Abstract
Background/Objectives
Patients with atopic dermatitis (AD) are predisposed to infection with Staphylococcus aureus which worsens their skin disease; it has been postulated that the relative lack of antimicrobial peptides due to aberrant allergic inflammation in AD skin could mediate this enhanced bacterial susceptibility. We sought to characterize the amounts of S. aureus and biological products found in infected AD lesions and whether treatment with topical corticosteroids and oral cephalexin as only antimicrobial improved outcomes.
Methods
59 children with clinically and S. aureus-positive impetiginized lesions of AD were enrolled in this study. A lesion was graded clinically using the Eczema Area and Severity Index, and wash fluid was obtained from the lesion for quantitative bacterial culture and antibiotic sensitivities, and measurement of bacterial products and cytokines. Subjects were re-evaluated two weeks following treatment.
Results
Improvement in the clinical and inflammatory characteristics of impetiginized lesions were noted, even in the 15% of lesions infected with MRSA. In a subgroup of subjects whose lesions did not contain S. aureus two weeks after initiating treatment, beta-defensin levels were elevated at both visits in comparison to normal skin.
Conclusions
These studies indicate that treatment of uncomplicated impetiginized pediatric AD with topical corticosteroids and the antibiotic cephalexin results in significant clinical improvement, even in subjects infected with MRSA. We propose that the inhibition of abnormal inflammation by the treatment regimen resulting in the increased relative levels of defensins is involved in the improvement of AD, and that systemic antibiotics do not appear to be necessary in secondary impetiginized AD.
Keywords: Staphylococcus aureus, atopic dermatitis, lipoteichoic acid, staphylococcal protein A, Tumor necrosis factor-alpha, methicillin-resistant Staphylococcus aureus
Introduction
Staphylococcus aureus infections are known triggers for skin inflammation and can modulate immune responses. Patients with atopic dermatitis (AD) are predisposed to staphylococcal colonization/infections which worsen their skin disease [1,2]. The mechanisms by which AD patients exhibit increased susceptibility to bacterial and viral infections are not completely defined, but several lines of evidence suggest a role for cationic antimicrobial defensin and cathelicidin peptides (antimicrobial peptides; AMPs) [3,4]. First, AMP levels are decreased in AD lesions [5]. Second, the T helper 2 (Th2) cytokines IL-4 and IL-13, which are elevated in AD skin lesions, have been reported to inhibit AMP expression in human keratinocytes cultures in vitro [6–8]. Moreover, several agents which have reported efficacy for diminishing staphylococcal colonization/infection in the setting of AD including systemic vitamin D and topical pimecrolimus can upregulate AMP production in keratinocytes [9,10]. Finally, the inhibition of abnormal allergic (Th2) inflammation could explain the isolated reports that topical corticosteroids alone can decrease S. aureus numbers in AD lesions [11].
Methacillin-resistant S. aureus (MRSA) is emerging as an important health concern [8,12]. Given that patients with AD are predisposed to staphylococcal infection, the incidence and clinical significance of MRSA in this special population is of importance. Previously, we reported a group of 89 pediatric AD patients from whom we characterized the wash fluid obtained from a quantitative culture of a clinically impetiginized skin lesion for amounts of S. aureus, antibiotic susceptibilities, and quantified lipoteichoic acid (LTA) and staphylococcal protein A (SPA) as well as a panel of cytokines [13,14] [25,28]. The present study examined a sub-population of this group whose lesions were positive for S. aureus, and were treated with a regimen of topical corticosteroids, oral antihistamines, and the oral antibiotic cephalexin as the only antimicrobial to assess the effectiveness of this therapy. This study indicates that treatment of pediatric AD patients with this regimen resulted in significant improvement of clinical and inflammatory parameters, even in subjects found to harbor cephalexin-resistant MRSA.
Methods
Atopic Dermatitis Subjects
Pediatric subjects with AD diagnosed using criteria of Hanifin and Rajka [15] with clinical evidence of impetiginized skin lesions were enrolled in these studies approved by the Indiana University Institutional Review Committee. As outlined in our previous study, subjects were not exposed to oral or topical antibiotics for a period of one month before the study [13]. None of the subjects had evidence of systemic involvement (e.g, fevers, chills) from their skin infection. Subjects enrolled into the study underwent a clinical assessment of a clinically-infected lesion of dermatitis using the Eczema Area and Severity Index (EASI), as well as an EASI scoring of entire body [16]. For consistency, both authors J.B.T. and A.K. conducted all of the EASI scoring. Wash fluid was removed from the lesion and the subject treated with topical corticosteroids (daily desonide ointment mild areas of dermatitis on body, face, or intertriginous areas; triamcinolone 0.1% ointment for thicker areas of dermatitis on body); oral antihistamines (hydroxyzine 1–2 mg/kg or doxepin 0.5–1 mg/kg daily) and oral cephalexin (approximately 25–50 mg/kg day divided BID to TID) for two weeks. Following two weeks, the subjects returned and the procedures were repeated. None of the subjects in this cohort used topical antibiotics nor bleach or other antiseptics in their bath water for one month before and during the two week treatment time.
