attenuate reinstatement.
The amygdaloid complex is a major component of the limbic system that regulations various aspects of learning, memory, attention, and motivation. Relevant to addiction, the amygdala mediates the formation of stimulus-reward associations and emotional learning (Balleine and Killcross, 2006; Cador et al., 1989; Cardinal et al., 2002). Various human imaging studies have shown that the amygdala is activated by drug-associated cues (Bonson et al., 2002; Childress et al., 1999), including those associated with ethanol (Schneider et al., 2001). Amongst its various subdivisions, the BLA has been characterized as receiving afferent inputs from numerous cortical and subcortical structures. The BLA sends excitatory glutamatergic projections to the NAc, and these projections mediate cue-evoked motivated behaviors (Ambroggi et al., 2008; Cador et al., 1989; Di Ciano and Everitt, 2004; Stuber et al., 2011). Human imaging studies have also shown that the NAc is activated by ethanol-associated cues (Myrick et al., 2008; Vollstadt-Klein et al., 2011), and this region also receives glutamatergic input from the medial prefrontal cortex (mPFC) and subcortical structures such as the hippocampus. There is evidence to suggest that glutamatergic projections from the mPFC to the NAc also mediate cue-induced drug-seeking (Knackstedt and Kalivas, 2009; Lalumiere and Kalivas, 2008). Thus, one limitation of the current study is that local infusions of MTEP into the BLA and NAc do not delineate the interconnected glutamatergic circuitry where mGluR5 receptors mediate cue-induced ethanol-seeking, and future studies are needed to investigate this.
Other studies that have examined the influence of these regions in the reinforcing properties of drugs of abuse have shown that subregions of the NAc differentially modulate drug-seeking behavior. While there are conflicting data, the shell subregion is thought to mediate the rewarding or hedonic effects of abused drugs (McKinzie et al., 1999; Pecina & Berridge, 2000; Gonzales et al., 2004; Carlezon et al., 2005). Alternatively, the core appears to mediate the conditioning factors associated with environmental cues related to drug-reward (Ito et al., 2000; Ito, et al., 2004, but see Shabashov et al., 2011).
While the results from this study suggest that the NAc is involved in the alcohol-seeking induced by alcohol-associated cues, they do not provide a direct comparison of MTEP treatments in specific NAc subregions. Therefore, it is difficult to determine if the effects observed in the NAc are specific are mediated by the core, shell, or both NAc subregions. Future studies could incorporate a similar experimental design to provide a better understanding of the unique roles of these subregions in cue-induced alcohol-seeking behavior.
In summary, we have demonstrated that mGluR5 receptors in the BLA and NAc mediate cue-induced ethanol-seeking behavior. Future studies are needed to determine whether these effects are altered in ethanol-dependent animals, and whether the subregions of the NAc have differential roles in the various components of alcohol-seeking behavior. Regardless, these findings provide insight into the neuroanatomical and neurochemical substrates of cue-induced ethanol-seeking behavior, and will hopefully lead to more advanced therapeutics for preventing relapse in human alcoholic patients.
Highlights.
We examined the role of glutamate in alcohol seeking behavior.
An mGluR5 antagonist (MTEP) was microinjected into the NAc core and basolateral amygdala prior to cue-induced alcohol seeking behavior.
MTEP significantly reduced alcohol-seeking behavior when administered into either brain region.
Glutamatergic transmission in these brain regions may underlie cue-induced alcohol seeking behavior.
