SUMMARY
Progression of lung disease is a major event in children with cystic fibrosis (CF), but regional differences in its evolution are unclear. We hypothesized that regional differences occur beginning in early childhood. We examined this issue by evaluating 132 patients followed in the Wisconsin Neonatal Screening Project between 1985 and 2010. We scored chest x-rays obtained every 1–2 years with the Wisconsin chest x-ray system, in which we divided the lungs into quadrants, and gave special attention to ratings for bronchiectasis (BX) and nodular/branching opacities. We compared the upper and lower quadrant scores, and upper right and left quadrant scores, as patients aged using a multivariable generalized estimation equation (GEE) model. We did a confirmatory analysis for a subset of 81 patients with chest computerized tomography (CT) images obtained in 2000 and scored using the Brody scoring system. The chest x-ray analysis shows that the upper quadrants have higher BX (p<0.001) and nodular/branching opacities (p<0.001) scores than the lower quadrants. CT analysis likewise reveals that the upper quadrants have more BX (p=0.02). Patients positive for mucoid PA showed significantly higher BX scores than patients with nonmucoid PA (p= 0.001). Chest x-ray scoring also revealed that the upper right quadrant has more BX (p< 0.001) than the upper left quadrant, and CT analysis was again confirmatory (p< 0.001). We conclude that pediatric patients with CF develop more severe lung disease in the upper lobes than the lower lobes in association with mucoid PA infections and also have more severe lung disease on the right side than on the left side in the upper quadrants. A variety of potential explanations such as aspiration episodes may be clinically relevant and provide insights regarding therapies.
Keywords: cystic fibrosis, lung disease, bronchiectasis, upper lobes, mucoid Pseudomonas aeruginosa
INTRODUCTION
Progression of lung disease is a prominent feature of cystic fibrosis (CF) and continues to be the leading cause of mortality.1 Some,2–4 but not all,5 imaging studies suggest that CF lung disease is more prominent in the upper lobes, although there has not been a prospective, longitudinal assessment of the regional progression of lung disease in CF. Maffessanti et al.2 published a cross-sectional study of 36 subjects, averaging 13 years old, with computerized tomography (CT) and chest radiography. Image analysis revealed that the severity of bronchial lesions and parenchymal destructive changes was unevenly distributed, with the upper lobes more involved than the lower lobes, and the right lung worse than the left. To explore the possible reasons for more severe disease in the upper lobes of CF patients, Meyer et al.6 performed bronchoscopy on 12 patients averaging 24 years of age. They found that evidence of inflammation, as indicated by neutrophil and unopposed neutrophil elastase, was greater in the upper lobe than in the lower lobe.
The Wisconsin CF Neonatal Screening Project7,8 is a longitudinal study beginning at diagnosis (either after a positive newborn screening test, or after a clinical diagnosis) that includes systematic chest imaging. In scoring chest radiographs with the Wisconsin system,9,10 we have made longitudinal measurements of lung disease lesions and their location based on repeated evaluation of disease components such as air trapping, bronchiectasis (BX), and nodular/branching opacities. Based on previous cross-sectional studies and impressions over more than a decade of scoring, we took advantage of this unique opportunity to address the hypothesis that there were regional differences in the evolution of CF lung disease in children.
MATERIALS and METHODS
Study Design and Operation
In brief, the Wisconsin CF Neonatal Screening Project is a randomized clinical trial conducted to assess the benefits and risks of neonatal screening for CF.7,11 Blood specimens of newborns born in Wisconsin between 1985 and 1994 were assigned either to an early CF diagnosis (screened) group or to a standard diagnosis (control) group. In the early diagnosis method, patients had screening tests performed beginning April 15, 1985, using either trypsinogen analysis (prior to 1991) or a combination of trypsinogen and DNA analysis (from July 1991 through June 30, 1994, when randomization was completed). A sweat chloride level of ≥60 mEq/L at one of Wisconsin’s two CF Centers (the University of Wisconsin and the Children’s Hospital of Wisconsin) was required to establish the diagnosis.
During the first CF center visit, after describing positive sweat test results and their implications to parents, we informed them about the Wisconsin CF Neonatal Screening Project and requested consent for enrollment in this longitudinal investigation, which was approved by the Human Subjects Committee at the University of Wisconsin and the Research and Publications Committee/Human Rights Board at the Children’s Hospital of Wisconsin, affiliated with the Medical College of Wisconsin. Informed consent was given by 93% of parents. We then prospectively followed 132 CF children enrolled during April 1985 to June 1994 up to June 30, 2010 using an Evaluation and Treatment Protocol that standardized respiratory care.