Quantitative Analysis of Dermatitis Lesions
Wash fluid derived from lesions was removed from a 2.5 cm diameter polypropylene chamber as previously described [13,17] using the methodology established by Williamson and Kligman [18]. Briefly, a sterile 2.5 cm diameter ring of PVC tubing (Nalgene® Labware, Rochester, NY) was placed over the skin lesion of patient, then, 1 ml sterile rinse solution (0.069M Na2HPO4, 0.0064M NaH2PO4, and 0.1% Tx-100) was administered inside the ring chamber that was held tightly on the skin to prevent leakage. The rinse solution was stirred around in the chamber with a sterile Teflon® rod (Scientific Commodities Inc., Lake Havasu City, AZ) for 15–20 times and collected. This collection was repeated and 2 ml total rinse solution was obtained. This methodology has been shown to be 95% quantitative for aerobic surface bacteria [18]. The wash solution was then aliquotted for immunoblotting analysis of LTA, ELISA measurement of SPA and cytokines, and bacterial quantitation. S. aureus colonies were quantified in the microbiology lab in Indiana University Hospital by limiting dilution assay, and antibiotic susceptibilities assessed by standard methodology.
Measurement of LTA, SPA and defensins in wash fluid specimens
Quantitative measurements of LTA and SPA proteins were as previously described [13,14,17]. Briefly, for LTA, 32 μl rinse solution from each patient sample was separated on 18% Tris-HCl gradient gel (Bio-Rad Laboratories, Hercules, CA), along with standards of 10, 5, 2.5, and 1 ng LTA protein (Sigma-Aldrich, St. Louis, MO) dissolved in same rinse solution loaded on the same electrophoresis gel. LTA protein was determined by immunoblotting with LTA monoclonal antibody (QED Bioscience, San Diego, CA) and enhanced chemiluminescence (Amersham Pharmacia Biotech, Piscataway, NJ). The arbitrary optical densities were measured by Image J Software (NIH, Bethesda, MD). The quantification of LTA was determined according to the standard curve drawn. Quantitative measurement of SPA was performed using ELISA (Assay Designs Inc., Ann Arbor, MI) as previously described [13]. Human beta-defensins (HBD) 2 and HBD3 proteins were quantified by ELISA (Phoenix Pharmaceuticals Inc., Burlingame, CA). Defensins and bacterial products LTA and SPA were quantified based upon area (ng/cm2) of the chamber that then was converted to volume (ng/cm3) based upon estimation of 0.1 cm effective epidermal thickness. Defensin levels were also expressed relative to the total amounts of protein measured in wash fluid. Total wash fluid protein was measured using the BCA Protein Assay (Thermo Scientific, Rockford, IL).
Cytokine multiplex analysis of wash fluid specimens
Patient wash fluids were collected and levels of IL-1β, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12, IL-13, IL-17, IFN-γ and TNF-α were measured using the Multiplex Bead Immunoassays as per manufacturer’s protocol (Millipore, Billerica, MA). A Luminex 200 instrument (Luminex Corporation, Austin, TX) was used for data acquisition and analysis. Cytokine concentrations were calculated using Bio-Plex Manager 2.3 software with a five parameter curve-fitting algorithm applied for standard curve calculations. Cytokines in wash fluid were quantified based upon area (ng/cm2) of the chamber that then was converted to volume (ng/cm3) based upon estimation of 0.1 cm effective epidermal thickness.
Statistical analysis
For all correlation analyses, Spearman rank correlation coefficient was used. Wilcoxon rank sum test was applied to compare clinical outcomes at visit 1 and changes in these outcomes at visit 2 from visit 1 between MRSA- and MSSA-positive skin lesions. Wilcoxon signed rank test was used to detect a significant change in outcomes of interest at visit 2 from visit 1. In computing changes in outcomes, we used value 0 whenever outcomes were not detectable.
Results
Correlation between lesional concentration of S. aureus bacteria and clinical inflammation
Fifty-nine children (ages 4 months-6 years) with AD from our original cohort of 89 subjects [13] who fulfilled the study criteria (positive for S. aureus, followed the treatment regimen, returned to clinic in 2 weeks) outlined in Methods were enrolled in these studies to clinically define eczema severity and quantitatively determine the levels of lesional cytokines and bacterial products before and after defined treatment. The experimental procedure, including a photograph of a typical patient at first and second visits is depicted in Fig 1. Lesion wash fluid was obtained and used for quantitative S. aureus culture or aliquotted and stored at −80 °C before it was used for measurement of other proteins/cytokines. An isolate of the S. aureus was assessed for antibiotic sensitivities.
Fig. 1. Outline of research protocol.