Acknowledgments
This work was supported by grants AA013852 (MFO) and AA017820 (JTG) from the National Institute on Alcohol Abuse and Alcoholism.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams CL, Cowen MS, Short JL, Lawrence AJ. Combined antagonism of glutamate mGlu5 and adenosine A2A receptors interact to regulate alcohol-seeking in rats. Int J Neuropsychopharmacol. 2008;11:229–41. doi: 10.1017/S1461145707007845. [DOI] [PubMed] [Google Scholar]
- Adinoff B. Neurobiologic processes in drug reward and addiction. Harv Rev Psychiatry. 2004;12:305–20. doi: 10.1080/10673220490910844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambroggi F, Ishikawa A, Fields HL, Nicola SM. Basolateral amygdala neurons facilitate reward-seeking behavior by exciting nucleus accumbens neurons. Neuron. 2008;59:648–61. doi: 10.1016/j.neuron.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backstrom P, Bachteler D, Koch S, Hyytia P, Spanagel R. mGluR5 antagonist MPEP reduces ethanol-seeking and relapse behavior. Neuropsychopharmacology. 2004;29:921–8. doi: 10.1038/sj.npp.1300381. [DOI] [PubMed] [Google Scholar]
- Backstrom P, Hyytia P. Involvement of AMPA/kainate, NMDA, and mGlu5 receptors in the nucleus accumbens core in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2007;192:571–80. doi: 10.1007/s00213-007-0753-8. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Killcross AS, Dickinson A. The effect of lesions of the basolateral amygdala on instrumental conditioning. J Neurosci. 2003;23:666–75. doi: 10.1523/JNEUROSCI.23-02-00666.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Killcross S. Parallel incentive processing: an integrated view of amygdala function. Trends Neurosci. 2006;29:272–9. doi: 10.1016/j.tins.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metcalfe J, Weyl HL, et al. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002;26:376–86. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- Boothby LA, Doering PL. Acamprosate for the treatment of alcohol dependence. Clin Ther. 2005;27:695–714. doi: 10.1016/j.clinthera.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Breese GR, Sinha R, Heilig M. Chronic alcohol neuroadaptation and stress contribute to susceptibility for alcohol craving and relapse. Pharmacol Ther. 2011;129:149–71. doi: 10.1016/j.pharmthera.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cador M, Robbins TW, Everitt BJ. Involvement of the amygdala in stimulus-reward associations: interaction with the ventral striatum. Neuroscience. 1989;30:77–86. doi: 10.1016/0306-4522(89)90354-0. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–52. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Carr GD. Alcoholism: a modern look at an ancient illness. Primary Care. 2011;38:9–21. v. doi: 10.1016/j.pop.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Devine DP, Wise RA. Habit-forming actions of nomifensine in nucleus accumbens. Psychopharmacology (Berl) 1995;122:194–7. doi: 10.1007/BF02246095. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–8. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayas CV, Liu X, Simms JA, Weiss F. Distinct patterns of neural activation associated with ethanol seeking: effects of naltrexone. Biol Psychiatry. 2007;61:979–89. doi: 10.1016/j.biopsych.2006.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G. Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behav Brain Res. 2002;137:75–114. doi: 10.1016/s0166-4328(02)00286-3. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Direct interactions between the basolateral amygdala and nucleus accumbens core underlie cocaine-seeking behavior by rats. J Neurosci. 2004;24:7167–73. doi: 10.1523/JNEUROSCI.1581-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ciano P, Robbins TW, Everitt BJ. Differential effects of nucleus accumbens core, shell, or dorsal striatal inactivations on the persistence, reacquisition, or reinstatement of responding for a drug-paired conditioned reinforcer. Neuropsychopharmacology. 2008;33:1413–25. doi: 10.1038/sj.npp.1301522. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. The neurocircuitry of addiction: an overview. Br J Pharmacol. 2008;154:261–74. doi: 10.1038/bjp.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, McLaughlin RJ, Haluk DM. Opposing roles for the nucleus accumbens core and shell in cue-induced reinstatement of food-seeking behavior. Neuroscience. 2008;154:877–84. doi: 10.1016/j.neuroscience.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MC, See RE. Differential involvement of the core and shell subregions of the nucleus accumbens in conditioned cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2004;176:459–65. doi: 10.1007/s00213-004-1895-6. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, See RE. Basolateral amygdala inactivation abolishes conditioned stimulus-and heroin-induced reinstatement of extinguished heroin-seeking behavior in rats. Psychopharmacology (Berl) 2002;160:425–33. doi: 10.1007/s00213-001-0997-7. [DOI] [PubMed] [Google Scholar]