For children less than 5 years of age, families were instructed to perform chest physiotherapy and postural drainage in six different positions including Trendelenberg, which was the standard of care at the time that these children were enrolled in the study. The Trendelenberg position was discontinued in 2004 after the report by Button et al.12 At that time, all of the patients in the study had already transitioned to other forms of chest physiotherapy such as positive expiratory pressure valves and high frequency chest compression. Oral and intravenous antibiotics were used as needed. We attempted eradication of the first acquisition of Pseudomonas aeruginosa using nebulized tobramycin beginning in 2003. The staff of the two CF Centers met regularly during the study to discuss new therapies such as recombinant human DNase, chronic use of azithromycin in patients chronically colonized with Pseudomonas aeruginosa, nebulized tobramycin for 28 day on-28 day off cycles and nebulized hypertonic saline. These new therapies were used according to recommendations. During the year 2000, we added a chest CT to the ongoing pulmonary outcomes evaluation and obtained written consent for a subset of patients for chest CT scans in a protocol described previously,13 which was also approved by the above institutional review boards.
Outcomes
Observations from systematic assessments were recorded on standardized study forms designed in 1984; data were entered soon after each clinic visit into a computerized database. To quantitatively monitor pulmonary outcome measures, chest radiographs were obtained at diagnosis, and at the 6-month and yearly clinic visits in the first 4 years of life. Children aged 4 years and older had protocol-mandated chest radiographs once per year at the clinic visit closest to their birth date. The original films obtained at diagnosis, age 2 years, age 4 years, and every year thereafter were scored independently by a pediatric pulmonologist and a radiologist using the Wisconsin Chest Radiograph Scoring System.9,10
Each film was assigned with a random number and grouped into sets of approximately 100 films each that were scored in sessions (1997, 1999, 2001, 2002, 2004 and 2008). In addition, a set of 16 repeat chest films was interspersed throughout the radiographs being rated, to further evaluate raters’ scoring reproducibility and rater agreement. We compared the 16 repeats over all six sessions using a mixed model with repeated measure and found that the raters’ scores are not statistically different over sessions (p=0.34) and there was no statistical difference between raters (p=0.74). The scores from both raters were averaged using the Wisconsin additive method’s formula, as recommended by Koscik et al.9
As described in previous reports, 9,10 there are six components in the Wisconsin Chest X-ray Scoring System, namely, hyperinflation, peribronchial thickening, bronchiectasis, nodular/branching opacities, large round/ill-defined opacities, and atelectasis. In scoring bronchiectasis and nodule/branching opacities, the lungs were divided into upper and lower quadrants, and right side and left side. Up to June 30, 2010, we had a total of 1,415 Wisconsin chest X-ray scores available; from these, we used 1,348 Wisconsin chest x-ray scores for subjects aged ≤18 years in this analysis due to relatively few patients and/or lack of patients’ chest x-ray scores for subjects aged >18 years.
During the year 2000, we obtained high resolution chest CT images from a subset of 81 children with CF, averaging 11.5 years (range, 6.6–17.6 years) who were still being followed in the original study and who gave consent. As described in detail elsewhere13 they had demographic and clinical characteristics that were not significantly different from the overall cohort. CT images were scored by three radiologist raters on a lobe-specific basis.13 Each lobe, with the lingula scored as a separate lobe, was evaluated for the extent and severity of bronchiectasis, mucous plugging, peribronchical thickening, parenchymal opacity, ground glass density, cysts or bullae, and air trapping. For bronchiectasis, the score reflects the average size of dilated bronchi, the size of the largest bronchus, and the amount of each lobe containing dilated bronchi.
Respiratory secretions were obtained from patients every 6 months by protocol and at all non-protocol visits requiring cultures for clinical assessment purposes. Results for Pseudomonas aeruginosa (PA) and Staphylococcus aureus (SA) were examined in the cultures. If PA was present, the morphology of the colony was identified as either nonmucoid or mucoid.