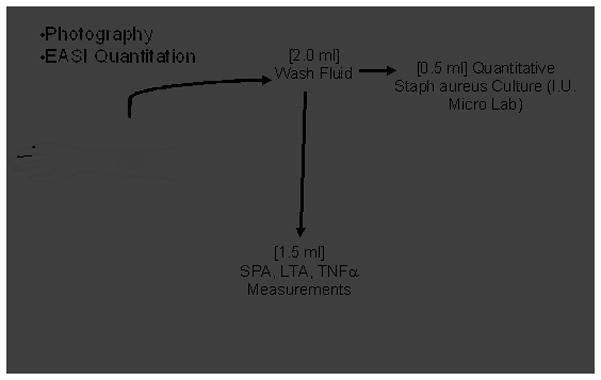
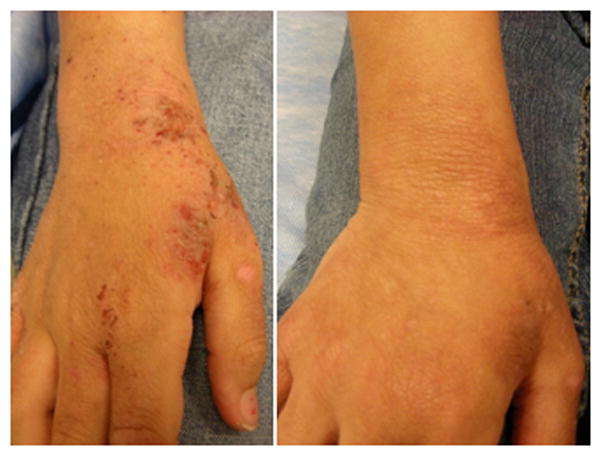
1A. Outline of clinical protocol involving wash fluid derived from clinically impetiginized AD lesions. 1B. Typical subject with clinically impetiginized (MRSA in this case) AD lesion at first visit, and second visit two weeks later following treatment with topical corticosteroid and oral cephalexin.
The lesions varied among the subjects, with 47 lesions from extremities, 8 from trunk/back/abdomen, and 4 from the face. No significant differences were seen in staphylococcal CFU or lesional EASI scores among samples taken from various locations (data not shown). Previous studies [19,20] have reported that the amount of skin inflammation tends to correlate with the numbers of S. aureus bacteria, with levels above 106 CFU/ml indicative of clinical infection. As depicted in Fig 2 the S. aureus CFU correlated with the lesional EASI scores at visit 2 (Spearman Correlation coefficient, r = 0.37, p = 0.004). These studies indicate that the levels of staphylococcal CFU significantly correlate with clinical inflammation in this cohort of pediatric AD patients.
Fig. 2. Amounts of S. aureus bacteria versus clinical assessment of inflammation in AD Subjects.
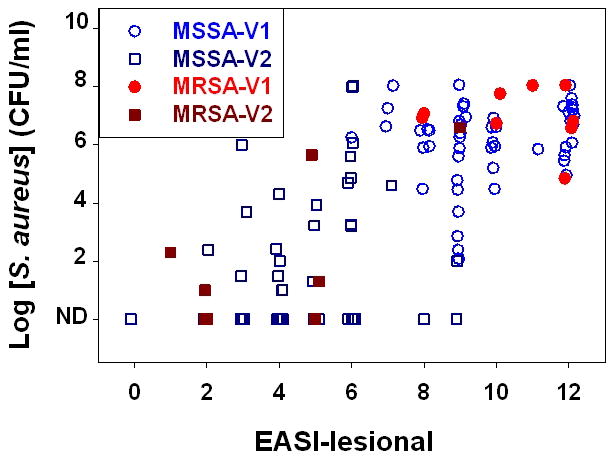
Wash fluid obtained from 59 subjects at first visits (open circle for MSSA and filled circle for MRSA) and at two-week follow-up visits (open square for MSSA and filled square for MRSA) were assessed for amounts of S. aureus bacteria and this was compared to Lesional EASI scores.
Cytokine and bacterial product analysis of wash fluid specimens from impetiginized AD lesions
Staphylococcal products including the bacterial lipoprotein LTA and protein SPA have potent immunomodulatory effects [13,17, 21,22]. Our previous studies have discovered that significant levels of these mediators are found in impetiginized AD skin lesions [13,14,17]. The next studies examined the amounts of LTA and SPA in the wash fluid derived from the skin lesions from our cohort of subjects. In this cohort of pediatric AD subjects, there was a significant positive correlation between amounts of LTA and staphylococcal bacteria (Spearman Correlation coefficients visit 1: r = 0.47, p < 0.001). Similarly, there was a strong positive correlation between SPA levels and staphylococcal CFU (Spearman Correlation coefficients visit 1: r = 0.68, p < 0.0001; visit 2: r = 0.46, p < 0.001;). The amounts of LTA and SPA also correlated with the EASI lesional score at visit 1; since the LTA and SPA levels were not detectable for greater than 90% of visit 2 samples, further analysis was not performed.