- Gass JT, Olive MF. Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol. 2008;75:218–65. doi: 10.1016/j.bcp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Olive MF. Role of protein kinase C epsilon (PKCΣ) in the reduction of ethanol reinforcement due to mGluR5 antagonism in the nucleus accumbens shell. Psychopharmacology (Berl) 2009;204:587–97. doi: 10.1007/s00213-009-1490-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Sinclair CM, Cleva RM, Widholm JJ, Olive MF. Alcohol-seeking behavior is associated with increased glutamate transmission in basolateral amygdala and nucleus accumbens as measured by glutamate-oxidase-coated biosensors. Addict Biol. 2011;16:215–28. doi: 10.1111/j.1369-1600.2010.00262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales RA, Job MO, Doyon WM. The role of mesolimbic dopamine in the development and maintenance of ethanol reinforcement. Pharmacol & ther. 2004;103:121–46. doi: 10.1016/j.pharmthera.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Heinz A, Beck A, Grusser SM, Grace AA, Wrase J. Identifying the neural circuitry of alcohol craving and relapse vulnerability. Addiction biology. 2009;14:108–18. doi: 10.1111/j.1369-1600.2008.00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res Rev. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Howes SR, Robbins TW, Everitt BJ. Dissociation in conditioned dopamine release in the nucleus accumbens core and shell in response to cocaine cues and during cocaine-seeking behavior in rats. J Neurosci. 2000;20:7489–95. doi: 10.1523/JNEUROSCI.20-19-07489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Robbins TW, Everitt BJ. Differential control over cocaine-seeking behavior by nucleus accumbens core and shell. Nat neurosci. 2004;7:389–97. doi: 10.1038/nn1217. [DOI] [PubMed] [Google Scholar]
- Jupp B, Krstew E, Dezsi G, Lawrence AJ. Discrete cue-conditioned alcohol-seeking after protracted abstinence: pattern of neural activation and involvement of orexin receptors. Br J Pharmacol. 2011;162:880–9. doi: 10.1111/j.1476-5381.2010.01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Lalumiere RT, Knackstedt L, Shen H. Glutamate transmission in addiction. Neuropharmacology. 2009;56 (Suppl):169–73. doi: 10.1016/j.neuropharm.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Kalivas PW. Glutamate and reinstatement. Curr Opin Pharmacol. 2009;9:59–64. doi: 10.1016/j.coph.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruzich PJ, See RE. Differential contributions of the basolateral and central amygdala in the acquisition and expression of conditioned relapse to cocaine-seeking behavior. J Neurosci. 2001;21:RC155. doi: 10.1523/JNEUROSCI.21-14-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaresan V, Yuan M, Yee J, Famous KR, Anderson SM, Schmidt HD, Pierce RC. Metabotropic glutamate receptor 5 (mGluR5) antagonists attenuate cocaine priming-and cue-induced reinstatement of cocaine seeking. Behav Brain Res. 2009;202(2):238–244. doi: 10.1016/j.bbr.2009.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalumiere RT, Kalivas PW. Glutamate release in the nucleus accumbens core is necessary for heroin seeking. J Neurosci. 2008;28:3170–7. doi: 10.1523/JNEUROSCI.5129-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meil WM, See RE. Lesions of the basolateral amygdala abolish the ability of drug associated cues to reinstate responding during withdrawal from self-administered cocaine. Behav Brain Res. 1997;87:139–48. doi: 10.1016/s0166-4328(96)02270-x. [DOI] [PubMed] [Google Scholar]
- McKinzie DL, Rodd-Henricks ZA, Dagon CT, Murphy JM, McBride WJ. Cocaine is self-administered into the shell region of the nucleus accumbens in Wistar rats. Ann NY Acad Sci. 1999;877:788–91. doi: 10.1111/j.1749-6632.1999.tb09323.x. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Randall PK, Voronin K. Effect of naltrexone and ondansetron on alcohol cue-induced activation of the ventral striatum in alcohol-dependent people. Arch Gen Psychiatry. 2008;65:466–75. doi: 10.1001/archpsyc.65.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6. San Diego: Academic Press; 2007. [Google Scholar]