Statistical Methods
We examined the correlation between BX scores obtained from the chest CT images and the concurrent Wisconsin chest x-ray BX component scores. We estimated the annual changes in the upper quadrant and lower quadrant BX scores and nodular/branching opacities scores with a simple generalized estimating equations (GEE) model that adjusted for age to account for repeated measures. In an effort to explain any quadrant differences (upper, lower, right, left), we developed a multivariable GEE model, controlling for patient group (screened or control), CF center (Madison or Milwaukee), gender, genotype (homozygous F508del or other), pancreatic status, meconium ileus, PA status (no PA, nonmucoid PA, and mucoid PA), SA status, and age. Using the same strategy, we estimated the difference between the right upper and left upper quadrant BX scores and nodule/branching opacities scores. To confirm our findings, we used analysis of covariance to compare the chest CT bronchiectasis and parenchyma scores between the upper and lower quadrants, and between the right upper and left upper quadrants, adjusting for age.
RESULTS
Table 1 shows the characteristics of the 132 study subjects enrolled in the Wisconsin Neonatal Screening Project study. The subset of 81 patients that obtained a chest CT had similar characteristics and were representative of the overall cohort, as described in detail elsewhere.11 At the time of the CT scan, the patients ranged in age from 6.6 to 17.6 years (mean, 11.5 and median, 11.3 years). There was excellent agreement between BX scores in WCXR and BX scores in CT with r=0.85 (p<0.001, Pearson correlation coefficient).
Table 1.
Characteristics of study subjects
| Characteristic | Category | N (%) (total N=132) |
|---|---|---|
| Patient group | Control | 63 (48) |
| Screened | 69 (52) | |
| Center | Madison | 70 (53) |
| Milwaukee | 62 (47) | |
| Gender | Male | 78 (59) |
| Female | 54 (41) | |
| Genotype | F508del/F508del | 70 (53) |
| F508del/Other | 49 (37) | |
| Other/Other | 13 (10) | |
| Pancreatic status | Pancreatic Sufficiency | 21 (16)a |
| Pancreatic Insufficiency | 107 (84) | |
| Meconium ileus | No | 103 (78) |
| Yes | 29 (22) |
Pancreatic status is unknown for 4 subjects.
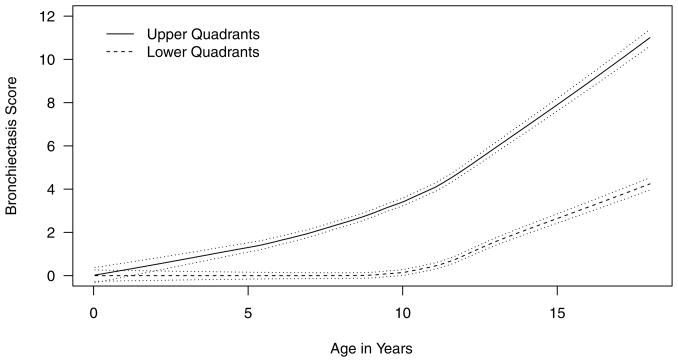
Longitudinal analysis of the Wisconsin chest x-ray scores indicates that the upper quadrants have significantly higher nodule/branching opacity scores (estimated difference (SE) from the simple GEE model: 0.36 (0.01), p<0.001) and BX scores (estimated difference (SE) from the simple GEE model: 2.48 (0.13), p<0.001, Figure 1) than the lower quadrants. The difference in BX scores between the upper and lower quadrants are statistically significant for children aged 2 and over (p<0.001, t-test). Similarly, on chest CT, we found that mean (SE) BX score for the upper quadrants (2.35 (0.33)) was significantly higher than the mean (SE) BX score for the lower quadrants (1.75 (0.27)), with a mean (SE) difference of 0.60 (0.25), p = 0.02.
Figure 1.
Quadrant difference in bronchiectasis scores over time. The solid lines represent the raw BX data with locally weighted regression and a smoothing scatterplot function. The dashed lines represent the 95% confidence limits.