Wash fluid samples were also subjected to multiplex analysis to measure protein levels of IL-1β, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12, IL-13, IL-17, IFN-γ and TNF-α. As outlined in Table I, the cytokines found in the greatest abundance in 1st visit lesions were IL-6, IL-8, with lesser amounts of TNF-α and IL-1β. Of note, increased levels of Th2 cytokines IL-4 and IL-13 were found in 1st visit wash fluid samples in comparison to essentially no Th1 cytokines IL-12 and IFN-γ. Significant Spearman Correlation coefficients were found for the following cytokines versus EASI lesional scores at visit 1: IL-1β (r = 0.33, p = 0.01); IL-6 (r = 0.34, p = 0.009); IL-8 (r = 0.38; p = 0.003); IL-13 (r = 0.29; p = 0.025); and TNF-α (r = 0.43; p < 0.001). For these same outcomes, stronger correlation was observed at visit 2. These findings indicate that significant levels of LTA and SPA and pro-inflammatory cytokines are found in impetiginized AD lesions.
Table I. Comparison of clinical assessment of inflammation, amounts of S. aureus, and levels of LTA and SPA and cytokines in MRSA versus MSSA-positive AD lesions.
The EASI score of the tested lesion and total body EASI scores, concentration of S. aureus bacteria, LTA, SPA and cytokine levels were compared in samples which the bacterial isolate was sensitive (MSSA) or resistant (MRSA) to the antibiotic oxacillin. Each of the above are the mean, (SD) and [median].
| Variable | MSSA-Visit 1 (N = 50) | MRSA-Visit 1 (N = 9) | MSSA-Visit 2 (N = 50) | MRSA-Visit 2 (N = 9) |
|---|---|---|---|---|
| EASI-Lesional | 9.8 (1.7) [9.0] | 10.6 (1.7) [11] | 4.5 (1.8) [4.0]* | 3.7 (2.5) [2.0]* |
| EASI-Total | 19 (11) [22] | 33.4 (24) [28] | 4.8 (4.4) [3.3]* | 6.3 (5.2) [5.0]* |
| Log [SA] (CFU/ml) | 6.1 (1.4) [6.4] | 7.0 (1.0) [6.9]# | 1.7 (2.3) [0]* | 1.9 (2.5) [1.0]* |
| [LTA] (pg/cm3) | 1024 (1892) [274] | 1466 (2150) [624] | ND: 92%* | ND: 78%* |
| [SPA] (pg/cm3) | 17 (52) [1.1] | 15 (28) [8.4] | 1.5 (8) [0.02]* | 1.5 (3.4) [0]* |
| [IL-1β] (pg/cm3)) | 133 (457) [40] | 96 (126) [52] | 15 (33) [4.0]* | 18 (21) [16]* |
| [IL-4] (pg/cm3) | 21 (42) [3.8] | 9.8 (14) [0.8] | ND: 88%* | ND: 56% |
| [IL-5] (pg/cm3) | 58 (231) [2.9] | 1.8 (1.9) [1.7] | ND: 84%* | ND: 100% |
| [IL-6] (pg/cm3) | 338 (502) [131] | 582 (874) [338] | 23 (48) [3.4]* | 28 (55) [1.0]* |
| [IL-8] (pg/cm3) | 10190 (15034) [4935] | 7924 (10826) [4598] | 2054 (6364) [180]* | 678 (779) [545]* |
| [IL-10] (pg/cm3) | 2.2 (3.6) [0.1] | 1.6 (2.3) [0.3] | ND: 96%* | ND: 89% |
| [IL-12] (pg/cm3) | ND: 92% | ND: 100% | ND: 96% | ND: 100% |
| [IL-13] (pg/cm3) | 214 (861) [15] | 22 (23) [11] | 30 (101) [0.6]* | 2.3 (4.8) [0]* |
| [IL-17] (pg/cm3) | 23 (65) [2.5] | 0.5 (1.1) [0] | ND: 60%* | ND: 67% |
| [TNF-α] (pg/cm3) | 47 (48) [29] | 47 (31) [47] | 8.2 (15.7) [3.0]* | 4.4 (5.9) [1.3]* |
| [IFN-γ] (pg/cm3) | ND: 94% | ND: 96% | ND: 96% | ND: 100% |
Denotes that the CFU of MRSA is statistically (p = 0.049) greater than MSSA at visit 1;
Denotes that the reduction at visit 2 from visit 1 within each group was significant, p < 0.05. ND: non-detectable.
Treatment outcomes in subjects with MSSA versus MRSA
Of the 59 staphylococcal isolates from our cohort of subjects, 9 (15%) were MRSA. As shown in Table I, there were no statistically significant differences in the various parameters except a slight but statistically (p = .049) increased MRSA vs MSSA CFU at visit 1. Children with MRSA tended to have increased total EASI scores but this was not statistically significant. Treatment with our regimen of topical corticosteroids and oral cephalexin resulted in significant improvement of EASI lesional and EASI total scores, as well as S. aureus CFU in the majority of impetiginized lesions. All nine subjects with MRSA exhibited improvement in EASI lesional and EASI total scores, and in seven subjects the staphylococcal CFU was decreased (and in four of the seven no S. aureus was measured at visit 2). In one of the nine subjects with MRSA the staphylococcal CFU was almost unchanged, and in one the staphylococcal CFU increased by more than sixfold. In the subjects harboring MSSA, 48 of 50 exhibited improvement in lesional EASI, with two subjects unchanged. Forty nine of the 50 exhibited improved EASI total scores at visit 2, with one almost unchanged. The staphylococcal CFU decreased in 47 of the 50 subjects with MSSA (and in 27 of the 47 no S. aureus was measured at visit 2). In three of the 50 subjects with MSSA the staphylococcal CFU was increased 2–10 fold.