- Pecina S, Berridge KC. Opioid site in nucleus accumbens shell mediates eating and hedonic ‘liking’ for food: map based on microinjection Fos plumes. Brain res. 2000;863:71–86. doi: 10.1016/s0006-8993(00)02102-8. [DOI] [PubMed] [Google Scholar]
- Rocha A, Kalivas PW. Role of the prefrontal cortex and nucleus accumbens in reinstating methamphetamine seeking. The European journal of neuroscience. 2010;31:903–9. doi: 10.1111/j.1460-9568.2010.07134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano C, Sesma MA, McDonald CT, O’Malley K, Van den Pol AN, Olney JW. Distribution of metabotropic glutamate receptor mGluR5 immunoreactivity in rat brain. J Comp Neurol. 1995;355:455–69. doi: 10.1002/cne.903550310. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Mingote SM, Weber SM. Beyond the reward hypothesis: alternative functions of nucleus accumbens dopamine. Curr Opin Pharmacol. 2005;5:34–41. doi: 10.1016/j.coph.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exp Res. 1986;10:436–42. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- Schneider F, Habel U, Wagner M, Franke P, Salloum JB, Shah NJ, et al. Subcortical correlates of craving in recently abstinent alcoholic patients. Am J Psychiatry. 2001;158:1075–83. doi: 10.1176/appi.ajp.158.7.1075. [DOI] [PubMed] [Google Scholar]
- Schroeder JP, Spanos M, Stevenson JR, Besheer J, Salling M, Hodge CW. Cue-induced reinstatement of alcohol-seeking behavior is associated with increased ERK1/2 phosphorylation in specific limbic brain regions: blockade by the mGluR5 antagonist MPEP. Neuropharmacology. 2008;55:546–54. doi: 10.1016/j.neuropharm.2008.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RE. Neural substrates of cocaine-cue associations that trigger relapse. Eur J Pharmacol. 2005;526:140–6. doi: 10.1016/j.ejphar.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Shabashov D, Shohami E, Yaka R. Inactivation of PKMzeta in the NAc Shell Abolished Cocaine-Conditioned Reward. J mol neurosci. 2011 doi: 10.1007/s12031-011-9671-7. (epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Shalev U, Highfield D, Yap J, Shaham Y. Stress and relapse to drug seeking in rats: studies on the generality of the effect. Psychopharmacology (Berl) 2000;150:337–46. doi: 10.1007/s002130000441. [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Nomura S, Ohishi H, Sugihara H, Nakanishi S, Mizuno N. Immunohistochemical localization of a metabotropic glutamate receptor, mGluR5, in the rat brain. Neurosci Lett. 1993;163:53–7. doi: 10.1016/0304-3940(93)90227-c. [DOI] [PubMed] [Google Scholar]
- Sidhpura N, Weiss F, Martin-Fardon R. Effects of the mGlu2/3 agonist LY379268 and the mGlu5 antagonist MTEP on ethanol seeking and reinforcement are differentially altered in rats with a history of ethanol dependence. Biol Psychiatry. 2010;67:804–11. doi: 10.1016/j.biopsych.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Li CS. Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug Alcohol Rev. 2007;26:25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- Spanagel R. Alcoholism: a systems approach from molecular physiology to addictive behavior. Physiol Rev. 2009;89:649–705. doi: 10.1152/physrev.00013.2008. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Sparta DR, Stamatakis AM, van LWA, Hardjoprajitno JE, Cho S, et al. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475:377–80. doi: 10.1038/nature10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobler PN, Fiorillo CD, Schultz W. Adaptive coding of reward value by dopamine neurons. Science. 2005;307:1642–5. doi: 10.1126/science.1105370. [DOI] [PubMed] [Google Scholar]
- Vollstadt-Klein S, Loeber S, Kirsch M, Bach P, Richter A, Buhler M, et al. Effects of cue-exposure treatment on neural cue reactivity in alcohol dependence: a randomized trial. Biol Psychiatry. 2011;69:1060–6. doi: 10.1016/j.biopsych.2010.12.016. [DOI] [PubMed] [Google Scholar]
- Weiss F, Maldonado-Vlaar CS, Parsons LH, Kerr TM, Smith DL, Ben-Shahar O. Control of cocaine-seeking behavior by drug-associated stimuli in rats: Effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens. Proc Nat Acad Sci USA. 2000;97:4321–6. doi: 10.1073/pnas.97.8.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrase J, Kahnt T, Schlagenhauf F, Beck A, Cohen MX, Knutson B, et al. Different neural systems adjust motor behavior in response to reward and punishment. Neuroimage. 2007;36:1253–62. doi: 10.1016/j.neuroimage.2007.04.001. [DOI] [PubMed] [Google Scholar]