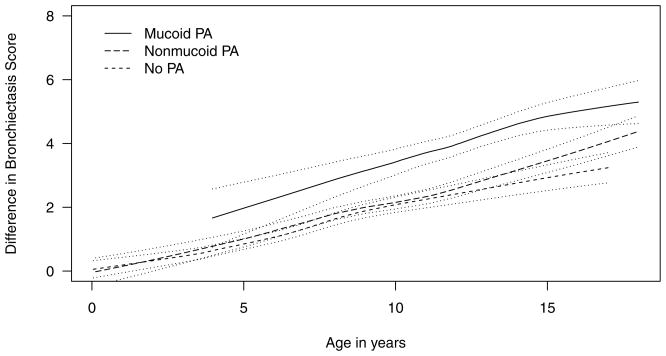
From the multivariable GEE model, PA status (p=0.0082) and age (p<0.001) were the only covariates significantly associated with upper versus lower quadrant differences in BX scores (Table 2). Having mucoid PA was associated with a greater upper versus lower quadrant difference in BX score, but there were no differences between having no PA and nonmucoid PA (Figure 2). None of the other variables were significantly associated with the difference in BX or nodule/branching opacities score between the upper and lower quadrants.
Table 2.
GEE model estimating the associations between covariates and the difference in Wisconsin chest x-ray bronchiectasis and nodular/branching opacities scores between the upper and lower quadrants. Positive estimates indicate the presence of more abnormalities in the upper quadrants
| Characteristic | Category | Bronchiectasis estimate (SE) | P-value | Nodular/branching opacities estimate (SE) | P-value |
|---|---|---|---|---|---|
| Age | Year | 0.27 (0.03) | <0.001 | −0.002 (0.12) | 0.9 |
| PA status | Nonmucoid PA (vs no PA) | −0.10 (0.21) | 0.64 | 0.01 (0.09) | 0.88 |
| Mucoid PA (vs nonmucoid PA) | 1.07 (0.33) | 0.002 | −0.07 (0.17) | 0.68 | |
| SA status | SA | 0.19 (0.18) | 0.30 | 0.09 (0.11) | 0.45 |
| Patient group | Screened (vs Control) | 0.27 (0.24) | 0.25 | 0.05 (0.11) | 0.68 |
| CF center | Milwaukee (vs Madison) | −0.19 (0.25) | 0.44 | 0.10 (0.11) | 0.40 |
| Gender | Female | 0.10 (0.25) | 0.69 | −0.10 (0.12) | 0.41 |
| Genotype | F508del/F508del (vs Other) | 0.11 (0.50) | 0.83 | 0.03 (0.18) | 0.85 |
| F508del/Other (vs Other) | 0.09 (0.47) | 0.84 | 0.01 (0.18) | 0.97 | |
| Pancreatic status | Insufficient (vs Sufficient) | −0.32 (0.36) | 0.37 | −0.15 (0.12) | 0.23 |
| Meconium ileus | MI | −0.11 (0.28) | 0.70 | 0.13 (0.14) | 0.37 |
Figure 2.
Effect of Pseudomonas aeruginosa (PA) on quadrant difference in bronchiectasis scores. The solid lines represent the raw BX data with locally weighted regression and a smoothing scatterplot function. The dashed lines represent the 95% confidence limits.
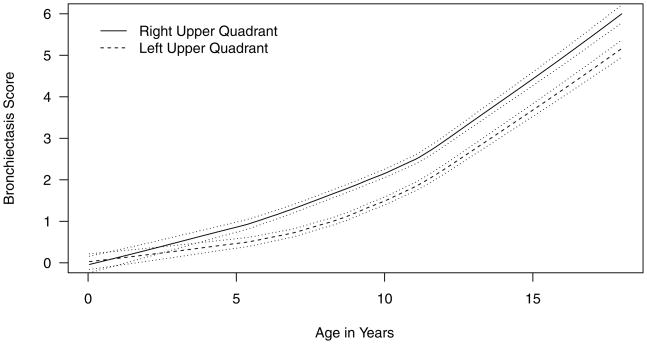
As compared to the upper left quadrant, the upper right quadrant had significantly worse BX scores by Wisconsin chest x-ray score (mean (SE) difference 0.48 (0.06), p<0.001, Figure 3). There was no difference in nodule/branching opacities score (p=0.4). Similarly, the chest CT bronchiectasis score for the right upper quadrant (mean (SE) 1.52 (0.21)) was significantly worse than the left upper quadrant (mean (SE) 0.83 (0.13)), with a mean (SE) difference of 0.68 (0.13), p<0.001. In the Wisconsin chest x-ray GEE model, age (p<0.001) was the only other factor significantly associated with right-versus left-sided upper quadrant differences in bronchiectasis scores (data not shown).
Figure 3.