As outlined in Table I, levels of bacterial products LTA and SPA and pro-inflammatory cytokines IL-1β, IL-4, IL-5, IL-6, IL-8, IL-10, IL-13, IL-17 and TNF-α were also decreased significantly following treatment in both MSSA- and MRSA-infected skin lesions. These findings indicate that the treatment regimen of topical corticosteroids and the oral antibiotic cephalexin was effective, even in subjects harboring MRSA.
Defensin analysis of wash fluid specimens from successfully treated impetiginized AD lesions
The next studies were designed to explore the mechanisms by which successful therapy was able to remove S. aureus. The first and second visit wash fluids from five subjects whose first visit demonstrated heavy (> 106 CFU) S. aureus at initial visit with no bacteria at follow-up visit were tested for the presence of human beta defensins (HBD) 2 and 3. As depicted in Table II, HBD2 and HBD3 were detected in wash fluid specimens derived from lesions at both 1st and 2nd visits. Defensin levels are expressed as effective concentrations (ng/cm3) as well as amounts relative to total wash fluid protein (ng/mg). It should be noted that wash fluids derived from clinically normal skin from five subjects with no history of skin disease revealed low levels of HBD2 (0.17–0.84 ng/cm3 and 3.7–12.4 ng/mg) and HBD3 (0–0.64 ng/cm3 and 0–3.9 ng/mg). In the pediatric AD subjects, HBD2 effective concentrations were similar in 1st and 2nd visits, yet the amounts relative to total wash fluid protein were greater at 2nd visits. HBD3 effective concentrations were greater at 1st in comparison to 2nd visits, but the amounts relative to total wash fluid protein were similar at 1st compared to second visit, and were clearly greater than those found in normal skin. Similarly, measureable amounts of Th2 cytokines IL-4 and IL-13 were found in first visit lesions, but were not present in treated skin lesions. The increased levels of HBD2 and HBD3 relative to normal skin and the lack of detectable Th2 cytokines at the 2nd visit fit with the notion that removing the Th2-mediated inhibition [3,4] contributes to “normalization” of AMP levels and resolution of staphylococcal infection. These studies indicate that sustained levels of endogenous antimicrobial cationic peptides HBD2 and HBD3 and loss of Th2 cytokines are associated with successfully treated secondarily impetiginized AD lesions.
Table II. Comparison of clinical assessment of inflammation, S. aureus CFU, levels of defensins, bacterial products LTA and SPA, and cytokines in 1st and 2nd visit samples from impetiginized AD subjects.
The EASI score of the tested lesion, concentration of S. aureus bacteria, LTA, SPA and cytokine levels were compared with HBD2 and HBD3 levels in 1st and 2nd visit (post-treatment) in a group of five subjects whose follow-up visits revealed no evidence of S. aureus. HBD levels are described as effective concentration (ng/cm3) as well as relative amounts per mg of total protein in the wash fluid samples. ND, non-detectable.
| Samples | EASI les |
Log SA |
Total Protein (mg/ml) |
HBD2 ng/cm3 |
HBD2 ng/mg |
HBD3 ng/cm3 |
HBD3 ng/mg |
LTA ng/cm3 |
SPA ng/cm3 |
IFN-γ pg/cm3 |
IL-12 pg/cm3 |
IL-4 pg/cm3 |
IL-5 pg/cm3 |
IL-13 pg/cm3 |
IL-1β pg/cm3 |
TNF-α pg/cm3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 Visit 1 |
9 | 7.0 | 1.77 | 8.0 | 1.1 | 145 | 20 | 918 | 1.1 | ND | ND | 16 | 45 | 452 | 19 | 86 |
| Patient 1 Visit 2 |
2 | ND | 0.026 | 7.8 | 75 | 3.5 | 34 | ND | 0.07 | ND | ND | ND | ND | 1.5 | ND | ND |
| Patient 2 Visit 1 |
9 | 6.7 | 2.41 | 7.4 | 0.8 | 104 | 11 | 1210 | 0.93 | ND | ND | 36 | 63 | 608 | 36 | 62 |
| Patient 2 Visit 2 |
2 | ND | 0.068 | 2.2 | 8.1 | 2.0 | 7.1 | ND | 0.05 | ND | ND | ND | ND | ND | ND | ND |
| Patient 3 Visit 1 |
12 | 6.1 | 2.05 | 10 | 1.2 | 135 | 16 | 9804 | 2.4 | ND | ND | 3.8 | 6 | 11 | 122 | 36 |
| Patient 3 Visit 2 |
4 | ND | 0.93 | 7.4 | 2.0 | 39 | 11 | ND | 0.03 | ND | ND | ND | ND | 1.4 | 4.1 | ND |
| Patient 4 Visit 1 |
12 | 6.7 | 1.59 | 9.0 | 1.4 | 86 | 13 | 6400 | 19 | 0.8 | ND | 0.8 | 27 | 98 | 25 | 34 |
| Patient 4 Visit 2 |
4 | ND | 0.45 | 9.7 | 5.4 | 16 | 9.0 | ND | 0.07 | ND | ND | ND | ND | ND | ND | ND |
| Patient 5 Visit 1 |
9 | 7.4 | 0.64 | 7.1 | 2.8 | 90 | 36 | 720 | 22 | ND | ND | 3.0 | ND | 1.4 | 24 | 8 |
| Patient 5 Visit 2 |
4 | ND | 0.43 | 8.7 | 5.1 | 55 | 32 | ND | ND | ND | ND | ND | ND | ND | 1.1 | ND |
Discussion
The present studies confirm previous reports of an association between high amounts of S. aureus and clinical worsening of AD lesions [19,20]. At first visit, subjects with MRSA had slightly higher S. aureus level compared to those with MSSA (p=0.049). There were no other statistically significant associations between antibiotic susceptibility pattern (MRSA vs. MSSA) and the following parameters: total or lesional EASI score, LTA, SPA or cytokines levels in impetiginized AD lesions.
The percentage of pediatric AD subjects with clinically-impetiginized lesions found to have MRSA was 15%. These findings are similar to the 7.4% MRSA of positive S. aureus skin cultures of pediatric AD subjects reported recently by Huang and colleagues [23], 14% by Martzi and colleagues [24] and 16% MRSA described by Suh and colleagues [25].
The clinical significance of MRSA in pediatric AD is unclear at this time. It is of interest that the present studies, as well as those populations previously reported [23–25] all indicate that the incidence of MRSA in pediatric AD patients appears lower than that of the general population. The reasons for this are unclear, but might be related to the fact that with the immune and barrier abnormalities associated with AD, there is probably not a tremendous selective advantage to MRSA in this setting unless there is significant exposure to oral antibiotics including cephalexin. Consistent with this notion, Huang et al described clinical experiences in which patients with MRSA converted to MSSA and vice-versa [23]. However, in pediatric patients with severe and/or chronic AD and exposure to chronic antibiotics, MRSA is reported to be more problematic [26,27,30].
MRSA isolates are reported to be resistant to cephalexin; thus, the clinical and microbiologic improvement seen in this study in AD lesions impetiginized with MRSA cannot be attributed to antimicrobial therapy [31,32]. Instead, we propose that improvement resulted from other components of the therapeutic regimen including topical corticosteroids, oral antihistamines and patient education regarding barrier care and daily baths with a mild soap. The findings of the present studies using measurements of clinical, microbiologic, and inflammatory cytokines agree with the conclusions of a recently published Cochrane review regarding the lack of necessity of systemic antibiotics for the treatment of secondarily impetiginized AD [33].
Though this interpretation that the improvement of MRSA-infected AD lesions following cephalexin as only antimicrobial indicated that the effects of topical care were adequate appears reasonable, it should be noted that there is not an absolute correlation between in vitro sensitivities and in vivo clinical improvement of infections. It has been reported that in clinical practice approximately 90% treated with antimicrobial drug which is active in vitro would be expected to improve; whereas 60% of patients treated with antibiotics lacking in vitro activity against a causative pathogen will have a clinical response [34]. This “90–60” rule described in immunocompetent individuals could be a partial explanation for why AD subjects with MRSA improved with cephalexin.
We hypothesize that the inhibition of the abnormal Th2 immune response associated with flaring AD by this regimen allowed normalization of barrier function as well as reversed AMP deficiencies, both of which served to help improve if not eliminate the staphylococcal skin infection. Indeed, a body of literature has been developing that demonstrates that key Th2 cytokines (IL-4, IL-13) can not only inhibit the synthesis of proteins such as filaggrin involved in skin barrier formation [28,29], they also serve to inhibit the production of endogenous antimicrobial peptides [6–8]. Our preliminary data examining levels of defensins and cytokines that upregulate (e.g., IL-1β, TNF-α) or inhibit (IL-4, IL-13) their synthesis from five subjects with >106 CFU S. aureus at visit one but no evidence of bacteria at second visit fit with this hypothesis.
The limitations of this study include the low numbers of subjects, and the considerable variability in the amounts of bacteria and bacterial products found in the skin lesions. Possible reasons for the variability of bacteria/bacterial products in wash fluid specimens could include the time since the lesion tested had been disturbed (e.g., washed) before being tested. One other variable could be the positive effects associated with being a part of a clinical study, which resulted in superior compliance with treatment compared to those seen in the “usual” clinical setting.
Limiting the use of systemic antibiotics in the setting of a chronic disease like AD could reduce the development of antibiotic resistance and the frequency of side effects from these drugs. While our data suggest that oral antibiotics are not required to treat uncomplicated AD impetiginized with MRSA, this should be interpreted with caution until larger studies can test this hypothesis. Of course, complicated staphylococcal skin infections, especially involving MRSA, are a significant source of morbidity and mortality and should be treated with appropriate systemic antibiotics [26,27].