Difference in bronchiectasis scores between the right upper and left upper quadrant on Wisconsin chest x-ray score. The solid lines represent the raw BX data with locally weighted regression and a smoothing scatterplot function. The dashed lines represent the 95% confidence limits.
DISCUSSION
Our principal finding is that, throughout childhood, the upper lung quadrants in children with CF have significantly worse bronchiectasis on chest x-ray and chest CT, suggesting that patients with CF develop more severe lung disease in those regions. In the only other large longitudinal evaluation of chest imaging in children with CF, Cleveland et al.5 used the Brasfield scoring system to demonstrate that disease severity tends to be more prominent in the upper lobes, but only after age 10. Before age 10, if one lobe had more prominent disease, it involved a middle lobe 48% of the time, a lower lobe 30% of the time, and an upper lobe only 22% of the time. However, the study reported by Cleveland et al.5 was published in 1998, and all children in the cohort were born before the routine use of many of the standards of care used today. Additionally, our cohort is made of children with CF identified early after a positive newborn screening test, or shortly thereafter; Cleveland’s cohort was before the era of newborn screening, and included children who typically presented with respiratory symptoms.
Maffessanti et al.2 in their cross-sectional study had found that the distribution of lesions in patients with CF is uneven, the upper portions being more extensively involved than the lower. Our results confirm and extend their data showing that there is a long-term quadrant difference in CF children. Moreover, our results reveal that mucoid PA infection, but not nonmucoid PA, leads to more quadrant difference in the development of bronchiectasis. Previously we observed that mucoid PA plays a more important role than nonmucoid PA in lung disease progression.13,14 In the current study, we have shown that the burden of lung disease progression associated with mucoid PA occurs in the upper quadrants. One possible reason might be that mucoid PA aggregates in the upper lobes rather in the lower lobes and thus leads to more damage there. Meyer et al.6 in their cross-sectional study reported that absolute number of neutrophils and unopposed neutrophil elastase activity were generally greater in bronchoalveolar lavage fluid from the right upper lobe than from the right lower lobe. They also found PA more often in the right upper lobe than either the right middle or lower lobe. Their results may help explain the zone difference. Our results reaffirm the importance of preventing infection with PA, and especially preventing PA transition from nonmucoid to mucoid status.
Our results show that bronchiectasis was more progressive on the right side over time. Maffessanti et al2 also found that the right upper lung was usually more involved than the left upper lung. In addition, Davis et al.4 found a right upper lobe dominance in 17 infants and children who received a chest CT and bronchoalveolar lavage at the time of a pulmonary exacerbation. Our results again confirm these cross-sectional findings and show a longitudinal side difference in bronchiectasis. Other than age, there were no other factors associated with the longitudinal side difference in BX scores.
The upper lobe predominance in the development of lung disease among children with CF is so striking and early that we must consider a variety of explanations and their clinical implications. Tomashefski et al.15 and Meyer et al.6 speculated on anatomic and physiologic variables that might play a role in the combination of respiratory infection and inflammation that is characteristic of CF and occurs as early as 4 weeks of age.16,17 Potential physiologic determinants include poor lymphatic drainage and relatively inefficient clearance of secretions, the higher partial pressure of oxygen in the upper lobe regions, relative hypoventilation and reduced cough clearance that might favor infection, retained secretions, and atelectasis. In addition, Meyer et al.6 in relating their findings of increased inflammation in upper lobe BAL fluid to a combination of anatomic and physiologic factors suggested that aspiration, possibly associated with gastroesophageal reflux (GER) and reduced CFTR-related bicarbonate secretion, might be incriminated, especially in infants and young children with CF. Our finding that the right upper lobe region has the worst disease in early childhood is consistent with this concept. Such an explanation might account for the observations16–18 that early inflammation is present during infancy. This explanation is consistent with findings that asymptomatic infants who received airway clearance therapy (ACT) in the Trendelenburg position, during which more episodes of GER occurred, had more respiratory symptoms in the first year of life and worse pulmonary function tests and chest x-ray scores at age 5.12 The authors of that study concluded that further prospective evaluation of chest physiotherapy in asymptomatic children with CF was needed. This is especially true in this new era of early diagnosis through newborn screening coupled to aggressive treatment.19 Our results indicate that a more cautious strategy for ACT may be needed to avoid iatrogenic insults to the developing lung in children with CF, especially in view of the limited evidence of benefits.