In summary, the current studies confirm the importance of S. aureus as a trigger for AD flares. Evaluation of wash fluid samples by quantitative bacterial culture from clinically impetiginized AD lesions before and after defined treatment revealed improvement in the staphylococcal CFU and EASI scores and reduced levels of staphylococcal products and inflammatory cytokines. A subset of patients whose AD lesions were impetiginized with MRSA improved with a treatment regimen which included oral cephalexin as the only antimicrobial. The presence of increased levels of defensins in wash fluid specimens of AD lesions after the treatment provides a mechanism by which the S. aureus bacterial superinfection is resolved.
Acknowledgments
This research was supported in part by grants from the Riley Memorial Association, and the National Institutes of Health grant U19 AI070448 (MK, JBT) and Veteran’s Administration Merit Award (JBT).
Footnotes
Conflict of Interest Statement:
The authors declare that they have no conflicts of interest.
References
- 1.Leung DY. Infection in atopic dermatitis. Curr Opin Pediatr. 2003;15:399–404. doi: 10.1097/00008480-200308000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Baker BS. The role of microorganisms in atopic dermatitis. Clin Exp Immunol. 2006;144:1–9. doi: 10.1111/j.1365-2249.2005.02980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kolls JK, McCray PB, Jr, Chan YR. Cytokine-mediated regulation of antimicrobial proteins. Nat Rev Immunol. 2008;8:829–35. doi: 10.1038/nri2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schauber J, Gallo RL. Antimicrobial peptides and the skin immune defense system. Journal of Allergy & Clinical Immunol. 2009;124(3 Suppl 2):R13–8. doi: 10.1016/j.jaci.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 5.Ong PY, Ohtake T, Brandt C, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–60. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 6.Howell MD, Boguniewicz M, Pastore S, Novak N, Bieber T, Girolomoni G, Leung DY. Mechanism of HBD-3 deficiency in atopic dermatitis. Clinical Immunology. 2006;121:332–8. doi: 10.1016/j.clim.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 7.Albanesi C, Fairchild HR, Madonna S, Scarponi C, De Pita O, Leung DY, Howell MD. IL-4 and IL-13 negatively regulate TNF-alpha- and IFN-gamma-induced beta-defensin expression through STAT-6, suppressor of cytokine signaling (SOCS)-1, and SOCS-3. J Immunol. 2007;179:984–92. doi: 10.4049/jimmunol.179.2.984. [DOI] [PubMed] [Google Scholar]
- 8.Kisich KO, Howell MD, Boguniewicz M, Heizer HR, Watson NU, Leung DY. The constitutive capacity of human keratinocytes to kill Staphylococcus aureus is dependent on beta-defensin 3. J Invest Dermatol. 2007;27(10):2368–80. doi: 10.1038/sj.jid.5700861. [DOI] [PubMed] [Google Scholar]
- 9.Buchau AS, Schauber J, Hultsch T, Stuetz A, Gallo RL. Pimecrolimus enhances TLR2/6-induced expression of antimicrobial peptides in keratinocytes. J Invest Dermatol. 2008;128:2646–54. doi: 10.1038/jid.2008.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller J, Gallo RL. Vitamin D and innate immunity. Dermatologic Therapy. 2010;23:13–22. doi: 10.1111/j.1529-8019.2009.01287.x. [DOI] [PubMed] [Google Scholar]
- 11.Nilsson EJ, Henning CG, Magnusson J. Topical corticosteroids and Staphylococcus aureus in atopic dermatitis. J Amer Acad Dermatol. 1992;27:29–34. doi: 10.1016/0190-9622(92)70151-5. [DOI] [PubMed] [Google Scholar]
- 12.Elston DM. Community-acquired methicillin-resistant Staphylococcus aureus. J Amer Acad Dermatol. 2007;56:1–16. doi: 10.1016/j.jaad.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 13.Travers JB, Kozman A, Mousdicas N, et al. Infected atopic dermatitis lesions contain pharmacologic amounts of lipoteichoic acid. J Allergy & Clin Immunol. 2010;125:146–52. doi: 10.1016/j.jaci.2009.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao Y, Kozman A, Al-Hassani M, Saha C, Yi Q, Yao W, et al. Identification of Staphylococcal Protein A in infected atopic dermatitis lesions. J Invest Dermatol. 2010;130:2502–04. doi: 10.1038/jid.2010.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol (Stockh) 1980;92:44–7. [Google Scholar]
- 16.Hanifin JM, Thurston M, Omoto M, et al. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. Exp Dermatol. 2001;10:11–8. doi: 10.1034/j.1600-0625.2001.100102.x. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Q, Mousdicas N, Yi Q, Al-Hassani M, Billings S, Perkins SM, et al. Staphylococcal Lipoteichoic Acid Inhibits Delayed-Type Hypersensitivity Reactions via the Platelet-activating Factor Receptor. J Clin Invest. 2005;115:2855–61. doi: 10.1172/JCI25429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williamson P, Kligman AM. A new method for the quantitative investigation of cutaneous bacteria. J Invest Dermatol. 1965;45:498–503. doi: 10.1038/jid.1965.164. [DOI] [PubMed] [Google Scholar]
- 19.Leyden JJ, Marples RR, Kligman AM. Staphylococcus aureus in the lesions of atopic dermatitis. Br J Dermatol. 1974;90:525–30. doi: 10.1111/j.1365-2133.1974.tb06447.x. [DOI] [PubMed] [Google Scholar]
- 20.Williams RE, Gibson AG, Aitchison TC, Lever R, Mackie RM. Assessment of a contact-plate sampling technique and subsequent quantitative bacterial studies in atopic dermatitis. Br J Dermatol. 1990;123:493–501. doi: 10.1111/j.1365-2133.1990.tb01455.x. [DOI] [PubMed] [Google Scholar]
- 21.Michelsen KS, Aicher A, Mohaupt M, et al. The role of toll-like receptors (TLRs) in bacteria-induced maturation of murine dendritic cells (DCS). Peptidoglycan and lipoteichoic acid are inducers of DC maturation and require TLR2. J Biol Chem. 2001;276:25680–25686. doi: 10.1074/jbc.M011615200. [DOI] [PubMed] [Google Scholar]
- 22.Gomez MI, Lee A, Reddy B, et al. Staphylococcus aureus protein A induces airway epithelial inflammatory responses by activating TNFR1. Nature Med. 2004;10:842–848. doi: 10.1038/nm1079. [DOI] [PubMed] [Google Scholar]
- 23.Huang JT, Abrams M, Tlougan B, Rademaker A, Paller AS. Treatment of Staphylococcus aureus colonization in atopic dermatitis decreases disease severity. Pediatrics. 2009;123:e808–14. doi: 10.1542/peds.2008-2217. [DOI] [PubMed] [Google Scholar]
- 24.Matiz C, Tom WL, Eichenfield LF, Png A, Friedlander SH. Children with atopic dermatitis appear less likely to be infected with community acquired methicillin-resistant Staphylococcus aureus: the San Diego experience. Ped Dermatol. 2011;28:6–11. doi: 10.1111/j.1525-1470.2010.01293.x. [DOI] [PubMed] [Google Scholar]
- 25.Suh L, Coffin S, Leckerman KH, Gelfand JM, Honig PJ, Yan AC. Methicillin-resistant Staphylococcus aureus colonization in children with atopic dermatitis. Ped Dermatol. 2008;25:528–34. doi: 10.1111/j.1525-1470.2008.00768.x. [DOI] [PubMed] [Google Scholar]
- 26.Hayakawa K, Hirahara K, Fukuda T, Okazaki M, Shiohara T. Risk factors for severe impetiginized atopic dermatitis in Japan and assessment of its microbiological features. Clin & Exp Dermatol. 2009;34:e63–65. doi: 10.1111/j.1365-2230.2008.03180.x. [DOI] [PubMed] [Google Scholar]
- 27.Hon KL, Leung AK, Kong AY, Leung TF, Ip M. Atopic dermatitis complicated by methicillin-resistant Staphylococcus aureus infection. J Nat Med Assoc. 2008;100:797–800. doi: 10.1016/s0027-9684(15)31373-0. [DOI] [PubMed] [Google Scholar]
- 28.Howell MD, Kim BE, Gao P, et al. Cytokine modulation of atopic dermatitis filaggrin skin expression. J Allergy & Clin Immunol. 2009;124(3 Suppl 2):R7–R12. doi: 10.1016/j.jaci.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 29.Sehra S, Yao Y, Howell MD, Nguyen ET, Kansas GS, Leung DY, Travers JB, Kaplan MH. IL-4 regulates skin homeostasis and the predisposition toward allergic skin inflammation. J Immunol. 2010;184:3186–90. doi: 10.4049/jimmunol.0901860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sircar KD, Bancroft E, Nguyen DM, Mascola L. Hospitalization of paediatric patients for methicillin-resistant Staphylococcus aureus skin and soft-tissue infection, 1998–2006. Epidemiol & Infect. 2010;138:677–82. doi: 10.1017/S095026880999121X. [DOI] [PubMed] [Google Scholar]
- 31.Moran GJ, Krishnadasan A, Gorwitz RJ, et al. Methicillin-resistant S. aureus infections among patients in the emergency department. New Engl J Med. 2006;355:666–74. doi: 10.1056/NEJMoa055356. [DOI] [PubMed] [Google Scholar]
- 32.Jacobs MR, Jones RN, Giordano PA. Oral β-lactams applied to uncomplicated infections of skin and skin structures. Diag Microbiol Infect Dis. 2007;57:55S–65S. doi: 10.1016/j.diagmicrobio.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 33.Bath-Hextall FJ, Birnie AJ, Ravenscroft JC, Williams HC. Interventions to reduce Staphylococcus aureus in the management of atopic eczema: an updated Cochrane review. Br J Dermatol. 2010;163:12–26. doi: 10.1111/j.1365-2133.2010.09743.x. [DOI] [PubMed] [Google Scholar]
- 34.Rex JH, Pfaller MA. Has antifungal susceptibility testing come of age? Clin Infect Dis. 2002;35:982–989. doi: 10.1086/342384. [DOI] [PubMed] [Google Scholar]