Our study has several limitations. First, we do not have data on inflammation or lower airway microbiology from bronchoalveolar lavage, although a correlation between structural changes and regional inflammation has been previously shown.4 Previous studies have also shown that oropharyngeal cultures have high negative predictive value for BAL cultures,20 so our identification of P. aeruginosa in oropharyngeal cultures and cough swabs is likely an accurate representation of infection status in our patients. Second, we do not have information on the presence or absence of gastroesophageal reflux disease or dysphagia in our patients to further corroborate the findings by Button, et al. Additionally, we only follow patients who received chest physiotherapy in the Trendelenburg position early in life, so we cannot compare our findings after this practice was discontinued. Finally, infants with CF born today have more treatments available than when our cohort began in the 1980s. However, there is no data on how new treatment modalities may affect regional distribution of lung disease.
We conclude that, in longitudinal measurements, pediatric patients with CF develop more severe lung disease in the upper lobes than the lower lobes in association with mucoid PA infections. Lung disease is also more severe on the right side than on the left side in the upper quadrants. Given the predominance of disease in the right upper quadrant, the contributions of aggressive identification and treatment of gastroesophageal reflux and dysphagia to the prevention of lung disease should be evaluated in infants with CF, especially given the opportunities made possible by early diagnosis after newborn screening.
Acknowledgments
The authors thank the patients and families and all other collaborators in the Wisconsin CF Neonatal Screening Project. The authors also thank Keith Meyer, for assistance with the manuscript.
Grant sponsor: National Institutes of Health Grant number: DK 34108; Cystic Fibrosis Foundation: Grant number: A001-5-01
References
- 1.Ramsey B. Management of pulmonary disease in patients with cystic fibrosis. N Engl J Med. 1996;335(3):179–188. doi: 10.1056/NEJM199607183350307. [DOI] [PubMed] [Google Scholar]
- 2.Maffessanti M, Candusso M, Brizzi F, Piovesana F. Cystic fibrosis in children: HRCT findings and distribution of disease. J Thorac Imaging. 1996;11(1):27–38. doi: 10.1097/00005382-199601110-00002. [DOI] [PubMed] [Google Scholar]
- 3.Santis G, Hodson ME, Strickland B. High resolution computed tomography in adult cystic fibrosis patients with mild lung disease. Clin Radiol. 1991;44(1):20–22. doi: 10.1016/s0009-9260(05)80220-x. [DOI] [PubMed] [Google Scholar]
- 4.Davis SD, Fordham LA, Brody AS, Noah TL, Retsch-Bogart GZ, Qaqish BF, Yankaskas BC, Johnson RC, Leigh MW. Computed tomography reflects lower airway inflammation and tracks changes in early cystic fibrosis. Am J Respir Crit Care Med. 2007;175(9):943–950. doi: 10.1164/rccm.200603-343OC. [DOI] [PubMed] [Google Scholar]
- 5.Cleveland R, Neish A, Zurakowski D, Nichols D, Wohl M, Colin A. Cystic fibrosis: predictors of accelerated decline and distribution of disease in 230 patients. AJR Am J Roentgenol. 1998;171(5):1311–1315. doi: 10.2214/ajr.171.5.9798870. [DOI] [PubMed] [Google Scholar]
- 6.Meyer KC, Sharma A. Regional variability of lung inflammation in cystic fibrosis. Am J Respir Crit Care Med. 1997;156(5):1536–1540. doi: 10.1164/ajrccm.156.5.9701098. [DOI] [PubMed] [Google Scholar]
- 7.Farrell P, Kosorok M, Laxova A, Shen G, Koscik R, Bruns W, Splaingard M, Mischler E. Nutritional benefits of neonatal screening for cystic fibrosis. Wisconsin Cystic Fibrosis Neonatal Screening Study Group. N Engl J Med. 1997;337(14):963–969. doi: 10.1056/NEJM199710023371403. [DOI] [PubMed] [Google Scholar]
- 8.Farrell PM. Improving the health of patients with cystic fibrosis through newborn screening. Wisconsin Cystic Fibrosis Neonatal Screening Study Group. Adv Pediatr. 2000;47:79–115. [PubMed] [Google Scholar]
- 9.Koscik R, Kosorok M, Farrell P, Collins J, Peters M, Laxova A, Green C, Zeng L, Rusakow L, Hardie R, Campbell P, Gurney J. Wisconsin cystic fibrosis chest radiograph scoring system: validation and standardization for application to longitudinal studies. Pediatr Pulmonol. 2000;29(6):457–467. doi: 10.1002/(sici)1099-0496(200006)29:6<457::aid-ppul8>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 10.Weatherly M, Palmer C, Peters M, Green C, Fryback D, Langhough R, Farrell P. Wisconsin cystic fibrosis chest radiograph scoring system. Pediatrics. 1993;91(2):488–495. [PubMed] [Google Scholar]
- 11.Farrell P, Kosorok M, Rock M, Laxova A, Zeng L, Lai H, Hoffman G, Laessig R, Splaingard M. Early diagnosis of cystic fibrosis through neonatal screening prevents severe malnutrition and improves long-term growth. Wisconsin Cystic Fibrosis Neonatal Screening Study Group. Pediatrics. 2001;107(1):1–13. doi: 10.1542/peds.107.1.1. [DOI] [PubMed] [Google Scholar]
- 12.Button BM, Heine RG, Catto-Smith AG, Olinsky A, Phelan PD, Ditchfield MR, Story I. Chest physiotherapy in infants with cystic fibrosis: to tip or not? A five-year study. Pediatr Pulmonol. 2003;35(3):208–213. doi: 10.1002/ppul.10227. [DOI] [PubMed] [Google Scholar]
- 13.Farrell P, Collins J, Broderick L, Rock M, Li Z, Kosorok M, Laxova A, Gershan W, Brody A. Association between mucoid Pseudomonas infection and bronchiectasis in children with cystic fibrosis. Radiology. 2009;252(2):534–543. doi: 10.1148/radiol.2522081882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Z, Kosorok M, Farrell P, Laxova A, West S, Green C, Collins J, Rock M, Splaingard M. Longitudinal development of mucoid Pseudomonas aeruginosa infection and lung disease progression in children with cystic fibrosis. JAMA. 2005;293(5):581–588. doi: 10.1001/jama.293.5.581. [DOI] [PubMed] [Google Scholar]
- 15.Tomashefski JF, Bruce M, Goldberg HI, Dearborn DG. Regional distribution of macroscopic lung disease in cystic fibrosis. Am Rev Respir Dis. 1986;133(4):535–540. doi: 10.1164/arrd.1986.133.4.535. [DOI] [PubMed] [Google Scholar]
- 16.Khan TZ, Wagener JS, Bost T, Martinez J, Accurso FJ, Riches DW. Early pulmonary inflammation in infants with cystic fibrosis. Am J Respir Crit Care Med. 1995;151(4):1075–1082. doi: 10.1164/ajrccm/151.4.1075. [DOI] [PubMed] [Google Scholar]
- 17.Stick S, Brennan S, Murray C, Douglas T, von Ungern-Sternberg B, Garratt L, Gangell C, De Klerk N, Linnane B, Ranganathan S, Robinson P, Robertson C, Sly P CF) ARESTfCFA. Bronchiectasis in infants and preschool children diagnosed with cystic fibrosis after newborn screening. J Pediatr. 2009;155(5):623–628.e621. doi: 10.1016/j.jpeds.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Stafler P, Davies JC, Balfour-Lynn IM, Rosenthal M, Bush A. Bronchoscopy in cystic fibrosis infants diagnosed by newborn screening. Pediatr Pulmonol. 2011;46(7):696–700. doi: 10.1002/ppul.21434. [DOI] [PubMed] [Google Scholar]
- 19.Borowitz D, Robinson KA, Rosenfeld M, Davis SD, Sabadosa KA, Spear SL, Michel SH, Parad RB, White TB, Farrell PM, Marshall BC, Accurso FJ, Foundation CF. Cystic Fibrosis Foundation evidence-based guidelines for management of infants with cystic fibrosis. J Pediatr. 2009;155(6 Suppl):S73–93. doi: 10.1016/j.jpeds.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenfeld M, Emerson J, Accurso F, Armstrong D, Castile R, Grimwood K, Hiatt P, McCoy K, McNamara S, Ramsey B, Wagener J. Diagnostic accuracy of oropharyngeal cultures in infants and young children with cystic fibrosis. Pediatr Pulmonol. 1999;28(5):321–328. doi: 10.1002/(sici)1099-0496(199911)28:5<321::aid-ppul3>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]